Abstract
The bulbs of Fritillaria cirrhosa is wildly used in traditional Chinese medicine to treat lung-related disease, which has recently been found to have antitussive, anti-inflammatory, antihypertensive and anti-tumor activity. Steroidal alkaloids are the major effective ingredients of F. cirrhosa. In the current study, we demonstrated an efficient strategy for F. cirrhosa bulb regeneration in vitro by cytokinin/auxin induction. Our data showed that the regenerated bulbs accumulated higher alkaloid content that the wild ones. We further performed RNA-seq and bioinformatics analysis to study the gene expression profile, especially those related to alkaloids biosynthesis. KEGG pathway annotation identified genes related to “Metabolic pathways” were the most abundant (2644, 26.0%), followed by those for “Biosynthesis of secondary metabolites” (1319, 13.0%) among the 113,865 unigenes identified. Further analysis suggested MEP pathway, other than MVA pathway, might be the major route for steroidal alkaloid biosynthesis of F. cirrhosa, as all the key genes in this pathway were found to be unregulated in our study. We also showed that accumulation of different phytochemicals was linked to plant hormone addition. Our current study demonstrated that in vitro cultivation is a promising strategy for mass production of F. cirrhosa steroidal alkaloids for pharmacological industry.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1218-y) contains supplementary material, which is available to authorized users.
Keywords: Fritillaria cirrhosa, Transcriptome, High-throughput sequencing, In vitro cultures, Cytokinin/auxin
Introduction
Fritillaria cirrhosa D. Don (Family, Liliaceae) is a bulbous medicinal plant native to China, especially the southeastern margin of the Qinghai–Tibet Plateau region (Zhang et al. 2010). The bulbs of this plant, called Chuan Bei Mu, are commonly used to treat cough in traditional Chinese medicine (TCM) for over 2000 years. Recent studies revealed that the extracts of F. cirrhosa bulbs possess anti-inflammatory and anti-cancer activities (Luo et al. 2012). A series of ingredients with proved pharmacological activities have been detected in F. cirrhosa bulbs, including steroidal alkaloid, saponins, terpenoids and glycosides (Jian et al. 2012). Due to the lack of effective cultivation techniques, the Chuan Bei Mu has mainly been collected from natural resources (Li et al. 2012). However, F. cirrhosa is endangered as a consequence of long-term overexploitation (Li et al. 2012; Zhang et al. 2010).
In recent years, huge amount of efforts has been put into the development of in vitro cultivation technique for medicinal plants producing bioactive compounds (Park and Paek. 2014; Qi et al. 2009). Attempt of F. cirrhosa tissue culture suggested a promising way for mass propagation and accumulation of major phytochemicals within a short period of time (Chen et al. 1995). Efficient production of major phytochemicals requires understanding of their biosynthetic pathways, and our present knowledge to F. cirrhosa phytochemical biosynthesis is limited to only a few genes involved in these pathways (Sun et al. 2011).
High-throughput RNA sequencing (RNA-Seq) is capable of generating the whole transcriptome information at single transcript level, enabling the characterization of secondary metabolite biosynthetic pathways (Pal et al. 2015). Initial efforts have been made to generate expressed sequence tags (ESTs) from the bulbs of F. cirrhosa collected in the wild (Sun et al. 2011). However, no large-scale transcriptome information validated by expression profiling is available for in vitro cultivated bulbs of F. cirrhosa. The key genes regulating major phytochemicals biosynthesis of F. cirrhosa have never been characterized functionally.
In this study, we performed RNA-Seq to generate and compare the transcriptomes of the wild and in vitro-regenerated F. cirrhosa bulbs. We comprehensively analyzed the gene expression profiles of F. cirrhosa with emphasis on genes related to the biosynthesis of steroidal alkaloid biosynthetic pathways, which are the major bioactive ingredients responsible for the pharmacological features (Jian et al. 2012; Wang et al. 2016). Our results demonstrated that steroidal alkaloid biosynthesis-related genes were universally upregulated in the in vitro-generated bulbs. This is consistent with the observation that the total alkaloid contents are higher in the regenerated bulbs than the wild bulbs. The current study provided supporting evidence that in vitro cultivation of F. cirrhosa could serve as a promising source for targeted steroidal alkaloid biosynthesis.
Materials and methods
Plant material preparation and measurement of the total alkaloid
Wild bulbs (WB) were collected from 3-year-old F. cirrhosa plants growing in the Kangding fold–thrust belt mountains (located at 30°3′44.9″N, 101°58′3.81″E, altitude 4300 m) in Sichuan Province, China. Regenerated bulbs (RB) were induced from wild bulb explants of F. cirrhosa on Murashige and Skoog (MS) medium supplemented with 30 g/L sucrose, 2 mg/L 6-BA and 0.5 mg/L NAA in a 16 h light/8 h dark cycle at 20 °C. Relative transcript abundance analysis used the same plant material as for the transcriptome analysis.
Total alkaloid extraction was performed as previously described with minor modifications (Liu et al. 2004). UV spectrophotometer was adopted to determine the content of total alkaloids using peiminine as the standard.
RNA extraction, cDNA library construction, and sequencing
Total RNA was extracted using RNeasy® Plant Mini Kit (Qiagen) according to the manufacturer’s protocol. The integrity of RNA was assessed by formaldehyde agarose gel electrophoresis. A total of 30 μg mixed RNA from three biological replicates detected by 2100 Bio-analyzer (Agilent, USA) was digested with DNase I (TAKARA), and then purified by Dynabeads® Oligo (dT)25 (Life, USA). 100 ng derived mRNAs were fragmented and reverse transcribed into first-strand cDNAs with random hexamer. The second-strand cDNAs were synthesized using a NEBNext® Ultra™ RNA Library Prep Kit for Illumina (NEB). The double-stranded cDNAs were purified and ligated to adaptors for Illumina paired-end sequencing. The cDNA libraries were sequenced on the Illumina HiSeq™ 2000 platform using the paired-end technology of Gene Denovo Co. (Guangzhou, China).
