Abstract
An efficient and high yielding expression system is required to produce recombinant proteins. Furthermore, the transient expression system can be used to identify the localization of proteins in plant cells. In this study, we demonstrated that combination of a geminiviral replication and a double terminator dramatically enhanced the transient protein expression level in plants. The GFP protein was expressed transiently in lettuce, Nicotiana benthamiana, tomatoes, eggplants, hot peppers, melons, and orchids with agroinfiltration. Compared to a single terminator, a double terminator enhanced the expression level. A heat shock protein terminator combined with an extensin terminator resulted in the highest protein expression. Transiently expressed GFP was confirmed by immunoblot analysis with anti-GFP antibodies. Quantitative analysis revealed that the geminiviral vector with a double terminator resulted in the expression of at least 3.7 mg/g fresh weight of GFP in Nicotiana benthamiana, approximately 2-fold that of the geminiviral vector with a single terminator. These results indicated that combination of the geminiviral replication and a double terminator is a useful tool for transient expression of the gene of interest in plant cells.
Introduction
Transgenic plants are generally used to obtain recombinant proteins or identify the localization of proteins. However, substantial time is required to generate transgenic plants, and the yield of the expressed protein is relatively low. Alternatively, transient expression systems with virus-based vectors have the advantage of rapid and high-level expression of the recombinant proteins. The replication system of plant viruses results in high-level expression of foreign proteins within a few days1. Thus, plant virus expression vectors are attractive, and the production level of the gene of interest can be easily amplified by increasing the number of host plants2. A tobamovirus (TMV)-based deconstructed viral system (magnICON) has been extensively engineered to achieve high levels of recombinant protein accumulation in tobacco leaves3. ZMapp for Ebolavirus infections, which was used during a recent outbreak, was also produced by the magnICON system4. Thus, the magnICON system is very useful system in tobacco, including Nicotiana benthamiana. However, the system may not be applicable to lettuce leaves5. Because of TMV-based viral vector, host factors from a plant species must recognize and interact with TMV elements or factors.
Bean yellow dwarf virus (BeYDV), a Mastrevirus of the Geminiviridae family, contains a single-stranded circular DNA genome and uses a rolling circle mechanism to replicate its genome, resulting in a very high yield of copies. This mechanism has been used for boosting protein expression in transgenic plants6 and for transient expression of foreign proteins with efficiency in N. benthamiana leaves7 and in lettuce leaves5. Some geminivirus species can replicate in non-host plant cells and BeYDV has a broad host range in dicotyledonous plants8. A recent study demonstrated that introduction of PsaK 5′-UTR, extensin (Ext) terminator, and the tobacco Rb7 matrix attachment region into the geminivirus replicon vector greatly improved the expression level of protein with the Agrobacterium strain EHA1059. The Ext terminator enhanced protein production, compared to vspB terminator9.
