Abstract
It is a well-known fact that sirolimus (SRL) undergoes degradation process via hydrolysis in aqueous media, leading to incorrect assessment of drug amount and thus release characteristics of formulations. The main objective of the present study was to evaluate the effect of nonionic surfactants in media on in-vitro release profiles for sirolimus eluting stents (SES) coated with biodegradable polymeric matrix. Phosphate buffer and acetate buffer incorporating nonionic surfactants with varying concentrations were examined for adequate solubility and stability (by RP-HPLC). Good sink condition was achieved in phosphate buffer (at pH 4.0) with 1.0% Tween 20, 1.0% Brij 35% and 0.5% Brij 58. Hydrodynamic size (by DLS) and the micelle-water partition coefficient (P) with standard free energy of solubilization (∆Gs°) of drug were evaluated to get some understanding about the solubilization phenomena. About 80% of drug release during the period of 48 h was achieved in optimized drug release media which was 1.0% Tween 20 in phosphate buffer pH 4.0. The obtained accelerated SRL release profile in optimized medium correlated well with the real time in-vitro release in phosphate buffer (pH 7.4). Surface morphology changes (by SEM), changes in gravimetric weights and molecular weight change (by GPC) were examined before and after drug release to understand the drug release mechanism which explains that the polymer did not undergo degradation during the drug release.
Keywords: Nonionic surfactant, In-vitro release, Stents, Biodegradable polymers
1. Introduction
Coronary stent implantation has been proven to be the most effective technique for the prevention of restenosis in coronary arteries as compared to angioplasty alone. The restenosis rates after bare metal stent implantation are still as high as 20%–30% at 6 months [1], [2]. However, from the data gathered it can be observed that the targeted drug delivery from the drug eluting stent (DES) has been an extremely effective, successful, accepted and most promising method for preventing restenosis after stent implantation procedures which can reduce restenosis from 20% to 30% to the single digits [3], [4], [5]. Targeted drug release formulations such as DES are basically matrix systems in which drug compounds are physically blended in the polymer matrix and are coated on the device surface to form thin films. Non-degradable polymers such as poly-N-butylmethacrylate (PBMA), polyethylene-vinylacetate (PEVA), styrene–isobutylene–styrene (SIBS), and phosphorylcholine methacrylate have been used to deliver the drug from DES. However, after the drug has been released from the polymeric coatings, the remaining non-degradable polymers in coating may lead to complications such as exaggerated inflammatory response, delayed healing and late thrombosis at the stent implant site. Hence, in the recent years more attention has been paid to the utilization of biodegradable polymers [6], [7]. Biodegradable polymers such as polylactide (PLA), polyglycolide (PGA), poly (lactide-co-glycolide) (PLGA), poly vinyl pyrrolidone (PVP) and polyanhydrides can be used as controlled drug delivery vehicle. The lactide/glycolide polymer chains are cleaved by hydrolysis to form natural metabolites (lactic and glycolic acids) which are eliminated from the body in the form of carbon dioxide and water through the tricarboxylic acid cycle (TCA cycle) entering natural metabolic pathways [8], [9]. Hence biodegradable polymers are considered to be the best choice to deliver drug from DES coatings to overcome the detrimental effects of non-degradable polymers [10], [11], [12].
Drugs like paclitaxel, sirolimus (SRL), everolimus and ABT-578 are generally being used with the polymeric matrix in DES, which can effectively inhibit restenosis [13], [14]. This class of compounds possesses potent anti-inflammatory, anti-proliferative, immunosuppressive, anti-fungal and anti-tumor activities [15], [16]. Immunosuppressant drug SRL and its derivatives with combination of biodegradable or non-degradable polymers are mainly used in coatings of coronary stents due to their established safety and efficacy in the management of restenosis [17], [18]. This class of compounds possesses lipophilic property and crosses the cell membranes to bind the FK binding protein-12 (FKBP-12). SRL inhibits proliferation of vascular smooth muscle cells by reducing cell-cycle kinase activity blocking of G1/S cell cycle transition. The mechanism of inhibition is cytostatic rather than cytotoxic since the affected cells remain viable [19], [20].