De novo assembly and annotation
Raw reads were first processed using in-house Perl scripts. The raw reads were filtered by removing adapter sequences, reads containing poly-N sequences, and low-quality sequences. Clean reads were de novo assembled using the Trinity Program (Grabherr et al. 2011). Unigenes were defined after removing redundancy and short contigs from the assembly. The unigenes were predicted by “GetORF” in the EMBOSS.
The unigenes were packaged (Rice et al. 2000) and aligned to the protein sequence database NCBI NR (non-redundant protein database), Swiss-Port (Annotated protein sequence database), KEGG (Kyoto encyclopedia of genes and genomes) and KOG (Clusters of orthologous groups of protein) by Blastp with an E-value threshold of 1 × 10−5.
The number of unique-match reads was calculated and normalized to RPKM (reads per kb per million reads) for gene expression analysis. Comparison of unigene expression between WB and RB was performed according to DESeq as described by Abders and Huber (Anders and Huber 2010). The differentially expressed genes (DEGs) between two samples were restricted with FDR (false discovery rate) ≤ 0.001 and the absolute value of log2 Ratio ≥ 1.
To examine the expression profile of DEGs, the expression data υ between two samples were normalized to 0, log2 (υRB/WB), and then clustered by Short Time-series Expression Miner software (STEM) (Ernst and Bar-Joseph 2006). The clustered profiles with P value ≤ 0.05 were considered as significantly expressed. Then the DEGs in all or in each profile were subjected to gene ontology (GO) classifications using WEGO (Ye et al. 2006), and KEGG (Kanehisa et al. 2000) pathway enrichment analysis.
Validation of differential expression using qRT-PCR
The cDNA was generated from 1 μg total RNA isolated from the bulbs using a Prime-Script ™ 1st Strand cDNA Synthesis Kit (TAKARA, Japan). Primers for quantitative real-time PCR (qRT-PCR) were designed using Primer Premier 5.0 software (Premier, Canada) and synthesized by Sangon Biotech (Shanghai) Co., Ltd. The 18S (Gen-Bank accession number: AY616727.1) was selected as the reference. The primer sequences are listed in Supplementary Table S1. qRT-PCR was performed on a Bio-Rad iQ5 Optical System Real Time PCR System (Bio-R ad, USA). Each reaction mixture was 20 μL containing 10 μL of SYBR Green PCR Master Mix (TaKaRa, Japan), 250 nM of each primer, and 6 μL of diluted first-strand cDNAs. The qRT-PCRs were run as follows: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s in 96-well optical reaction plates. The Ct values were determined for three biological replicates, with three technical replicates for each value. Expression levels of the tested reference genes were determined by Ct values and calculated by 2−△△Ct.
Statistical analysis
All data were statistically analyzed by means of the SPSS with LSD to identify differences. Significant differences (P < 0 0.05) between treatments were indicated by different letters.
Results and discussion
Regeneration of F. cirrhosa bulbs in vitro and the quantification of total alkaloid
F. cirrhosa is a medicinal plant with high pharmaceutical value, and the bulbs produce major secondary metabolites responsible for most of the pharmacological activity of this plant (Wang et al. 2016). The accumulation of secondary metabolites in alpine growing plants can be influenced by various environmental factors as well as the age of the plant at harvest (Verma and Shukla 2015). The growing demand in the pharmaceutical industry led us to search for an alternative approach for mass production of F. cirrhosa bulbs. In vitro cultivation techniques have been shown to be a useful tool for mass multiplication (Chen et al. 1995; Sairkar et al. 2009; Senthil et al. 2015).
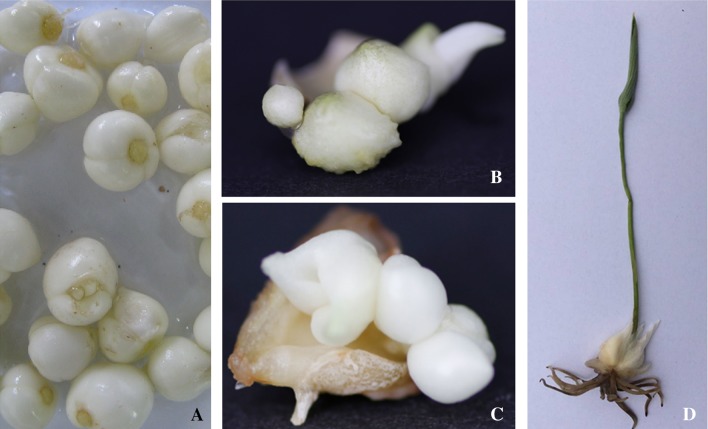
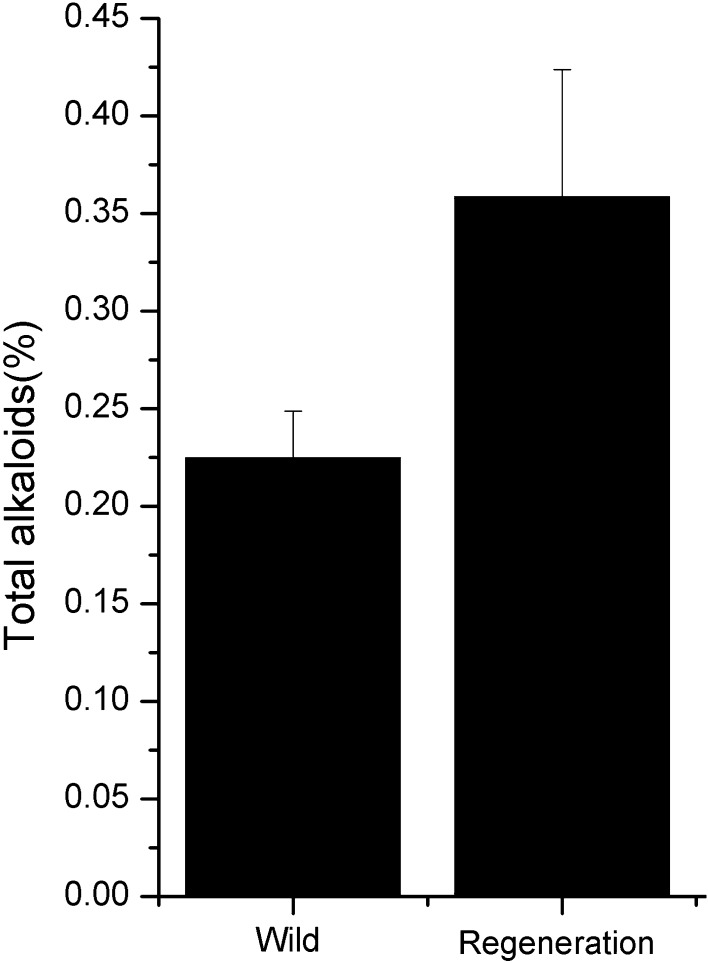
Recent years, cytokinin/auxin combinations media have been successfully exploited for the induction of organogenesis (Khanam et al. 2000). In our current study, treatments were compared for the effects of cytokinin/auxin combinations (6-BA and NAA) on the induction and multiplication from WB explants. As shown in Fig. 1b, c, the regenerated bulbs were induced successfully from bulb explants of F. cirrhosa, as observed by swelling and enlargement of immature bulbs 45–60 days after culture initiation, and subsequent direct bulb formation during the fourth month (Fig. 1d). We then used decocting method to determine the total alkaloid content of WB and RB. As show in Fig. 2, the total alkaloids were more abundant in RB than that in WB. The results of these in vitro cultivation assays indicated that cytokinin/auxin treatment was efficient in inducing the initial proliferation of F. cirrhosa.