Previous research has demonstrated that the terminator of the heat shock protein (HSP) gene increased the gene expression level in Arabidopsis plants10, tomatoes11, and lettuce12. Furthermore, the expression level of the gene was increased with double transcription terminators, the CaMV 35S terminator and NOS terminator13,14; however, these results were obtained from stable transformants. The introduction of a second terminator possibly detects read-through transcripts and traps it by the hairpin structure15. Read-through transcripts may cause inhibition of 3′-end cleavage/polyadenylation processing. The less efficient polyadenylation of mRNA leads to reduction of translatable mRNAs and, consequently, decrease in protein production16,17. In this study, combining the geminiviral replication system with a double terminator increased the transient protein expression level. In particular, when the HSP and Ext terminators were used as a double terminator, the expression level was the highest and reached approximately 3.7 mg/g fresh weight (FW) in N. benthamiana. Furthermore, this system could be applicable to not only N. benthamiana, but also tomatoes, eggplants, lettuce, hot peppers, melons, and orchids. These results indicated that this system is a useful tool, with which to express specific proteins in plant cells.
Results
A comparison of the expression level of GFP between pBYR2HS and pBYR2fp geminiviral replicon vector
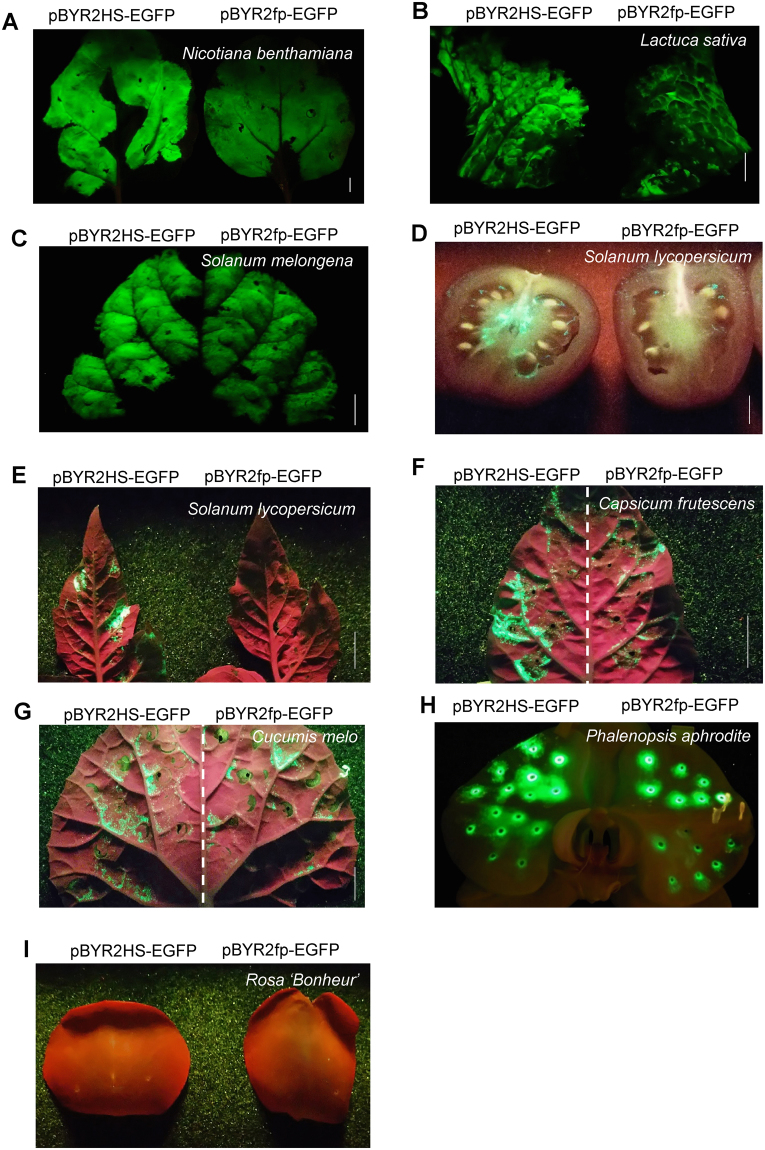
In the pBYR2HS vector, the alcohol dehydrogenase (AtADH) 5′-UTR region was replaced with tobacco mosaic virus (TMV) Ω and the HSP terminator was inserted into pBYR2fp, resulting in a double terminator construct (Fig. 1). Each vector was transformed into Agrobacterium tumefaciens GV3101 and green fluorescent protein (GFP) was transiently expressed in N. benthamiana (Fig. 2A), lettuce Lactuca sativa (Fig. 2B), eggplants Solanum melongena (Fig. 2C), tomato Solanum lycopersicum fruits (Fig. 2D), tomato leaves (Fig. 2E), hot peppers Capsicum frutescens (Fig. 2F), melons Cucumis melo (Fig. 2G), orchids Phalaenopsis aphrodite (Fig. 2H), and roses (Fig. 2I). Transfection with pBYR2HS-EGFP improved expression of GFP in these plants except for the rose, compared with pBYR2fp-EGFP. In particular, GFP fluorescence emission was only observed in tomato fruits and leaves agroinfiltrated with pBYR2HS-EGFP (Fig. 2D,E). No fluorescence was detected in the rose (Fig. 2I).
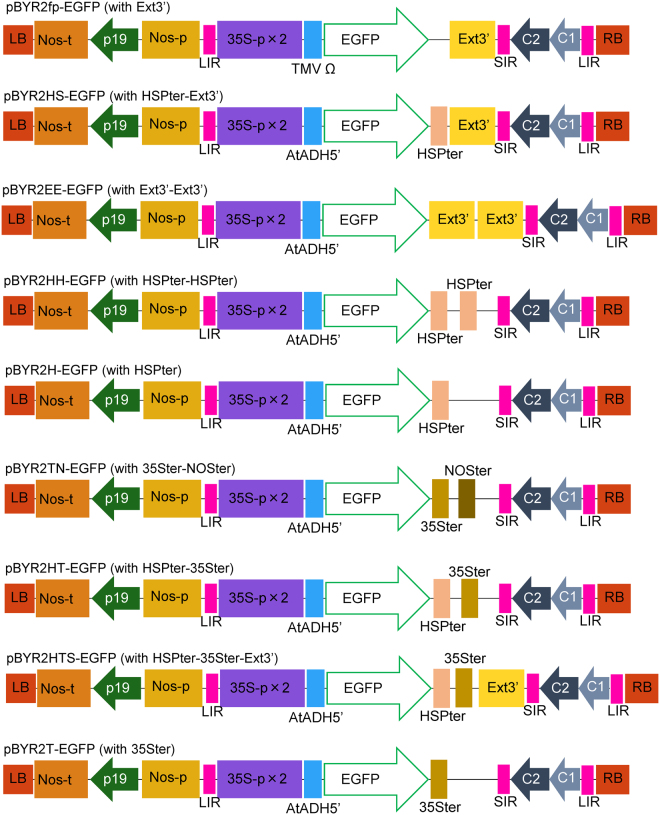
Figure 1.
Schematic diagram of the T-DNA region of the plasmids pBYR2fp-EGFP, pBYR2HS-EGFP, pBYR2EE-EGFP, pBYR2HH-EGFP, pBYR2H-EGFP, pBYR2TN-EGFP, pBYR2HT-EGFP, pBYR2HTS-EGFP, and pBYR2T-EGFP. 35S-p x 2, CaMV 35 S promoter with double-enhanced element; AtADH5′, 5′-untranslated region (UTR) of Arabidopsis thaliana alcohol dehydrogenase gene; TMV Ω, 5′-leader sequence of tobacco mosaic virus; EGFP, enhanced green fluorescence protein; HSPter, terminator of heat shock protein gene; Ext3′, tobacco extension gene 3′ element; 35Ster, terminator of CaMV 35S; NOSter, NOS terminator; LIR, long intergenic region of bean yellow dwarf virus (BeYDV) genome; SIR, short intergenic region of BeYDV genome; C1/C2, BeYDV ORFs C1 and C2 encoding for replication initiation protein (Rep) and RepA, respectively; LB and RB, the left and right borders of the T-DNA region, respectively; Nos-p and Nos-t, NOS promoter and terminator, respectively; and p19, a gene-silencing suppressor gene from tomato bushy stunt virus.
Figure 2.
Introduction of HSP terminator improved transient expression of EGFP. Agrobacterium tumefaciens harboring pBYR2HS-EGFP and pBYR2fp-EGFP were transfected into Nicotiana benthamiana (A), lettuce Lactuca sativa var. crispa (B), eggplant Solanum melongena cv. ‘Dewakonasu’ (C), tomato fruits Solanum lycopersicum cv. ‘M82’ (D), tomato leaves Solanum lycopersicum cv. ‘Micro-Tom’ (E), hot pepper Capsicum frutescens cv. ‘Shima-togarashi’ (F), melon Cucumis melo cv. ‘Earl’s Favorite Harukei No.3′ (G), orchid Phalaenopsis Aphrodite (H), and a rose Rosa sp. ‘Bonheur’ (I). These plants were incubated for 3 days after agroinfiltration. Then, after blue-light excitation, GFP emission was observed with an ultraviolet-absorbing filter Fujifilm SC-52. Bars indicate a 1-cm length.