SRL contains no ionizable functional groups to get solubilized in the aqueous media and hence becomes practically insoluble in aqueous solutions, but it is slightly soluble in few acceptable parenteral excipients [21], [22], [23]. This hydrophobic behavior in aqueous environment hinders development of SRL based formulations in spite of SRL's promising pharmacological activities. The safety and effectiveness of any controlled release formulation are determined by the drug dose and its release characteristics [24]. An ideal way to determine the pharmacokinetic characteristic of drug eluting devices is to assess in-vivo drug release using animal models, but it requires more time and is highly resource intensive. Hence in-vitro simulated conditions are often chosen to shorten the formulation development period and to avoid high developmental costs. The in-vitro release profile of SRL from DES is one of the most crucial parameters in designing and optimizing the formulation. To improve the SRL's solubility and stability in aqueous in-vitro release medium, several techniques have been developed like addition of suitable solvents, hydrotropes, surfactants, and block copolymers [25], [26], [27], [28], [29]. Surfactants form colloidal-sized clusters in solutions known as micelles which are capable of encapsulating the drug molecules, resulting in reduction in the interfacial tension and improved solubility of the drug in the medium, and hence are capable of increasing the solubility of sparingly soluble drug substances in aqueous solution [30], [31].
The concentration of nonionic surfactants in aqueous solution is a very important and critical parameter along with the temperature and agitation in development of drug release media for the hydrophobic drug like SRL as it has no ionizable group [30], [32]. Accelerated in-vitro release media could be beneficial for rapid assessment of the formulation and manufacturing process parameters in the early development stage of DES as well as quality control method development [33], [34]. Such accelerated methods are also recommended by regulatory agencies in the assessment of drug releasing properties of DES [35]. We have designed a systematic study plan to evaluate Pluronics micelles, nonionic surfactant micelles, Tetronic micelles and mixed micelles which can be used to develop drug release media (DRM) for DES and their release profile can be correlated with the real time release data. For this purpose in our previously published research work, different block co-polymers (Pluronics) had been evaluated for the solubilization and release of SRL from the DES [29]. In the same context of research flow, the main objective of the present work was to evaluate application of some nonionic surfactant micelles in the aqueous media to evaluate accelerated in-vitro release for SRL eluting stents coated with blend biodegradable polymers. We have tried to optimize the DRM using nonionic surfactant micelles, the results showed that they would adequately solubilize and stabilize SRL for the quantification throughout the release time interval and then obtained accelerated in-vitro drug release profiles were correlated with the real time release data.
2. Materials and methods
2.1. Materials
The salts required for buffer preparations like disodium hydrogen phosphate, sodium chloride, potassium dihydrogen phosphate and HPLC grade organic solvents like dichloromethane, methanol and tetrahydrofuran were purchased from the Thermo Fisher Scientific, India. HPLC grade water and non-ionic surfactant Brij 35 were purchased from Merck, India, whereas Tween 20, Tween 80, Brij 56 and Brij 58 were purchased from Sigma Aldrich, India. The molecular characteristics of surfactants are given in Table 1.
Table 1.
Physicochemical characteristics of nonionic surfactants.
| Nonionic surfactants | Molecular formula | MW (Mn)a | HLBa | CMC (mM)a at 25 °C | CP (°C)a at 1% |
|---|---|---|---|---|---|
| Tween 20 | C58H114O26 | ~1228 | 16.7 | 0.06 | ~76 |
| Tween 80 | C64H124O26 | ~1310 | 15 | 0.012 | ~65 |
| Brij 35 | (C2H4O)23C12H25OH | ~1198 | 16.9 | 0.09 | >100 |
| Brij 56 | (C2H4O)10C16H33OH | ~683 | 12 | 0.035 | ~73 |
| Brij 58 | (C2H4O)20C16H33OH | ~1124 | 16 | 0.08 | >100 |
The average molecular weights (MW), HLB values, CMC values and the cloud points (CP) were determined and provided by the manufacturers.
SRL (molecular formula C51H79NO13, molecular weight 914.19 g/mol), a semi-synthetic macrolide antibiotic, was purchased from Hangzhou Zhongmeihuadong Pharmaceuticals, China. Biodegradable polymer poly (lactide-co-glycolide) (50:50 PLGA) was procured from Lakeshore Biomaterials, USA and poly vinyl pyrrolidone (PVP K-90/D) was procured from ISP technologies Inc., Wayne, NJ, USA. Coronary stents made of 316LVM stainless steel alloy coated with SRL and PLGA/PVP biodegradable polymeric blend were provided by Sahajanand Medical Technologies Pvt. Ltd. Surat, India.