Fig. 1.
In vitro propagation and plantlet regeneration from F. cirrhosa bulbs explants. a Wild bulbs; b and c induced callus from explants of wild bulb; d regenerated plantlet with bulb
Fig. 2.
Determination of F. cirrhosa bulbs’ total alkaloid content
RNA-Seq and de novo assembly
RNA-seq provides a global overview of the gene expression at the transcriptome level. To understand the impact of in vitro cultivation on the genes expression profile of F. cirrhosa, especially those genes related to the phytochemical biosynthesis, we performed RNA-Seq with the wild and regenerated F. cirrhosa bulbs. We obtained 73,632,020 and 34,583,794 cleaned high-quality reads from the WB and RB, respectively (Table 1), which were remarkably more than the previously reported ESTs analysis with 1343 unique transcripts (Sun et al. 2011). All clean reads were pooled together and then de novo assembled by Trinity to generate a unique transcript library. 113,865 unigenes with a mean size of 528 bp and N50 number of 617 bp were assembled. The length of the genes ranged from 231 to 16,461 bp (Table 1).
Table 1.
Summary of the RNA-Seq data of the two libraries
| Libraries | No. of raw reads | No. of clean reads | No. of assembled unigenes | Average of unigene length (bp) | N50 of unigene length (bp) | Min of unigene length (bp) | Max of unigene length (bp) |
|---|---|---|---|---|---|---|---|
| WB | 76,711,942 | 73,632,020 | 113,865 | 528 | 617 | 231 | 16,461 |
| RB | 35,324,402 | 34,583,794 |
Functional annotation of the assembled transcripts
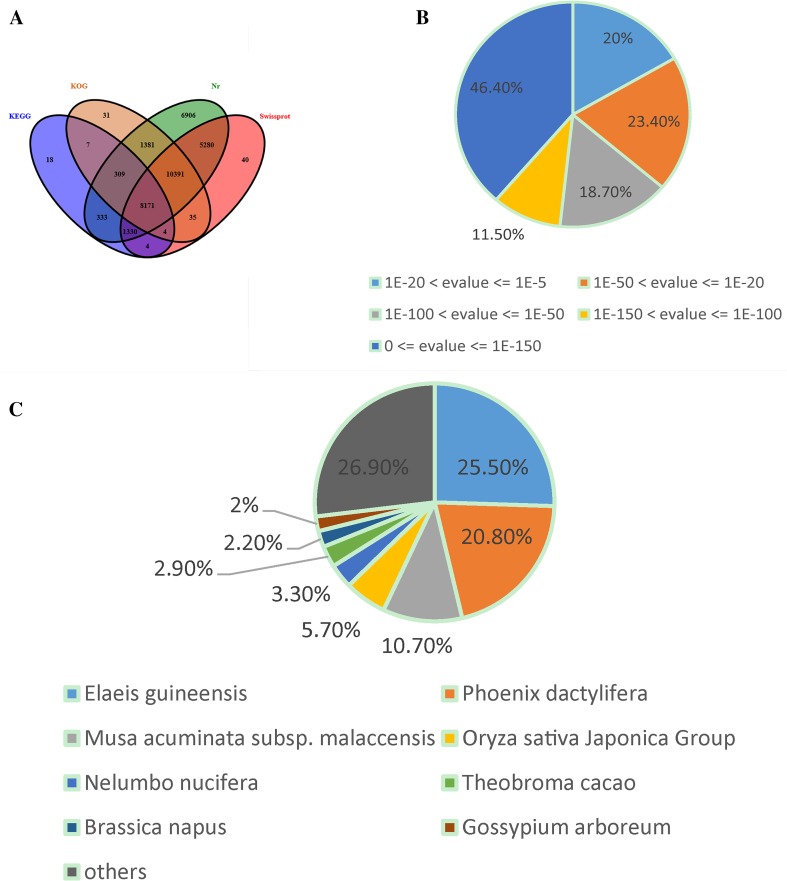
The annotation for F. cirrhosa unique sequences was based on BLASTP searches of four public databases. A final number of 34,101, 25,255, 20,329 and 10,176 unigenes (E value < 1e−5) had significant matches in NR, Swiss-prot, KOG and KEGG databases, respectively (Fig. 3a). Based on the NR annotation and the E value distribution, 79.8% of the mapped sequences showed strong homology (E < 10−20) and 56.6% showed very strong homology (E < 10−50) to the available plant sequences (Fig. 3b). With respect to species, 25.5 and 20.8% of the unique sequences had top matches to sequences from Elaeis guineensis and Phoenix dactylifera, respectively, with additional hits to Musa acuminata subsp. malaccensis (10.7%), Oryza sativa Japonica Group (5.7%), Nelumbo nucifera (3.3%), Theobroma cacao (2.9%), etc. (Fig 3c).