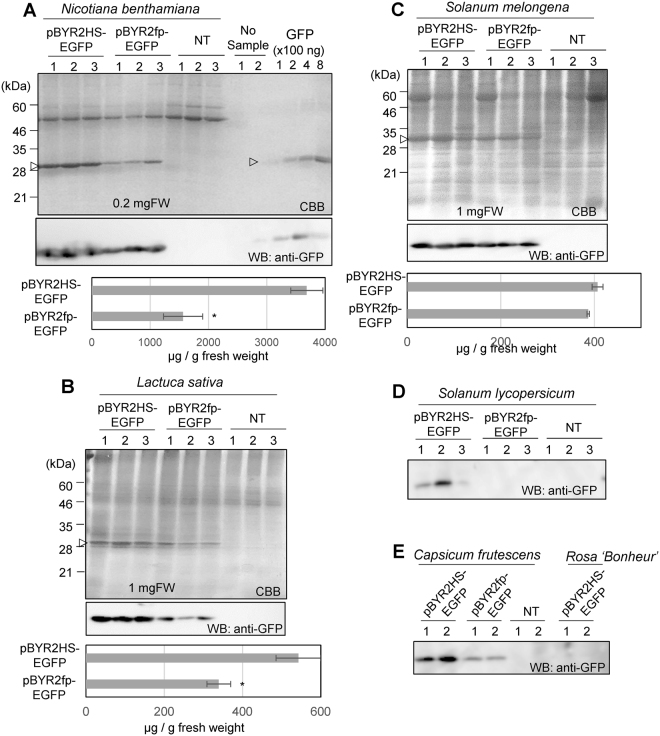
Then, total soluble proteins were prepared from 0.2 mg fresh weight (FW) of N. benthamiana and 1 mg FW of lettuce, eggplant, tomato, hot pepper, and rose. The total soluble proteins were detected with Coomassie Brilliant Blue (CBB) staining. The GFP was also detected by immunoblot analysis with anti-GFP antibodies. Expression levels of GFP from plants agroinfiltrated with pBYR2HS-EGFP were higher than that from plants agroinfiltrated with pBYR2fp-EGFP (Fig. 3). Calculation of expression level by image analyzer indicated that approximately 3.7 mg of GFP was expressed from 1 g FW (fresh weight) in N. benthamiana agroinfiltrated with pBYR2HS-EGFP (Fig. 3A). Conversely, approximately 1.5 mg of GFP was expressed in 1 g FW in N. benthamiana agroinfiltrated with pBYR2fp-EGFP. Similarly, the expression level of GFP from lettuce or eggplant agroinfiltrated with pBYR2HS-EGFP (0.37 mg/g FW or 0.46 mg/g FW, respectively) was higher than that of lettuce or eggplant agroinfiltrated with pBYR2fp-EGFP (0.20 mg/g FW or 0.42 mg/g FW, respectively, Fig. 3B,C). Because a clear GFP band with CBB staining was not observed in tomatoes, hot peppers, and roses, western blot analyses were performed. GFP expression levels of tomato leaves and hot pepper leaves agroinfiltrated with pBYR2HS-EGFP was also increased (Fig. 3D,E). GFP expression in roses agroinfiltrated with pBYR2HS-EGFP was not detected even when the western blot analysis was performed. These results indicated that introduction of the HSP terminator improved the expression level of GFP in the agroinfiltrated plants and this system can be used for several species.
Figure 3.
Effect of HSP terminator on transient GFP expression at 3 days post-agroinfiltration. Total soluble proteins were extracted from agroinfiltrated plant leaves with pBYR2HS-EGFP or pBYR2fp-EGFP. Coomassie Brilliant Blue (CBB) staining and immunoblot analysis with anti-GFP antibodies were performed by using agroinfiltrated leaves of N. benthamiana (A), lettuce (B), eggplants (C), tomatoes (D), hot peppers, and roses (E). The numbers at the top of the gels indicate different samples taken from different leaves from different plants. NT indicates non-transfected plants. The amount of protein was measured according to band intensity from CBB staining gel using ImageJ software. Arrowheads indicate bands corresponding to GFP protein. The band clearly seen at 55 kDa in a CBB staining gel is corresponding to large subunit of Rubisco. Data represent the means ± SD (n = 3 to 4). Significance was determined using unpaired Student’s t tests (*p < 0.05). Full-length gels and full-length blots are presented in Supplementary Figures S1 and S2.