2.2. Methods
2.2.1. Stent coating
The metallic (SS 316 LVM) coronary stents (16 mm long) (Sahajanand Medical Technologies, India) were coated with drug and polymer matrix solution. For controlled drug release, the drug SRL (20%, m/m) was blended with a matrix of biodegradable polymers 50:50 PLGA (75%, m/m) and PVP (5%, m/m) designed to deliver drugs at a predetermined rate. SRL and polymers were weighed accurately and dissolved in HPLC grade dichloromethane (DCM) to prepare drug coating solution having total solid concentration of 0.5% (m/v). The drug concentration over the entire surface area was kept at 1.0 μg/mm2. The drug-polymer solution was coated on stents using modified air suspension coating technique described in detail in earlier research work [36], [37]. The stents were spray-coated with drug coating solution to achieve a desired amount of drug-polymer matrix having even coating with the thickness of 5–7 µm measured by the scanning electron microscopy (SEM) on stents. Coated stents were vacuum dried for 24 h to ensure the complete removal of the residual solvent. The stents were weighed before and after the coating using analytical balance (Citizen CX-265 with 0.01 mg accuracy) to evaluate the total amount of drug-polymer matrix coated on the stent. The coating procedure was carried out in clean room conditions maintained at 20±3 °C and 50%±10% relative humidity.
2.2.2. High performance liquid chromatography(HPLC)
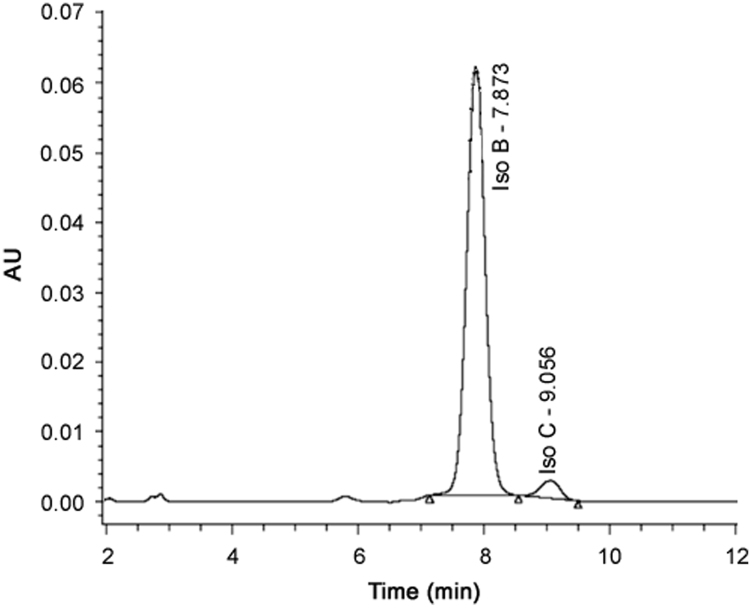
Quantification of the drug was carried out using reversed phase HPLC (LC-2010AHT; Shimadzu, Japan). The HPLC system consisted of a low pressure gradient separation module with configuration of quaternary solvent delivery pump, column heater and dual-wavelength UV detector. Chromatographic system operation and recording of data were performed with the use of LC Solution software. The HPLC separation was achieved using a Grace Smart C18 column (4.6 mm × 250 mm, 5.0 µm particle size, 100 Å pore size). The sample injection volume was 10 µL and flow rate was maintained at 1.0 mL/min. The mobile phase used was methanol:water at the ratio of 80:20 (%, v/v) with isocratic mode. The effluent was monitored with UV detector at 277 nm for total run time of 15 min. Two isomers of SRL, Isomer B and Isomer C can be separated adequately and detected by the HPLC at the retention time (RT) of approximately 7.8 min and 9.0 min, respectively, by the method used in current research work. Total areas of both the isomers (B and C) were used for the quantification of SRL which was based on the linear calibration curve with compliance to Beer-Lambert plot (R2= 0.999). Representative chromatogram of SRL standard in methanol is given in Fig. 1.
Fig. 1.
Typical HPLC chromatograms of SRL in methanol.
2.2.3. Optimization of DRM
Optimization of DRM containing nonionic surfactant was carried out based upon the solubility, stability and sink condition for SRL. In our previous work it was observed that the solubility and stability of SRL in phosphate buffer (PB) and acetate buffer (AB) at pH 4.0 each were better as compared to other buffers with different pH (PB pH 6.4, PB pH 7.4 and AB pH 4.5, AB pH 5.0) [29]. The results are in good agreement with the widely documented data exhibiting instability of SRL and some other drugs of the same class in neutral and in basic pH range because of hydrolysis of lactone moiety present in this class of drugs [38], [39]. The poor solubility and stability of SRL in PB with pH 7.4 affect the quantification of solubilized SRL during definite time intervals required for the drug release method to construct release or dissolution profile of SRL from DES. Hence in this study we developed an aqueous media that can solubilize 90% or more amounts of SRL and also that solubilized drug should remain stable in the media during drug release time period. Since nonionic surfactants generally have smaller critical micelle concentration (CMC) values and are known to be good solubilizers of SRL like hydrophobic substances; Brij 35, Brij 56, Brij 58, Tween20 and Tween 80 were selected for the initial study. The concentrations of surfactants were kept slightly above their respected CMC values to get adequate drug solubility in PB and AB (pH 4.0). Also, if the concentration of surfactant in the media is much higher than CMC, then it may react with the biodegradable polymers within coated film on the stent surface during the drug release study and/or it may also interfere with the chromatographic separation and quantification of SRL by HPLC. Thus, surfactant concentrations were kept as low as possible but slightly higher than CMC (ranging from 0.1% to 1.0%) for the initial studies.