Fig. 3.
Characteristics of homology search of F. cirrhosa unigenes. a Venn diagram of the number of unigenes annotated by BLASTx with an E-value threshold of 10−5 against protein databases. The number in the circles indicates the number of unigenes annotated by single or multiple databases. b E value distribution of the top BLASTx hits against the Nr database for each unigene. c Percentage of unigenes matching the top eight species using BLASTx in the Nr database
We used GO assignments to classify the functions of the predicted unigenes. GO annotated unigenes were categorized into three ontologies: biological process, cellular component, and molecular function. The biological process category included the largest number of unigenes (33,664), followed by cellular component (26,160), and then molecular function (12,142) (Supplementary Figure S1). Within the biological process category, “metabolic process,” “cellular process,” and “single-organism process” were the most enriched, while proteins related to “cell,” “cell part,” and “organelle” were enriched in the cellular component category. Among the molecular function category, “catalytic activity” and “binding” accounted for most of the unigenes (Supplementary Figure S1). Transcripts related to GO term “metabolic process” was the most abundant in the biological process category, which is similar to the previously reported GO annotation of medicinal tissues (Han et al. 2013; Tang et al. 2016). Furthermore, 35,970 unigenes with annotations were assigned to KOG classifications. Among the 25 KOG categories, the cluster for “general function prediction only” represented the largest category (6398, 17.8%), followed by the “post-translational modification, protein turnover, chaperones” (4611, 12.8%), “signal transduction mechanisms” (3305, 9.2%), and “RNA processing and modification” (2379, 6.7%) (Supplementary Figure S2).
We then performed KEGG pathway annotation to identify the biological function and gene interaction. 10176 unigenes with annotation could be distributed into 123 KEGG pathways. Genes related to “Metabolic pathways” were found to be the most abundant in number (2644, 26.0%), followed by those for “Biosynthesis of secondary metabolites” (1319, 13.0%) and “Ribosome” (562, 5.5%) (Supplementary Table S2). Of the transcripts assigned to secondary metabolite biosynthetic pathways, a large pool with 210 (2.06%) members was mapped to phenylpropanoid biosynthesis with terpenoid backbone biosynthesis (81, 0.8%), and N-Glycan biosynthesis also included many transcripts (74, 0.73%) (Supplementary Table S3). The overall annotation of the F. cirrhosa transcriptome provided a valuable resource for investigating specific processes, functional descriptions and pathways. On the basis of the applied criteria (fold change ≥ 2, FDR < 0.001), 7045 genes were identified as significantly DEGs between the WB and RB, of these, 3509 were up-regulated and 3536 were down-regulated.
Expression of the major steroidal alkaloid biosynthesis-related genes
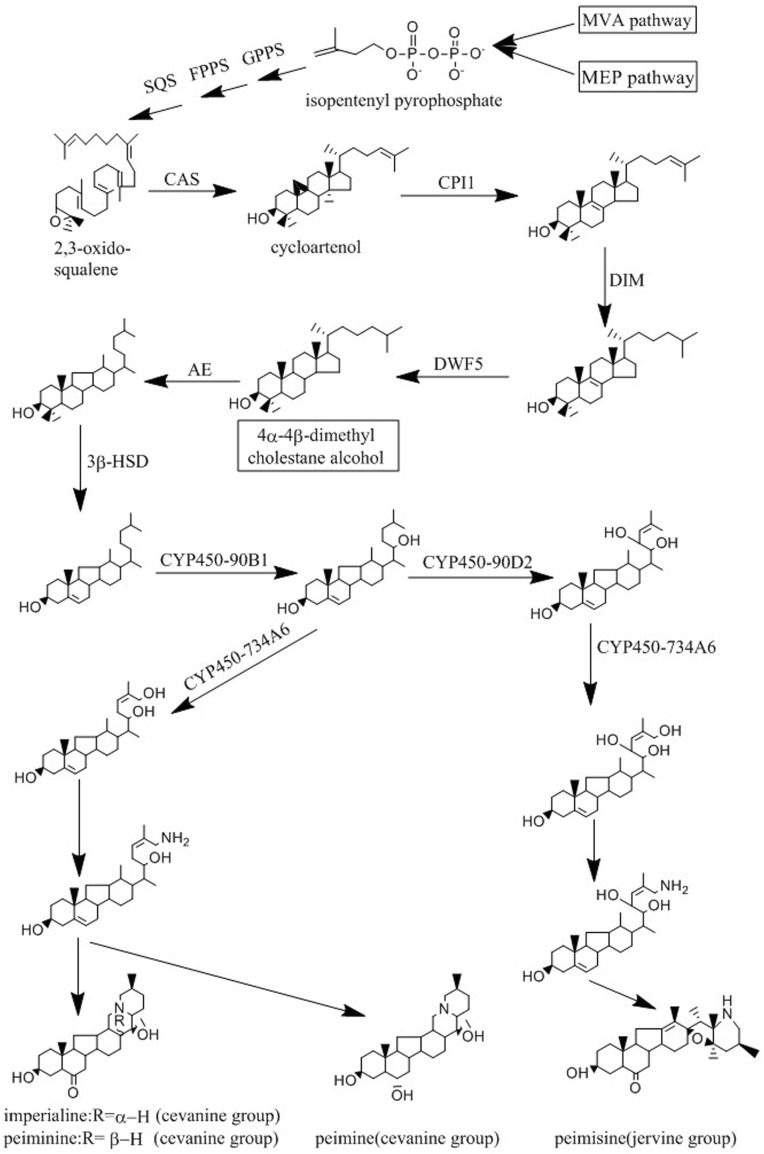
The major phytochemicals (steroidal alkaloid) of F. cirrhosa includes peimine, imperialine, peiminine, peimisine. The biosynthesis pathway of these steroidal alkaloids is still largely unknown. Steroid are synthesized via two pathways in plants: the classical mevalonate (MVA) pathway and the methylerythritol phosphate (MEP) pathway that leads to the synthesis of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) (Kul’Kova et al. 1999). These intermediates serve as the substrates that undergo a cascade of chemical conversions along with the formation of metabolite intermediates, such as geranyl pyrophosphate (GPP), farnesyl pyrophosphate (FPP), squalene, cycloartenol, through terpenoid backbone biosynthesis (Schaller 2010). Cycloartenol is a common intermediate for the biosynthesis of steroidal alkaloid (Dev 1984). The steroidal alkaloids of F. cirrhosa, which have a cevanine or jervine-type framework, are considered to arise from cycloartenol by catabolic processes with nitrogen incorporation or hydroxylation reactions (Hao et al. 2013). The possible biosynthetic pathway of F. cirrhosa steroidal alkaloids was proposed by analyzing the chemical structures of different types of steroid alkaloids and the function of their biocatalytic enzymes in the functionalization of steroid skeleton (Fig. 4).