A double terminator with geminiviral replication enhances transient protein expression
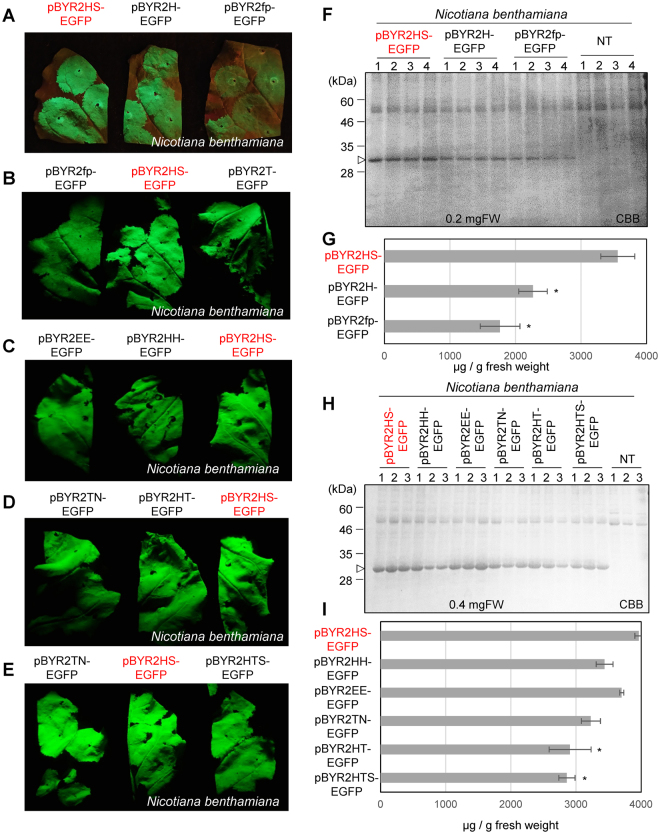
To confirm whether the HSP terminator improved expression of the protein or double terminator enhanced expression, several kinds of plasmids were prepared (Fig. 1). First, pBYR2H-EGFP containing only the HSP terminator, pBYR2fp-EGFP containing only Ext 3′, and pBYR2HS-EGFP containing both the HSP terminator and Ext 3′ were compared. These plasmids were used for agroinfiltration into N. benthamiana and the expression of GFP was compared (Fig. 4A,F,G). The expression level of GFP from N. benthamiana with pBYR2H-EGFP (2.2 mg/g FW, Fig. 4G) was higher than with pBYR2fp-EGFP (1.7 mg/g FW, Fig. 4G), suggesting that the HSP terminator enhanced expression of GFP. However, if the plasmid contained both the HSP terminator and Ext 3′, the expression level was significantly higher than with plasmids containing the single terminator. Next, several combinations of double terminators were investigated (Fig. 4B–E). In conclusion, the expression level of GFP with pBYR2HS-EGFP (3.9 mg/g FW) was the higher than that obtained with other plasmids, such as pBYR2HH-EGFP (3.4 mg/g FW), pBYR2EE-EGFP (3.7 mg/g FW), pBYR2TN-EGFP (3.2 mg/g FW), pBYR2HT-EGFP (2.9 mg/g FW), and pBYR2HTS-EGFP (2.9 mg/g FW) (Fig. 4H,I). Interestingly, the expression level of GFP with pBYR2HTS-EGFP was statistically lower than that with pBYR2HS-EGFP (Fig. 4I). This suggests that triple terminators may decrease expression of the protein, compared with a double terminator. Taken together that expression level with pBYR2HH-EGFP was slightly lower than that with pBYR2EE-EGFP, it is plausible that too much terminator activity may decrease the protein expression level. These results indicated that the double terminator is sufficient to enhance expression of the protein, and a combination of HSP terminator and Ext 3′ was the best in this experiment.
Figure 4.
Effect of single or double terminator on transient EGFP expression at 3 days post-agroinfiltration. GFP emission from leaves of N. benthamiana agroinfiltrated with several kinds of plasmids (Fig. 1) was observed with an ultraviolet-absorbing filter, Fujifilm SC-52 (A–E). Total soluble proteins were extracted from agroinfiltrated N. benthamiana leaves with pBYR2HS-EGFP, pBYR2H-EGFP, or pBYR2fp-EGFP. Coomassie Brilliant Blue staining were performed (F) and the amount of protein was measured (G). Total soluble proteins were extracted from agroinfiltrated N. benthamiana leaves with pBYR2HS-EGFP, pBYR2HH-EGFP, pBYR2EE-EGFP, pBYR2TN-EGFP, pBYR2HT-EGFP, and pBYR2HTS-EGFP. Coomassie Brilliant Blue staining was performed (H) and the amount of protein was measured (I). The numbers at the top of the gels indicate different samples taken from different leaves from different plants. Data represent the means ± SD (n = 3 to 4). Significance was determined using unpaired Student’s t tests (*p < 0.05). Full-length gels are presented in Supplementary Figures S3 and S4.
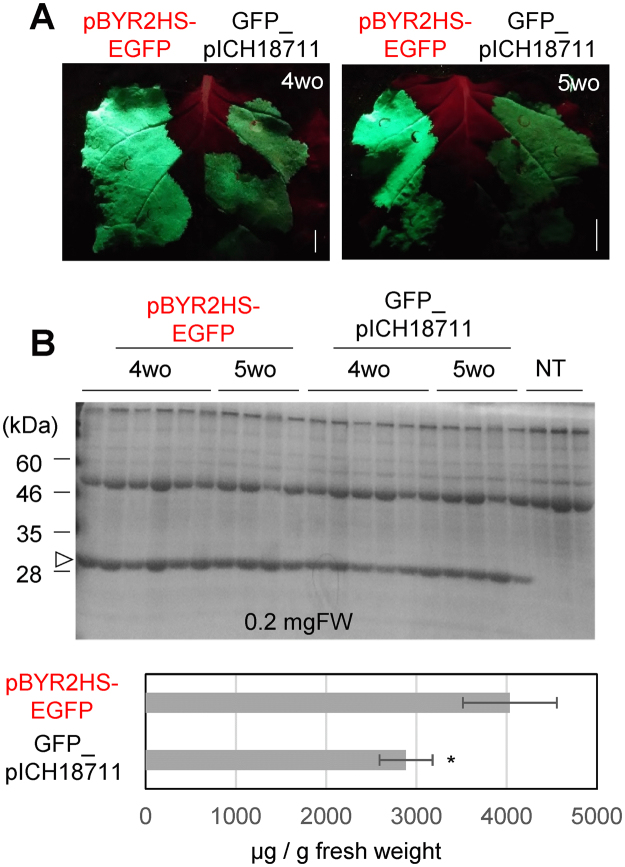
To examine whether this expression system works well, the system was compared to the magnICON system as a standard viral expression system. Both vectors were agroinfiltrated into the same leaf, side by side. After 3-day incubation, the expression of GFP was compared (Fig. 5A) and total soluble protein was separated with SDS-PAGE (Fig. 5B). The expression level of GFP from 4-week-old N. benthamiana with pBYR2HS-EGFP (4.0 mg/g FW, 36% TSP (total soluble protein), Fig. 5C) was significantly higher than that with GFP_pICH18711 (2.9 mg/g FW, 28% TSP, Fig. 5C). These results indicate that protein expression level with our system is higher than that with the magnICON system under our conditions, i.e. 3-day incubation at 25 °C with 16 h light and 8 h dark.