The solubility and stability of SRL in the media were determined by spiking (day 0) minimum volume (≤50 µL) of highly concentrated stock solution prepared in methanol into the aqueous media with and without nonionic surfactant and then incubating it up to 48 h at 37 °C with 50 rpm in orbital shaking incubator (Orbitek LT, Scigenic Biotech). The solubility of SRL was calculated from the amount of SRL recovered by HPLC at zero time point with respect to theoretical amount of SRL taken. Similarly, stability was evaluated at zero time point and at 48 h by comparing the amount recovered at different time points with respect to amount of SRL obtained at zero time point by HPLC.
The sink conditions were determined by dispersing about 1.0 mg of SRL (powder form) in 2.0 mL water as well as in release medium with and without nonionic surfactants. The solutions were then sonicated in the ultrasonic bath (model 3510, Branson) for 60 min. Aliquots of the solution were withdrawn, filtered through a 0.45 µm nylon filter using syringe and analyzed by HPLC for the quantification.
2.2.4. Dynamic light scattering (DLS)
In order to determine the micelle size and poly-dispersity, DLS measurements were carried out at 90° scattering angle on solutions using Zetasizer 4800 (Malvern Instruments, UK) equipped with 192 channel digital correlator (7132) and coherent (Innova) air-ion laser at a wavelength of 514.5 nm. The average diffusion coefficients and hence the hydrodynamic size were obtained by the method of cumulants. Each measurement was repeated at least five times. All samples were filtered and proper care was taken to avoid contamination.
2.2.5. Accelerated in-vitro drug release and real time in-vitro drug release study
The accelerated in-vitro drug release studies were conducted in optimized drug release media using orbital shaking incubator (Orbitek LT, Scigenic Biotech) at the pre-determine time intervals. 8 mL of release medium (with surfactants) was added to a 10 mL volumetric flask containing DES and incubated at 37±1 °C with an agitation at 50 rpm. Stents were expanded using an inflation device (i30™, Sahajanand Medical Technologies) to its nominal expansion diameter of 3.0 mm prior for incubation. Entire release medium was changed at the end of every time point (0.5, 1.5, 3.0, 5.0, 24.0 and 48.0 h) to maintain sink conditions. Release media containing different surfactants and their varying concentrations were investigated; the agitation rate and temperature were maintained constant. At the end of each release time interval, the samples were withdrawn and directly transferred into HPLC vials and analyzed for SRL content in release media by HPLC. The in-vitro drug release analysis was carried out on 12 numbers of samples in each finalized medium at pre-determined time intervals to evaluate and compare the profiles of SRL from DES. After the completion of all the release time intervals, any drug remaining on DES was extracted in methanol by sonication.
The real time (long term)in-vitro drug release studies were conducted on 12 numbers of samples using orbital shaking incubator(Orbitek LT, Scigenic Biotech). All stents were expanded using an inflation device (i30™, Sahajanand Medical Technologies) to its nominal expansion diameter of 3.0 mm prior for incubation. Phosphate buffer saline (PBS) with pH 7.4 prepared as per Indian Pharmacopeia (IP) was selected as drug release media to simulate the in-vivo conditions. 8 mL of PBS was added to the DES in a 10 mL volumetric flask and incubated at 37±1 °C with an agitation of 50 rpm. Aliquots were collected after every 24 h up to 7 days and every 72 h after 7 days. Fresh 8 mL of PBS was added after each time interval when the aliquots had been taken to maintain the sink condition and kept it again in orbital shaking incubator up to next time interval and so on. The real time drug release study was continued up to 48 days. After completion of each specific release time interval PBS was collected and extraction of SRL was done from the buffer using organic solvent dichloromethane (DCM). DCM was then evaporated using water bath and SRL was dissolved in methanol to analyze for drug release by RP-HPLC method.
2.2.6. SEM and gravimetric analysis
SEM (XL-30 ESEM, Philips, the Netherlands) was used to examine the surface morphology changes of DES before and after exposure to the DRM at each time interval during drug release test.