Fig. 4.
Proposed F. cirrhosa steroidal alkaloid biosynthetic pathways
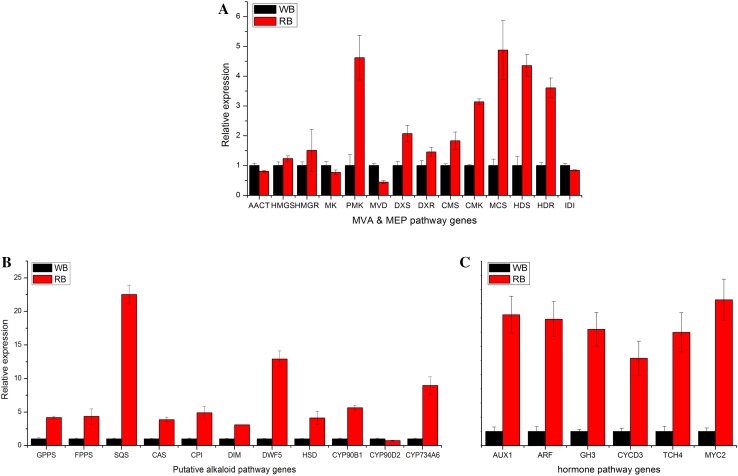
To validate the above proposed F. cirrhosa steroidal alkaloid biosynthetic pathways and establish a complete expression profile for genes involved in this process, we first extracted 6 and 8 unigenes annotated as different enzyme-coding genes from the MEP and MVA pathways, (Table 2). As showed in Table 2, expression levels of unigenes annotated as mevalonate kinase (MK) and diphosphomevalonate decarboxylase-like (MVD) of the MVA pathway were down-regulated, while unigene annotated as phosphomevalonate kinase (PMK) was up-regulated in the RB. For the MEP pathways, the expression levels of the majority unigenes (6 in 8) were significantly up-regulated in the RB. Therefore, unlike MVA pathway-associated unigenes that showed mixed expression trend, the expression of unigenes associated with MEP pathways showed unilateral upward trend comparing the WB and RB. These results were consistent with the qRT-PCR analysis of the MEP and MVA pathway genes identified (Fig. 5a). A previous study showed that the steroidal backbones were synthesized via the MVA pathways, and not via MEP pathways in plants (Suzuki and Muranaka 2007). While our current results are not sufficient to draw a conclusion regarding F. cirrhosa steroid synthesis, our data at least suggested that MEP pathway is the main route to the production of steroidal backbones for this particular plant. It is critical to confirm this result in future studies to understand F. cirrhosa sterol alkaloid biosynthesis comprehensively. Using chemical inhibitors to the rate-limiting enzymes on the two pathways might provide valuable information to answer this question (Bach and Lichtenthaler 1982; Zeidler et al. 1998).
Table 2.
Genes identified by transcriptome in putative alkaloid biosynthetic pathways
| Pathway | Annotation | Gene ID | RPKM | Trend | |
|---|---|---|---|---|---|
| Wild | Regeneration | ||||
| MVA | AACT(acetyl-CoA acetyltransferase) | Unigene0050126 | 128.618 | 121.771 | – |
| HMGS(hydroxymethylglutaryl-CoA synthase) | Unigene0048264 | 270.704 | 289.662 | – | |
| HMGR(3-hydroxy-3-methylglutaryl-coenzyme A reductase) | Unigene0052102 | 376.287 | 414.101 | – | |
| MK(mevalonate kinase) | Unigene0022250 | 10.8035 | 5.6103 | ↓ | |
| PMK(phosphomevalonate kinase) | Unigene0050127 | 11.2354 | 28.5526 | ↑ | |
| MVD(diphosphomevalonate decarboxylase-like) | Unigene0052125 | 109.969 | 37.8945 | ↓ | |
| MEP | DXS(1-deoxy-D-xylulose-5-phosphate synthase 2) | Unigene0039155 | 12.0899 | 49.5867 | ↑ |
| DXR(chloroplast 1-deoxy-D-xylulose-5-phosphate reductoisomerase) | Unigene0048178 | 63.5768 | 77.0203 | – | |
| CMS(2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase) | Unigene0020765 | 0.001 | 0.4418 | ↑ | |
| CMK(4-diphosphocytidyl-2-C-methyl-D-erythritol kinase) | Unigene0019637 | 0.1256 | 9.988 | ↑ | |
| MCS(2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase) | Unigene0017850 | 0.001 | 0.4999 | ↑ | |
| HDR(4-hydroxy-3-methylbut-2-enyl diphosphate reductase) | Unigene0017845 | 0.0598 | 13.6742 | ↑ | |
| HDS(4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase) | Unigene0048148 | 49.4889 | 80.3542 | ↑ | |
| IDI(isopentenyl diphosphate isomerase) | Unigene0045079 | 521.259 | 261.7421 | ↓ | |
| Downstream | GPPS(geranyl pyrophosphate synthase) | Unigene0038524 | 10.1704 | 95.2013 | ↑ |
| FPPS(farnesyl pyrophosphate synthase) | Unigene0037411 | 0.0197 | 44.9723 | ↑ | |
| SQS(squalene synthase) | Unigene0029799 | 2.5466 | 30.0693 | ↑ | |