Figure 5.
Comparison of expression levels at 3 days post-agroinfiltration. pBYR2HS-EGFP or GFP_pICH18711 was agroinfiltrated into the same leaves of 4-week-old (4wo) or 5-week-old (5wo) N. benthamiana, side by side. (A) GFP emission was observed. (B) Total soluble proteins were extracted from agroinfiltrated N. benthamiana leaves. Coomassie Brilliant Blue (CBB) staining were performed. Arrowheads indicate bands corresponding to GFP protein. (C) The amount of GFP protein from 4-week-old leaves, which were incubated 3 days after agroinfiltration, was measured according to band intensity from CBB staining gel using ImageJ software. The numbers at the top of the gels indicate different samples taken from different leaves from different plants. Data represent the means ± SD (n = 6). Significance was determined using unpaired Student’s t tests (*p < 0.05). Full-length gels and full-length blots are presented in Supplementary Figure S6.
Discussion
The HSP terminator with the extensin terminator strongly increased protein expression levels in lettuce, N. benthamiana, tomatoes, eggplants, hot peppers, and orchids P. aphrodite.
Generally, it takes substantial time to produce stable transgenic plants for production of a recombinant protein. Post-transcriptional gene silencing (PTGS) often occurs in plants to reduce the amount of protein expression. PTGS is a natural defense mechanism relying on RNA interference for plants against viruses and pathogenes18. Production of excessive RNA transcripts over a threshold level, such as overexpressing a transgene under CaMV 35S promoter, causes transgene silencing through activation of PTGS19–21. Thus, the yield of production of several kinds of recombinant proteins in plants is limited by PTGS. Conversely, transient expression in plant leaves solves these problems. After 3 to 7 days of agroinfiltration, recombinant proteins are produced. And when the vacuum-infiltration method is applied, large-scale production of recombinant proteins is possible.
In a previous study, the expression level of the gene was increased with double transcription terminators, CaMV 35S and NOS13,14. The double terminator was useful for the transient expression system (Fig. 4), and a combination of the HSP terminator and Ext terminator was the best in this study (Fig. 4I). For convenience, EGFP sequences were removed from pBYR2HS-EGFP and the SalI site was introduced between AtADH 5′ and the HSP terminator. The resulting vector was named pBYR2HS-SalI and a gene of interest could be introduced into pBYR2HS digested with SalI.
The results indicate that at least 3.7 mg of GFP per 1 g FW of leaf of N. benthamiana was able to be produced. To produce proteins in N. benthamiana, the magnICON expression system is often used. For example, yields of the Plasmodium yoelii merozoite surface protein 4/5, Norwalk virus-like particles, and hepatitis B virus core antigen virus-like particles were 1–2 mg/g FW22, 0.8 mg/g FW23, and 2.4 mg/g FW24, respectively. The magnICON system is shown to be capable of yield up to 4 mg/g FW of GFP25. Expression levels of GFP in our system were similar to those in the magnICON system. Under our conditions, i.e. 3-day incubation at 25°C with 16 h light and 8 h dark, protein expression level from N. benthamiana leaves agroinfiltrated with the magnICON vector was less than that with pBYR2HS-EGFP (Fig. 5). According to the paper25, the peak of GFP expression was at 6 to 10 days post-infiltration and expression level was not very high at 3 days post-infiltration. Thus, protein expression level with the magnICON system was not high under our conditions and the pBYR2HS system may accumulate protein in shorter periods. A recent study demonstrated that changing the 5′ and 3′ untranslated region in the geminivirus replicon system (pBYR2eP3) enhanced expression levels of a protein in N. benthamiana and the Norwalk virus capsid protein was produced at 1.8 mg/g FW9. It is possible that the introduction of the HSP terminator into pBYR2eP3 for the double terminator further enhances protein production.
Not only the magnICON system, but also several other systems, such as the E. coli expression system, Brevibacillus expression system, and the baculovirus-insect cell expression system, are available for production of recombinant proteins. Although a simple comparison is not accurate, the amount of protein production is compared as mL of liquid culture equivalent to 1 g. In the Brevibacillus expression system, yields of α-amylase, cyclodextrin glucanotransferase, human protein disulfide isomerase, and human epidermal growth factor were 3.7 mg/mL, 1.5 mg/mL, 1.0 mg/mL, and 1.5 mg/mL, with production based on the manufacture’s instruction26–28. The yield of GFP-ELP (elastin-like polypeptides) was 1.6 mg/mL in the E. coli expression system29. The maximum amount of GFPuv was 6.9 mg/g larvae mass with the baculovirus expression system30. These results suggest that our system is comparable to other protein expression systems in terms of the quantity of protein expression. The advantage of using plants is the ease of obtaining large quantities of plants at low cost, and no risk of contamination with retroviruses when compared to mammalian cell cultures.
Furthermore, geminivirus replicon has advantages because this replicon system can work in several species (Fig. 2). It is probably because geminivirus has a broad host range31. On the other hand, the magnICON system can primarily work in tobacco32. As shown in the previous article, the magnICON system may not be used in lettuce5. The geminiviral replicon system can be used for not only protein expression, but also determining the localization of the protein of interest in several species.
Methods
Vector construction
The pBYR2fp vector harbored a replication system from BeYDV33. Furthermore, a p19 RNAi suppressor expression cassette was included in this vector (Fig. 1). To compare expression levels, EGFP was amplified with the primers, pBYR2fp-EGFP-F and EGFP-pBYR2fp-R (Table S1), and the PCR product was inserted into XbaI-digested pBYR2fp with an In-Fusion HD Cloning Kit (Takara Bio).