Gravimetric analysis was carried out by weighing of DES. Weight of each DES was recorded using analytical balance (XS205DU, Mettler Toledo, USA) before and after placing them in the DRM for 48 h during drug release test and mass loss (%) was calculated.
2.2.7. Gel permeation chromatography (GPC)
The molecular weight of polymer matrix at initial time (0 h) and after the completion of last release time interval (48 h) was determined by GPC. Because the amount of polymer matrix present on single stent is very less for detection by GPC, polymer matrix was extracted from 3 stents at both the time-intervals and then analyzed for molecular weight. Polymer matrix along with drug was extracted by dissolving the stents in DCM, which was later evaporated using dry nitrogen gas. Molecular weight of the test samples was determined by GPC using an LC-2010AHT; Shimadzu HPLC system equipped with a differential refractive index detector (RID-10AShimadzu) and a column (PLgel, 5 µm, Agilent) maintained at 40 °C. Degassed tetrahydrofuran (THF) was used as the mobile phase at a flow rate of 1 mL/min. The average molecular weight of test samples was determined relative to polystyrene standards with molecular weights ranging from 162 to 5,000,000 g/mol (Polystyrene Easycal Vial, Agilent). The calibration was performed for each run.
2.2.8. Statistics
All data are presented as mean±SD. Accelerated in-vitro release profiles were compared using the similarity factor, f2 [40]. The compared profiles were considered equivalent when f2 value obtained was 50 or higher (50−100).
Where n=Time point, Rt=% cumulative drug release at each selected ‘n’ time interval of the reference set and Tt = % cumulative drug release at each selected ‘n’ time interval of the reference set.
The correlation between the short-term and long-term release studies was established for different drug release media by plotting different levels of release obtained in days (for real time release) vs. hours (for accelerated release). The correlation coefficients (R2) derived is the indicator of whether newly developed accelerated in-vitro release method correlates with the real-time release or not and the prediction of the long-term release from the accelerated release profile is possible or not [41].
3. Results and discussion
3.1. Optimization and development of DRM
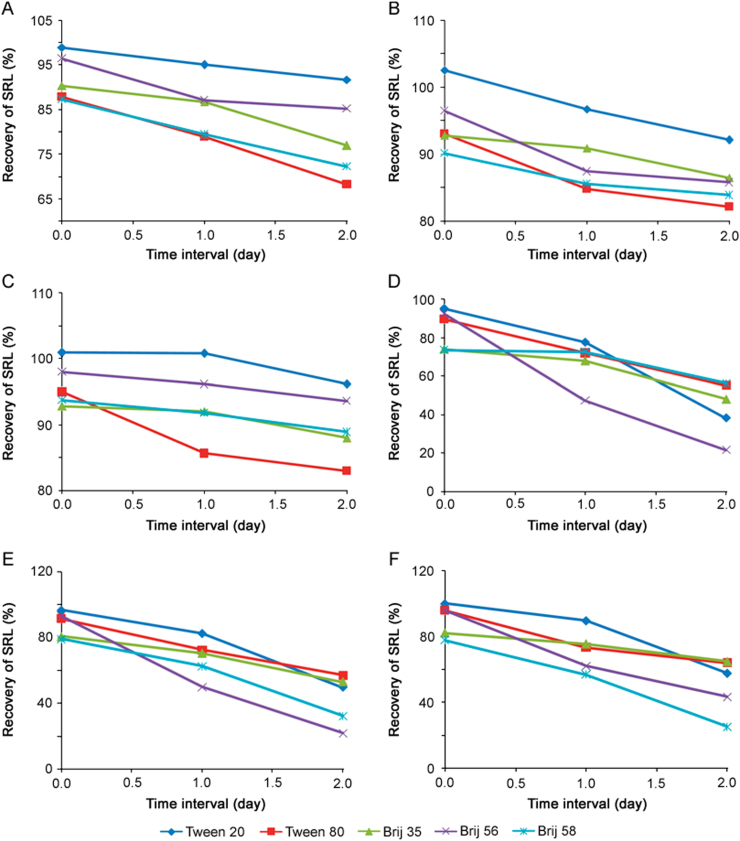
The percentage recovery of SRL from AB (pH 4.0) and PB (pH 4.0) was evaluated using different surfactants (Tweens and Brijs) and at different surfactant concentrations (0.1%, 0.5% and 1.0%) for time intervals of day 0, day 1 and day 2. The average % drug recovery from different nonionic surfactants in two buffer media (AB and PB) at pH 4 is given in Fig. 2. It is evident from Figs. 2A, B and C that solubility of SRL in Tween 20 was better than Tween 80 in PB at all three concentrations. In case of Brij, Brij 35 exhibited better solubility in PB than Brij 56 and Brij 58. Similarly, Figs. 2D, E and F depict that Tween 20, Brij 35 and Brij 58 exhibit good solubility in AB than the other Tween and Brij used in this study. It was also observed that SRL was more stable in PB for all three concentrations than in AB with all Tweens and Brijs evaluated during the study. Moreover, when the concentration of nonionic surfactants was increased, it led to an increase in hydrophobic interactions with drugs and also increased the number of micelles. This endows good stability to the system and micellar solubilization of SRL was observed at higher concentration of Tween 20, Brij 35 and Brij 58. However, in case of Brij 58, the solution became slightly hazy at 0.5% concentration and the opacity further increased as the concentration was raised to 1.0%, though an increase in solubility and stability of SRL was still observed with increase of concentrations. The solubility and stability of SRL in different Brij (35, 56 and 58) can be attributed to the difference in the poly (oxyethylene) chain length, in the order of Brij 35>Brij 58>Brij 56 (E23>E20>E10). Therefore, as the chain length increased in Brij it exhibited more solubility and stability of SRL in the media. Whereas Tweens (20 and 80) exhibited different solubility and stability behavior, which can be attributed to structural difference in the type of fatty acid associated with the poly (oxyethylene) sorbitan part of the molecule (monolaurate and monooleate), despite having the same (20) poly (oxyethylene) chain length.