| CAS(cycloartenol synthase) | Unigene0038300 | 36.3476 | 87.6244 | ↑ | |
| CPI1(cyclopropyl sterol isomerase1) | Unigene0033621 | 5.104 | 12.0916 | ↑ | |
| DIM(delta(24)-sterol reductase) | Unigene0048260 | 46.8104 | 171.8682 | ↑ | |
| DWF5(7-dehydrocholesterol reductase) | Unigene0030355 | 22.8576 | 140.7592 | ↑ | |
| 3β-HSD(3β-hydroxysteroid decarboxylase) | Unigene0036411 | 0.1364 | 0.9741 | ↑ | |
| C-22 hydroxylase(cytochrome P450-90B1) | Unigene0037699 | 59.1627 | 362.3193 | ↑ | |
| C-23 hydroxylase(cytochrome P450-90D2) | Unigene0041552 | 132.8696 | 55.9507 | ↓ | |
| C-26 hydroxylase(cytochrome P450-734A6) | Unigene0040793 | 25.2658 | 57.0467 | ↑ | |
| Hormone | AUX1(gibberellin receptor GID1C) | Unigene0028406 | 0.001 | 19.3756 | ↑ |
| ARF(auxin response factor 11) | Unigene0028137 | 0.001 | 2.9979 | ↑ | |
| GH3(indole-3-acetic acid-amido synthetase) | Unigene0033714 | 1.0939 | 8.6828 | ↑ | |
| CYCD3(cyclin-D3) | Unigene0038335 | 4.0042 | 31.6655 | ↑ | |
| TCH4(xyloglucan endotransglucosylase) | Unigene0020230 | 0.157 | 3.0823 | ↑ | |
| MYC2(transcription factor MYC2) | Unigene0030546 | 2.8326 | 15.746 | ↑ | |
Fig. 5.
qRT-PCR analysis of the expression levels of MVA & MEP-related (a), putative alkaloid biosynthesis-related (b) and hormone signal transduction-related (c) genes
Meanwhile, all the 11 enzyme-coding genes (downstream pathway) known to be involved in the alkaloid downstream biosynthetic pathways were identified based on the functionalization of steroidal backbones (Table 2) (Kutchan et al. 2008). Among these enzymes, cyclase, oxidases, isomerases, hydroxylase and aminotransferases may take part in the conversion of cholesterol to sterol alkaloids. The sterol alkaloid biosynthesis starts with squalene oxidation and cyclization by different forms of (S)-2, 3-oxidosqualene cyclase. CAS catalyzes the biosynthesis of cycloartenol, which serves as a substrate for the production of phytosterols (Kutchan et al. 2008). Cytochrome P450s (CYP450) perform the multiple hydroxylation reactions of a wide variety of natural compounds, which is an important mechanism in secondary metabolism (Morant et al. 2003).
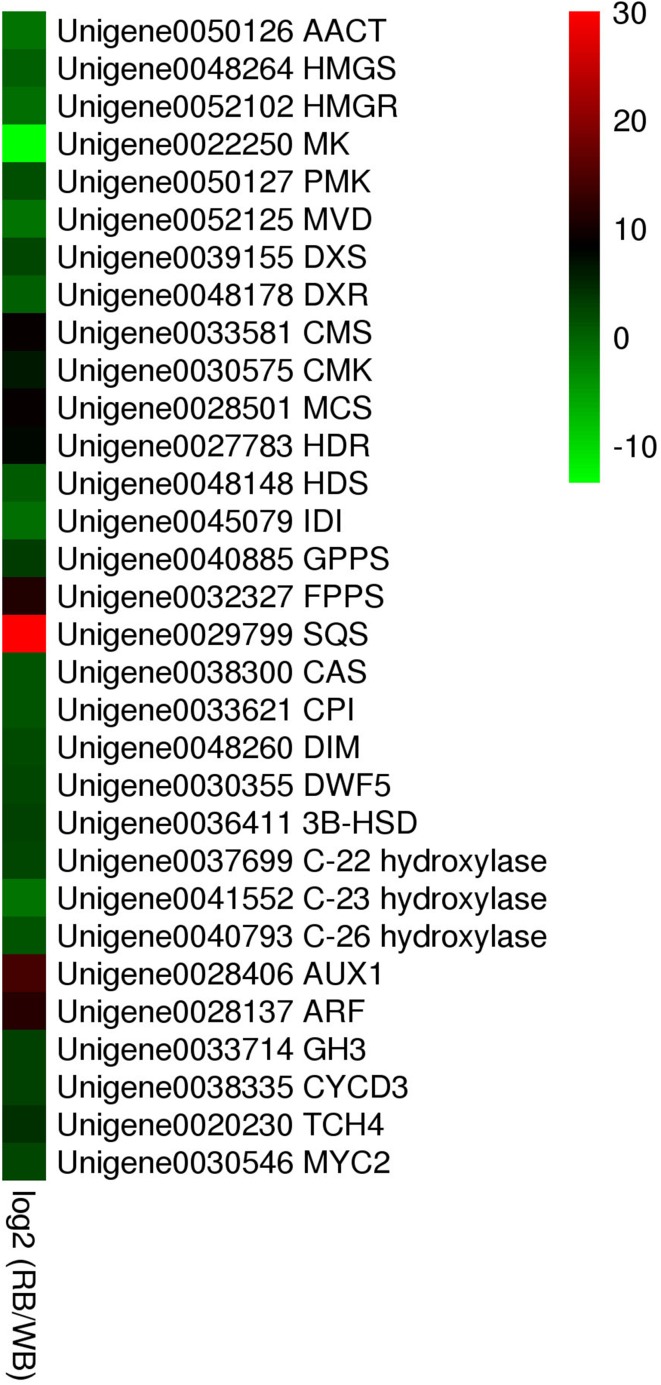
In the present study, out of 11 unigenes which were reported to be the rate-limiting enzymes in sterol biosynthesis (Morant et al. 2003; Suzuki and Muranaka 2007) showed up-regulated trend in the RB except for the cytochrome P450-90D2 (Table 2, Fig. 6). The upregulation of these genes were in parallel with the qRT-PCR results (Fig. 5b), and coinciding with the increased accumulation of alkaloid in the RB. There may be a positive correlation between high expression of the genes for the alkaloid downstream biosynthetic pathways and relatively higher accumulation of alkaloids in vitro bulbs. As the RB was induced by cytokinin/auxin combination, we further compared the expression level of six genes participating in plant hormone signal transduction between WB and RB (Table 2). The expression of these genes was significantly higher in RB than WB (Figs. 5c, 6). The expression level of transcription factor MYC2, which is responsible for regulating ORCA gene expression, which further affects the expression of a cascade of alkaloid biosynthesis genes (Zhang et al. 2011), was up-regulated in RB according to the RNA-seq and qRT-PCR results. Therefore, in F. cirrhosa, a series of genes required for the hormone signal transduction may activate some undetermined pathway (MVA or MEP) involved in steroidal alkaloid biosynthesis and finally affect the accumulation of major phytochemicals such as peimine, imperialine, and verticine.