To obtain higher yield of recombinant proteins, 5′-UTR of alcohol dehydrogenase gene and the terminator of heat shock protein, which enhanced gene expression10,34, was introduced. EGFP was amplified with the primers, pRI201-EGFP-F and EGFP-pRI201-R (Table S1). The PCR product was inserted into NdeI and SalI-digested pRI201-AN (Takara Bio). The resulting vector, pRI201-EGFP, was used as a template for amplification of the cassette (AtADH 5′-UTR-EGFP-HSP terminator) with the primers, pBYR2fp-AtADH-F and pBYR2fp-HSPter-R (Table S1). The cassette was introduced into XhoI and XbaI-digested pBYR2fp, and pBYR2HS-EGFP was constructed (Fig. 1).
Ext3′ was amplified with the primers, pBYR2EE-Ext3-F and pBYR2EE-Ext3-R (Table S1). The PCR product was inserted into SalI and XbaI-digested pBYR2HS-EGFP, resulting in the plasmid pBYR2EE-EGFP (Fig. 1).
To produce pBYR2H-EGFP, Ext3′-SIR-C2 sequences were removed from pBYR2HS-EGFP with XmaI and ClaI. SIR-C2 sequences were amplified with the primers, HSPter-SIR-F and C1-ClaI-C2-R (Table S1) and inserted into XmaI and ClaI-digested pBYR2HS-EGFP, resulting in the plasmid pBYR2H-EGFP (Fig. 1). HSPter was amplified with the primers, pBYR2H-HSPter-F and pBYR2H-HSPter-R (Table S1) and inserted into XbaI-digested pBYR2H-EGFP, resulting in the plasmid pBYR2HH-EGFP (Fig. 1).
35Ster or NOSter was amplified with the primers, pBYR2T-35Ster-F and 35Ster-NOSter-R or 35Ster-NOSter-F and pBYR2TN-NOSter-R (Table S1), respectively. These PCR products were used as templates with primers, pBYR2T-35Ster-F and pBYR2TN-NOSter-R to produce 35Ster-NOSter sequences. This product was inserted into SalI and XbaI-digested pBYR2H-EGFP, resulting in the plasmid pBYR2TN-EGFP (Fig. 1).
35Ster was amplified with the primers, pBYR2HS-35Ster-F and pBYR2HS-35Ster-R (Table S1), and inserted into XbaI-digested pBYR2H-EGFP or XbaI-digested pBYR2HS-EGFP, resulting in the plasmid pBYR2HT-EGFP or pBYR2HTS-EGFP (Fig. 1), respectively. 35Ster was also amplified with the primers, pBYR2T-35Ster-F and pBYR2HS-35Ster-R (Table S1), and then inserted into SalI and XbaI-digested pBYR2H-EGFP, resulting in the plasmid pBYR2T-EGFP.
Transient expression protocol in N. benthamiana, tomatoes, egg plants, hot peppers, melon, and a rose cultivar
The preparation of Agrobacterium and transient expression in N. benthamiana was performed as previously described35 with modifications. Briefly, the vectors described above and GFP_pICH18711 kindly provided by Dr. Victor Klimyuk (Icon Genetics GmbH) were transformed into A. tumefaciens GV3101. A. tumefaciens GV3101 harboring the binary vector was grown in L-broth media containing 10 mM MES (pH 5.6), 20 μM acetosyringone, 100 mg/L of kanamycin, 30 mg/L gentamycin, 30 mg/L of rifampin to the stationary phase at 28 °C. After centrifugation, A. tumefaciens was resuspended in the infiltration buffer (10 mM MgCl2, 10 mM MES (pH 5.6), 100 μM acetosyringone) to adjust OD600 = approximately 1. The suspension was infiltrated with a 1-mL syringe without a needle into the abaxial air spaces of 4-week-old leaves of N. benthamiana. The suspension was infiltrated into 4-week-old leaves of tomatoes, Solanum lycopersicum cv. ‘Micro-Tom’, 4-week-old leaves of eggplants, Solanum melongena cv. ‘Dewakonasu’, 4-week-old leaves of hot peppers, Capsicum frutescens L., 3-week-old leaves of melons, Cucumis melo cv. ‘Earl’s Favorite Harukei No. 3′, and petals of commercially produced orchids, Phalaenopsis aphrodite and the rose, Rosa sp. ‘Bonheur’. For infiltration into tomato fruits of S. lycopersicum cv. ‘M82’, a 1-mL syringe with a needle was used. Three days after infiltration, blue LED lamp was shed onto plants. GFP emission was detected with an ultraviolet absorbing filter (SC-52, Fujifilm).
Transient expression protocol for lettuce
Preparation of Agrobacterium tumefaciens suspension and transient expression in lettuce was performed as previously described36 with modifications. A. tumefaciens GV3101 containing the binary vector was grown in modified YEB media (6 g/L of yeast extract, 5 g/L of tryptone, 5 g/L of sucrose, and 2 mM MgSO4) with antibiotics (100 mg/L of kanamycin, 30 mg/L gentamycin, and 30 mg/L of rifampin) for 2 days at 28 °C. Then, 2-day cultures were diluted 100 times in the same modified YEB with antibiotics, 10 mM MES (pH 5.6), and 20 μM acetosyringone, and grown for 18–24 h at 28 °C on a rotary shaker at 140 rpm. OD595 was reached to approximately 2. Then, 55 g/L of sucrose and 200 μM acetosyringone were added into the bacterial culture. The suspension was incubated for 1 h at 22 °C. After incubation at 22 °C, 2,4-dichlorophenoxyacetic acid and Tween-20 were added to the final concentrations of 100 μg/mL and 0.005%, respectively, and the suspension was subjected to vacuum-infiltration.
Red-leaf lettuce was obtained commercially from a local grocery store, rinsed with distilled water, and water was removed with paper towels. Then, the base of rinsed lettuce was placed on wet paper towels. The lettuce was covered with plastic wrap and incubated for one day at 24 °C. Before vacuum-infiltration, the lettuce was incubated with a blue LED light for more than 30 min. A 1.2-L portion of the Agrobacterium suspension was placed into a 2-L glass beaker inside a vacuum desiccator (Fig. S6A). The lettuce head was immersed into the suspension (Fig. S6B) and vacuum-infiltrated (29 in. Hg) for 20 min. After releasing the vacuum, the lettuce head was rinsed in water. The water was removed with paper towels. The base of rinsed lettuce was wrapped with wet paper towels and the lettuce was placed in a bowl (Fig. S6C). The lettuce was incompletely wrapped with plastic wrap, leaving some holes (Fig. S6D). The lettuce was incubated for 3–5 days at 24 °C under a 16-h light and 8-h dark photoperiod.