Fig. 2.
The average % recovery of SRL after day 0, day 1 and day 2 in the different aqueous media composition: (A) 0.1% nonionic surfactants in PB (pH 4.0), (B) 0.5% nonionic surfactants in PB (pH 4.0), (C) 1.0% nonionic surfactants in PB (pH 4.0), (D) 0.1% nonionic surfactants in AB(pH 4.0), (E) 0.5% nonionic surfactants in AB(pH 4.0) and (F) 1.0% nonionic surfactants in AB(pH 4.0). Data represent the mean value for n=3.
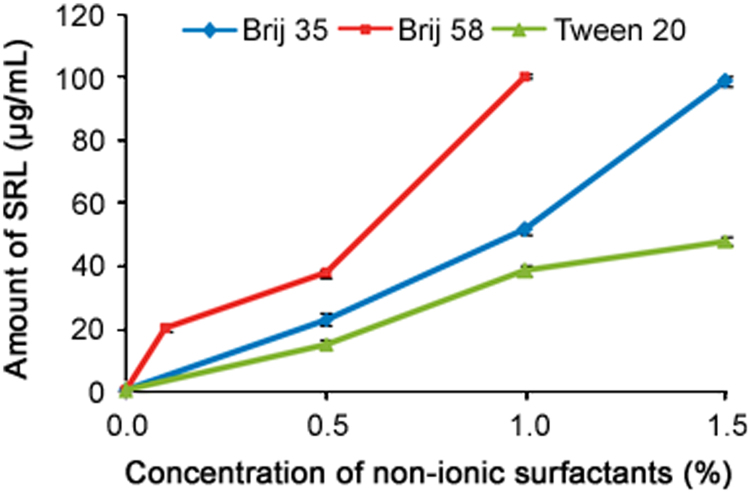
So, based upon these preliminary results, the different concentrations of Brij 35 (0.5%–1.5%), Brij 58 (0.1%–1.0%) and Tween 20 (0.5%–1.5%) were selected and evaluated to establish the sink conditions for SRL drug in aqueous release media. From the above results as well as from our previous work it is found that stability of SRL in PB at pH 4.0 is slightly better than in water and AB at pH 4.0 due to the less dissociation of SRL. Hence, 0.5%, 1.0% and 1.5% concentrations of Brij 35 and Tween 20 as well as 0.1%, 0.5% and 1.0% of Brij 58 were prepared in PB (pH 4.0) in triplicate and average solubility of SRL was evaluated. Sink condition for SRL was evaluated with and without the surfactants to ensure the adequate solubility of the drug in the release media, which was determined by HPLC. Average solubility of SRL with different concentrations of Tween and Brij in PB (pH 4.0) is given in Fig. 3. It was found that solubility of SRL in PB without surfactant (0.55 µg/mL) was better than in water (0.43 µg/mL), clearly indicating strong interactions between the drug and surfactants with a concentration-dependent manner. The solubilization of SRL in buffer increased with the addition of surfactants in a concentration-dependent manner.
Fig. 3.
Plotting of amount of solubilized SRL vs. nonionic surfactant (Brij 35, Brij 58 and Tween 20) concentration in phosphate buffer at pH 4.0. Data represent mean and S.D. for n=3.