Fig. 6.
Heat map analysis of the expression levels of MVA & MEP-related (a), putative alkaloid biosynthesis-related (b) and hormone signal transduction-related (c) genes
Conclusion
The current work demonstrated that the F. cirrhosa bulbs could be regenerated from callus induction with cytokinin/auxin combination and RB showed higher alkaloid accumulation than the wild bulbs. Our transcriptome analysis identified 113,865 unique genes. Among these unigenes, KEGG pathway annotation identified genes related to “Metabolic pathways” were the most abundant (2644, 26.0%), followed by those for “Biosynthesis of secondary metabolites” (1319, 13.0%). Further analysis suggested MEP pathway might be the one responsible for the steroidal alkaloids biosynthesis of F. cirrhosa, as all the key genes in this pathway were found to be unregulated in our study. We also showed that accumulation of different phytochemicals was linked to plant hormone addition. Altogether, our work provided valuable information on the gene expression profile of in vitro-generated F. cirrhosa and led to a proposal regarding the steroidal alkaloid biosynthetic pathway. This work demonstrated that the in vitro cultivation is a promising strategy for mass production of F. cirrhosa steroidal alkaloids for pharmacological industry.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1 Gene ontology (GO) classification of assembled in F. cirrhosa unigenes (JPEG 992 kb)
Fig. 2 Eukaryotic Orthologous Groups (KOG) classification of assembled in F. cirrhosa unigenes (JPEG 68 kb)
Acknowledgements
We are grateful to Dr. Nan Jiang from Ohio State University for helping us with language editing. This work was supported by the Natural Science Foundation of China (31600261).
Author contributions
Conceived and designed the experiments: WGW, JL. Performed the experiments: QZ, RL, KJH, YZ. Contributed reagents/materials/analysis tools: WGW, JL. Wrote the paper: QZ, RL, WGW, JL. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Qi Zhao and Rui Li authors contributed equally to this work.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1218-y) contains supplementary material, which is available to authorized users.
Contributor Information
Wenguo Wang, Phone: +86 28 85235971, Email: wangwenguo@caas.cn.
Jian Li, Phone: +86 28 85235971, Email: lijian01@cdu.edu.cn.
References
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach TJ, Lichtenthaler HK. Mevinolin: a highly specific inhibitor of microsomal 3-hydroxy-3-methylglutaryl-coenzyme a reductase of radish plants. Zeitschrift Für Naturforschung C. 1982;37(1–2):46–50. doi: 10.1515/znc-1982-1-209. [DOI] [PubMed] [Google Scholar]
- Chen M, Chen H, Zhong F, Wang B. Tissue culture of bulbus Fritillariae cirrhosae. China J Chin Materia Med. 1995;20(8):461. [PubMed] [Google Scholar]
- Dev S. Cycloartenol to Buxus alkaloids. J Chem Sci. 1984;93(6):1015–1030. [Google Scholar]
- Ernst RBJ, Bar-Joseph Z. STEM: a tool for the analysis of short time series gene expression data. BMC Bioinform. 2006;7(1):1–11. doi: 10.1186/1471-2105-7-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han XJ, Wang YD, Chen YC, Lin LY, Wu QK. Transcriptome sequencing and expression analysis of terpenoid biosynthesis genes in Litsea cubeba. PLoS One. 2013;8(10):e76890. doi: 10.1371/journal.pone.0076890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao DC, Gu XJ, Xiao PG, Peng Y. Phytochemical and biological research of Fritillaria medicine resources. Chin J Nat Med. 2013;11(4):330. doi: 10.3724/SP.J.1009.2013.00330. [DOI] [PubMed] [Google Scholar]
- Jian GU, Tan R, Jing LI, Zhangg E, Luo XW, Fan LN. Total saponins contents and anti-inflammatory effect of Fritillariae cirrhosae bulbus of different species. J Southwest Univ Nationalities. 2012;38(02):252–255. [Google Scholar]
- Kanehisa M, Goto S. Kegg: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;27(1):29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanam N, Khoo C, Khan AG. Effects of cytokinin/auxin combinations on organogenesis, shoot regeneration and tropane alkaloid production in Duboisia myoporoides. Plant Cell Tissue. 2000;62(2):125–133. doi: 10.1023/A:1026568712409. [DOI] [Google Scholar]
- Kul’Kova VV, Shakirov R, D’Yakonov AL. Steroid alkaloids of the plant and animal Kingdoms. Chem Nat Compd. 1999;35(2):107–149. doi: 10.1007/BF02234919. [DOI] [Google Scholar]
- Kutchan TM, Frick S, Weid M. Engineering plant alkaloid biosynthetic pathways: progress and prospects. Adv Plant Biochem. 2008;1(07):283–310. [Google Scholar]
- Li X, Song J, Wei J, Hu Z, Xie C, Luo G. Natural Fostering in Fritillaria cirrhosa: integrating herbal medicine production with biodiversity conservation. Acta Pharm Sin B. 2012;2(1):77–82. doi: 10.1016/j.apsb.2011.12.006. [DOI] [Google Scholar]