Protein extraction and immunoblot analysis
Soluble protein was prepared as described previously with modification37. Briefly, Plant leaves (200 mg) were ground by bead beating using Cell Destroyer PS-1000 (Pro Sense, Inc., Tokyo, Japan) at 2,500 rpm for 10 s after freezing with liquid nitrogen. Then, 1 mL of lysis buffer [50 mM Tris-HCl, pH 8.0, 120 mM NaCl, 0.2 mM sodium orthovanadate, 100 mM NaF, 10% glycerol, 0.2% Triton X-100, 5 mM DTT, and 1× protein inhibitor cocktail (Nacalai Tesque, Inc., Kyoto, Japan)] was added. Powdered leaves and lysis buffer were mixed completely by bead beating and incubated on ice with shaking for 1 h. The samples were spun and liquid solution was used as a soluble protein extract at the concentration of 0.2 mg fresh weight (FW)/µL. To load a crude extract from 1 mg FW, 5 µL of sample solution was applied onto an SDS-PAGE gel. To load a crude extract from 0.2 mg FW, a crude extract was 5-fold diluted with lysis buffer (0.04 mg/µL) and, then, 5 µL of sample solution was applied. The gel was stained with Coomassie Brilliant Blue (CBB). The protein was also transferred onto a PVDF membrane (Amersham Hybond P PVDF, GE Healthcare). The blot was probed with anti-GFP antibody and detected using Luminata Forte Western HRP substrate (Millipore).
To compare expression level, each protein should be applied onto the same gel. Actually, the results can easily vary. Thus, we did three or more independent experiments. After staining with CBB, the gel was compared. We picked up the gel, in which the expression from pBYR2HS-EGFP was the least one among these gels, for Fig. 3A and calculate concentration of GFP expression by measuring band intensities using ImageJ software. Then, other gels containing similar GFP expression level from pBYR2HS-EGFP, compared to that in Fig. 3A, was picked up for calculation.
The whole gels of western blot analyses and CBB staining are provided in Supplemental Figures S1 to S5. Standard lines for calculation are also provided in Supplemental Figures S2 to S5.
Electronic supplementary material
Acknowledgements
We thank Ms. Rieko Nozawa and Ms. Yuri Nemoto at the University of Tsukuba for technical support. We also thank Dr. Victor Klimyuk at Icon Genetics GmbH for providing the vector. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (JP16H01458) and KAKENHI (JP16K07390) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, by a Cooperative Research Grant from the Plant Transgenic Design Initiative, Gene Research Center, University of Tsukuba, by the National BioResource Project (NBRP) from Japan Agency for Medical Research and Development (AMED).
Author Contributions
H.E. and K.M. contributed to designing the experiments. T.Y., K.H., K.E., R.O., S.F. and M.T. performed the experiments, and collected and analyzed the data. H.S.M., H.E. and K.M. contributed to data interpretation and preparation of the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Tsuyoshi Yamamoto and Ken Hoshikawa contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23024-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hefferon KL. Plant virus expression vectors set the stage as production platforms for biopharmaceutical proteins. Virology. 2012;433:1–6. doi: 10.1016/j.virol.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Chen Q, et al. Agroinfiltration as an effective and scalable strategy of gene delivery for production of pharmaceutical proteins. Adv Tech Biol Med. 2013;1:103. doi: 10.4172/2379-1764.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klimyuk V, Pogue G, Herz S, Butler J, Haydon H. Production of recombinant antigens and antibodies in Nicotiana benthamiana using ‘magnifection’ technology: GMP-compliant facilities for small- and large-scale manufacturing. Curr Top Microbiol Immunol. 2014;375:127–154. doi: 10.1007/82_2012_212. [DOI] [PubMed] [Google Scholar]
- 4.Qiu X, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai H, He J, Engle M, Diamond MS, Chen Q. Robust production of virus-like particles and monoclonal antibodies with geminiviral replicon vectors in lettuce. Plant Biotechnol J. 2012;10:95–104. doi: 10.1111/j.1467-7652.2011.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Mason H. Bean yellow dwarf virus replicons for high-level transgene expression in transgenic plants and cell cultures. Biotechnol Bioeng. 2006;93:271–279. doi: 10.1002/bit.20695. [DOI] [PubMed] [Google Scholar]
- 7.Huang, Z. et al. High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol Bioeng106, 9–17 (2010). [DOI] [PMC free article] [PubMed]
- 8.Liu L, Pinner MS, Davies JW, Stanley J. Adaptation of the geminivirus bean yellow dwarf virus to dicotyledonous hosts involves both virion-sense and complementary-sense genes. J Gen Virol. 1999;80:501–506. doi: 10.1099/0022-1317-80-2-501. [DOI] [PubMed] [Google Scholar]
- 9.Diamos AG, Rosenthal SH, Mason HS. 5′ and 3′ untranslated regions strongly enhance performance of geminiviral replicons in Nicotiana benthamiana leaves. Front Plant Sci. 2016;7:200. doi: 10.3389/fpls.2016.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagaya S, Kawamura K, Shinmyo A, Kato K. The HSP terminator of Arabidopsis thaliana increase gene expression in plant cells. Plant Cell Phyiol. 2010;51:328–332. doi: 10.1093/pcp/pcp188. [DOI] [PubMed] [Google Scholar]
- 11.Hirai T, et al. The HSP terminator of Arabidopsis thaliana induces a high level of miraculin accumulation in transgenic tomatoes. J Agric Food Chem. 2011;59:9942–9949. doi: 10.1021/jf202501e. [DOI] [PubMed] [Google Scholar]