Nonionic surfactants displayed different solution behavior depending on their molecular weight and their hydrophilic/lipophilic balance (HLB). Brij 58, Tween 20 and Brij 35 had almost similar HLB values (Table 1) and displayed increased solubility with the increase of concentration. The solubility of SRL remained high in surfactants with low HLB (Brij 58) while surfactants with high HLB values (Tween 20 and Brij 35) showed an opposite behavior up to 1.0% concentration. Fig. 3 shows that in the entire concentration range, the amount of SRL solubilized follows the trend Brij 58>Brij 35>Tween 20, which may be attributed to the fact that Brij 58 has the lowest molecular weight (Mn~1124<1198<1228, respectively), while Tween 20 has the highest molecular weight though the difference in the molecular weights is not significant. This leads to an increased hydrophobic–hydrophobic interaction for SRL with these nonionic surfactants participating in solubilization process. From the results, it is clearly observed that Tween 20 and Brij 35 with 1.0% concentration showed almost similar solubility as Brij 58 with 0.5% concentration, which is the desired solubility for SRL in the media. Hence, PB of pH 4.0 containing 1.0% concentration of Tween 20 and Brij 35 as well as 0.5% concentration of Brij 58 was good enough to use as DRM for the SRL eluting stents and was further used for the evaluation of in-vitro release of SRL from DES. Before using these media for the in-vitro release profile of SRL from the DES, some basic properties of the media and solubilization process need to be understood. With this approach the following studies were carried out to understand the micellar behavior in the optimized media.
3.2. Characterization of surfactant micelle in the optimized media
3.2.1. Effect on hydrodynamic size of micelles
Hydrodynamic size (Dh) of the surfactant micelles in PB at pH 4.0 with and without solubilized SRL was measured using DLS and is shown in Table 2.
Table 2.
Hydrodynamic size (Dh) of micelles without and with SRL in different concentrations of nonionic surfactants, Tween and Brij at 37 °C.
| Surfactant concentration | Dh without SRL (nm) |
Dh with SRL (nm) |
||||
|---|---|---|---|---|---|---|
| Tween 20 | Brij 35 | Brij 58 | Tween 20 | Brij 35 | Brij 58 | |
| 0.5% | – | – | 8.16 | – | – | 10.92 |
| 1.0% | 5.34 | 4.92 | 8.31 | 6.15 | 5.47 | 11.55 |
| 1.5% | 5.69 | 5.52 | – | 6.67 | 5.78 | – |
From the results, it is evident that for Tween 20, Brij 35 and Brij 58 Dh is almost similar at two different concentrations without solubilized SRL but in presence of SRL Dh increases with increase of concentration. With increase in Dh, solubilization is also increased with increase of concentrations (Fig. 3). There is a slight increase in Dh with the increase of concentration (Table 2) while solubility increase is quite rapid (Fig. 3). The Dh of Tween 20 and that of Brij 35 are nearly the same (5±1 nm), which may be because both the surfactants have nearly the same HLB values (16.7 and 16.9, respectively) while Dh of Brij 58 is slightly higher (8), which is probably due to lower HLB value (16.0). From the DLS results, it can thus be said that the hydrodynamic size of micelles increased for all three surfactants when SRL (drug) was incorporated within micelle, which is in complete agreement with results showing increase in solubility of the drug with increase of concentration.
3.2.2. Partition coefficient and thermodynamics of solubilization
Ability of nonionic surfactants to solubilize SRL drug was evaluated and characterized using the following descriptors [42].
(i) Molar solubilization capacity, χ, i.e. the number of moles of the solute (SRL drug) that can be solubilized by one mole of nonionic surfactant, which characterizes the ability of the surfactant to solubilize SRL drug.
(ii) Micelle-water partition coefficient, P, which is the ratio of the drug (SRL) concentration in the micelle to the drug (SRL) concentration in water (i.e. aqueous media-phosphate buffer of pH 4.0), for a surfactant concentration.
(iii) Standard free energy of solubilization, ∆Gs°, estimated from the molar micelle-water partition coefficient, PM (i.e., P for Ccopolymer =1 M).
The results shown in Table 3 indicate that the solubilization capacity (χ) and partition coefficient (P) increase as the surfactant concentration is increased. The standard free energy of solubilization, ∆Gs°, in water (PB pH 4.0) for SRL was calculated and found to be negative. These negative values indicate that the migration of the drug molecules in the micelle to the air-water interface is a spontaneous process favored by their hydrophobicity. However, more negative standard free energy of solubilization (∆Gs°) for the surfactant with increased concentration indicates more favored solubilization of SRL. The free energy of solubilization is negative in all cases and becomes more negative with increased concentration of surfactant, which suggests a strong hydrophobic interaction that leads to higher solubilization. Free energy of solubilization(∆Gs°) in the order of Brij 58>Brij 35>Tween 20 indicates that SRL is more soluble in Brij 58 than in Brij 35 and Tween 20, which is in good agreement with the solubility data of SRL (Fig. 3).