- Liu B, Shi R. Research on the method for quantitatively determining total alkaloid in the effective fraction of the extract of Kushen Decoction. J Beijing Univ Tradit Chin Med. 2004;02:284–288. [Google Scholar]
- Luo H, Zhang L, Xu W, Yang J, Yang W, Yang A, Wen X, Cui H. Simultaneous determination of four main isosteroidal alkaloids of bulbus Fritillariae cirrhosae in rat plasma by LC–MS–MS. Chromatographia. 2012;75(13–14):729–737. doi: 10.1007/s10337-012-2247-z. [DOI] [Google Scholar]
- Morant M, Bak S, Møller BL, Werckreichhart D. Plant cytochromes P450: tools for pharmacology, plant protection and phytoremediation. Curr Opin Biotech. 2003;14(2):151–162. doi: 10.1016/S0958-1669(03)00024-7. [DOI] [PubMed] [Google Scholar]
- Pal T, Malhotra N, Chanumolu SK, Chauhan RS. Next-generation sequencing (NGS) transcriptomes reveal association of multiple genes and pathways contributing to secondary metabolites accumulation in tuberous roots of Aconitum heterophyllum Wall. Planta. 2015;242(1):239–258. doi: 10.1007/s00425-015-2304-6. [DOI] [PubMed] [Google Scholar]
- Park SY, Paek KY. Bioreactor culture of shoots and somatic embryos of medicinal plants for production of bioactive compounds. Heidelberg: Springer; 2014. pp. 337–368. [Google Scholar]
- Qi Z, Wu C, Wang W, Shu Y, Bao J, Fang C. In vitro plantlet regeneration system from rhizomes and mannose-binding lectin analysis of Polygonatum cyrtonema Hua. Plant Cell Tissue. 2009;99(3):269–275. doi: 10.1007/s11240-009-9600-4. [DOI] [Google Scholar]
- Rice P, Longden I, Bleasby A. EMBOSS: the European molecular biology open software suite. Trends Genet. 2000;16(6):276–277. doi: 10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Sairkar P, Chandravanshi MK, Shukla NP, Mehrotra NN. Mass production of an economically important medicinal plant Stevia rebaudiana using in vitro propagation techniques. IEEE Des Test Comput. 2009;24(3):246–254. [Google Scholar]
- Schaller H. 1.21—Sterol and steroid biosynthesis and metabolism in plants and microorganisms. Compr Nat Prod. 2010;2:755–787. [Google Scholar]
- Senthil K, Jayakodi M, Thirugnanasambantham P, Sang CL, Duraisamy P, Purushotham PM, Rajasekaran K, Charles SN, Roy IM, Nagappan AK. Transcriptome analysis reveals in vitro cultured Withania somnifera leaf and root tissues as a promising source for targeted withanolide biosynthesis. Bmc Genom. 2015;16(1):14. doi: 10.1186/s12864-015-1214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Sun Y, Song J, Li C, Li X, Zhang X, Li Y, Hu S, Luo H, Zhu Y. Discovery of genes related to steroidal alkaloid biosynthesis in Fritillaria cirrhosa by generating and mining a dataset of expressed sequence tags (ESTs) J Med Plants Res. 2011;5(21):5307–5314. [Google Scholar]
- Suzuki M, Muranaka T. Molecular genetics of plant sterol backbone synthesis. Lipids. 2007;42(1):47–54. doi: 10.1007/s11745-006-1000-5. [DOI] [PubMed] [Google Scholar]
- Tang N, Hélène SC, Sébastien R, Guillaume B, Zhao B, Christophe R. A survey of the gene repertoire of Gigaspora rosea unravels conserved features among Glomeromycota for obligate biotrophy. Front Microbiol. 2016;7:233. doi: 10.3389/fmicb.2016.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N, Shukla S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J Appl Res Med. 2015;2(4):105–113. [Google Scholar]
- Wang D, Yang J, Du Q, Li H, Wang S. The total alkaloid fraction of bulbs of Fritillaria cirrhosa displays anti-inflammatory activity and attenuates acute lung injury. J Ethnopharmacol. 2016;193:150–158. doi: 10.1016/j.jep.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, Li S, Li R, Bolund L. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006;34:293–297. doi: 10.1093/nar/gkl031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler J, Schwender J, Müller C, Wiesner J, Weidemeyer C, Beck E, Jomaa H, Lichtenthaler HK. Inhibition of the non-mevalonate 1-Deoxy-d-xylulose-5-phosphate pathway of plant isoprenoid biosynthesis by fosmidomycin. Z Naturforsch C. 1998;53(12):980–986. [Google Scholar]
- Zhang DQ, Gao LM, Yang YP. Genetic diversity and structure of a traditional Chinese medicinal plant species, Fritillaria cirrhosa (Liliaceae) in southwest China and implications for its conservation. Biochem Syst Ecol. 2010;38(2):236–242. doi: 10.1016/j.bse.2009.12.029. [DOI] [Google Scholar]
- Zhang H, Hedhili S, Montiel G, Zhang Y, Chatel G, Pré M, Gantet P, Memelink J. The basic helix-loop-helix transcription factor CrMYC2 controls the jasmonate-responsive expression of the ORCA genes that regulate alkaloid biosynthesis in Catharanthus roseus. Plant J. 2011;67(1):61–71. doi: 10.1111/j.1365-313X.2011.04575.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1 Gene ontology (GO) classification of assembled in F. cirrhosa unigenes (JPEG 992 kb)
Fig. 2 Eukaryotic Orthologous Groups (KOG) classification of assembled in F. cirrhosa unigenes (JPEG 68 kb)