- 12.Matsui T, et al. Production of double repeated B subunit of Shiga toxin 2e at high levels in transgenic lettuce plants as vaccine material for porcine edema disease. Transgenic Res. 2011;20:735–748. doi: 10.1007/s11248-010-9455-9. [DOI] [PubMed] [Google Scholar]
- 13.Luo Z, Chen Z. Improperly terminated, unpolyadenylated mRNA of sense transgenes is targeted by RDR6-mediated RNA silencing in Arabidopsis. Plant Cell. 2007;19:943–958. doi: 10.1105/tpc.106.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beyene G, et al. Unprecedented enhancement of transient gene expression from minimal cassettes using a double terminator. Plant Cell Rep. 2011;30:13–25. doi: 10.1007/s00299-010-0936-3. [DOI] [PubMed] [Google Scholar]
- 15.Abe H, Abo T, Aiba H. Regulation of intrinsic terminator by translation in Escherichia coli: transcription termination at a distance downstream. Genes Cell. 1999;4:87–97. doi: 10.1046/j.1365-2443.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- 16.Proudfoot NJ. Genetic dangers in poly(A) signals. EMBO Rep. 2001;2:891–892. doi: 10.1093/embo-reports/kve207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gehring NH, et al. Increased efficiency of mRNA 3′ end formation: a new genetic mechanism contributing of hereditary thrombophilia. Nat Genet. 2001;28:389–392. doi: 10.1038/ng578. [DOI] [PubMed] [Google Scholar]
- 18.Bazzini AA, Mongelli VC, Hopp HE, del Vas M, Asurmendi S. A practical approach to the understanding and teaching of RNA silencing in plants. Electron J Biotechnol. 2007;10:178–190. doi: 10.2225/vol10-issue2-fulltext-11. [DOI] [Google Scholar]
- 19.Wassenegger M, Pelissier T. A model for RNA-mediated gene silencing in higher plants. Plant Mol. Biol. 1998;37:349–362. doi: 10.1023/A:1005946720438. [DOI] [PubMed] [Google Scholar]
- 20.Meins F. RNA degradation and models for post-transcriptional gene silencing. Plant Mol. Biol. 2000;43:261–273. doi: 10.1023/A:1006443731515. [DOI] [PubMed] [Google Scholar]
- 21.Choi, M. S. et al. The effect of cucumber mosaic virus 2b protein to transient expression and transgene silencing mediated by agro-infiltration. Plant Pathol. J.24, 296–304 (2008).
- 22.Webster DE, et al. Production and characterization of an orally immunogenic Plasmodium antigen in plants using a virus-based expression system. Plant Biotechnol J. 2009;7:846–855. doi: 10.1111/j.1467-7652.2009.00447.x. [DOI] [PubMed] [Google Scholar]
- 23.Santi L, et al. An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine. 2008;26:1846–1854. doi: 10.1016/j.vaccine.2008.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Z, et al. Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice. Vaccine. 2006;24:2506–2513. doi: 10.1016/j.vaccine.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, Gleba Y. Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat Biotechnol. 2005;23:718–723. doi: 10.1038/nbt1094. [DOI] [PubMed] [Google Scholar]
- 26.Takano T, et al. Expression of the cyclodextrin glucanotransferase gene of Bacillus macerans in Bacillus brevis. Biosci Biotechnol Biochem. 1992;56:808–809. doi: 10.1271/bbb.56.808. [DOI] [PubMed] [Google Scholar]
- 27.Tojo H, et al. Production of human protein disulfide isomerase by Bacillus brevis. J Biotechnol. 1994;15:55–62. doi: 10.1016/0168-1656(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 28.Yamagata H, et al. Use of Bacillus brevis for efficient synthesis and secretion of human epidermal growth factor. Proc Natl Acad Sci USA. 1989;86:3589–3593. doi: 10.1073/pnas.86.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chow DC, Dreher MR, Trabbic-Carlson K, Chilkoti A. Ultra-high expression of a thermally responsive recombinant fusion protein in E. coli. Biotechnol Prog. 2006;22:638–646. doi: 10.1021/bp0503742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cha HJ, Pham MQ, Rao G, Bentley WE. Expression of green fluorescent protein in insect larvae and its application for heterologous protein production. Biotechnol Bioeng. 1997;56:239–247. doi: 10.1002/(SICI)1097-0290(19971105)56:3<239::AID-BIT1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.Hefferon KL. DNA virus vectors for vaccine production in plants: Spotlight on geminiviruses. Vaccines. 2014;2:642–653. doi: 10.3390/vaccines2030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marillonnet S, et al. In planta engineering of viral RNA replicons: Efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc Natl Acad Sci USA. 2004;101:6852–6857. doi: 10.1073/pnas.0400149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon KB, et al. Overexpression and self-assembly of virus-like particles in Nicotiana benthamiana by a single-vector DNA replicon system. Appl Microbiol Biotechnol. 2014;98:8281–8290. doi: 10.1007/s00253-014-5901-6. [DOI] [PubMed] [Google Scholar]
- 34.Sugio T, Satoh J, Matsuura H, Shinmyo A, Kato K. The 5′-untranslated region of the Oryza sativa alcohol dehydrogenase gene functions as a translational enhancer in monocotyledonous plant cells. J Biosci Bioeng. 2008;105:300–302. doi: 10.1263/jbb.105.300. [DOI] [PubMed] [Google Scholar]
- 35.Li X. Infiltration of Nicotiana benthamiana protocol for transient expression via Agrobacterium. Bio-protocol. 2011;Bio101:e95. [Google Scholar]
- 36.Negrouk V, et al. Highly efficient transient expression of functional recombinant antibodies in lettuce. Plant Sci. 2005;169:433–438. doi: 10.1016/j.plantsci.2005.03.031. [DOI] [Google Scholar]
- 37.Miura, K. et al. SlICE1 encoding a MYC-type transcription factor controls cold tolerance in tomato, Solanum lycopersicum. PlantBiotechnol29, 253–260 (2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.