Table 3.
SRL solubilization parameters in different copolymer solutions.
| Tween 20 |
Brij 35 |
Brij 58 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc. (%) | χ | P | ∆Gso (kJ/mol) | Conc. (%) | χ | P | ∆Gso (kJ/mol) | Conc. (%) | χ | P | ∆Gso (kJ/mol) |
| 0.5 | 3.99 × 10−3 | 26.6 | −4.72 | 0.5 | 6.00 × 10−3 | 40.73 | −5.69 | 0.1 | 2.69 × 10−2 | 36.22 | −6.84 |
| 1.0 | 4.26 × 10−3 | 68.91 | −4.88 | 1.0 | 6.77 × 10−3 | 92.86 | −5.99 | 0.5 | 1.24 × 10−2 | 67.58 | −7.58 |
| 1.5 | 5.13 × 10−3 | 86.04 | −5.36 | 1.5 | 8.63 × 10−3 | 178.31 | −6.62 | 1.0 | 0.93 × 10−2 | 181.2 | −9.58 |
3.3. In-vitro drug release analysis of SRL eluting stents (SES)
3.3.1. Accelerated in-vitro drug release
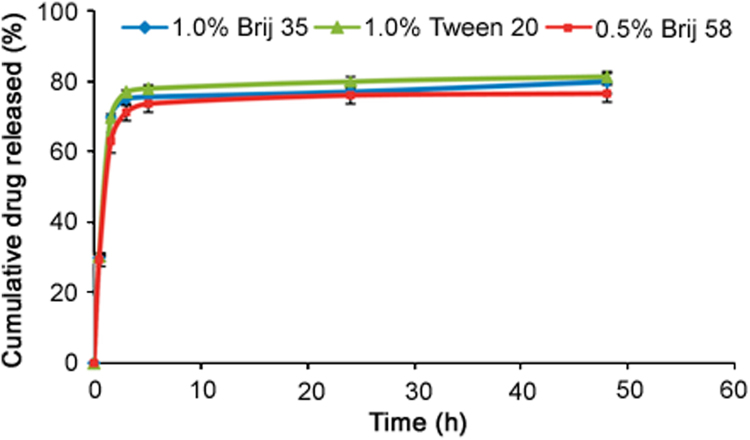
The choice of a suitable release medium is an important aspect in the development of in-vitro drug release method. The aqueous release media which exhibited adequate sink conditions (required solubility and stability) for SRL were selected to study accelerated in-vitro drug release. On the basis of the preliminary studies, release media, namely 0.5% Brij 58 in PB (pH 4.0), 1.0% Brij 35 in PB (pH 4.0) and 1.0% Tween 20 in PB (pH 4.0), were selected as in-vitro drug release media and evaluated at the predetermined time intervals of 0.5, 1.5, 3, 5, 24 and 48 h which were suited for intended application. The time intervals used were decided from the trial and error approach. Cumulative percentage of drug released into each selected release medium is plotted as a function of time. Cumulative release profiles of SRL from DES in all the three optimized media are shown in Fig. 4.
Fig. 4.
The in-vitro release profile of SRL from DES into aqueous media containing different nonionic surfactants in PB (pH 4.0). Data represent mean and S.D. for n=12.
It is well known that the surfactant lowers the interfacial tension between the product and the aqueous release medium, allowing for a more rapid and possibly more complete penetration of the release medium into coating matrix. At higher surfactant concentrations than CMC, a greater amount of surfactant is incorporated into the matrix, which results in greater wetting/solubilization of the drug, and consequently increases the drug release rate from the coating matrix [43], [44]. The above results up to 48 h are in good agreement with the data exhibiting almost similar solubility (Fig. 2) in 0.5% Brij 58, 1.0% Brij 35% and 1.0% Tween 20. But still Tween 20 was found to be the best drug release medium by giving more than 80% drug release (average 81.92%) while Brij35 gave close to but less than 80% (average 77.76%) and Brij 58 exhibited slightly less than 80% (average 75.46%) drug release in 48 h time period. The average residual drug content on these stents after the complete release time period was found to be about 9%, 12% and 11% for media containing Tween 20, Brij 35 and Brij 58, respectively. So for the stents kept in 1.0% Tween 20% and 1.0% Brij 35, about 90% of the total drug loaded was recovered whereas in the media containing 0.5% Brij 58, about 86% of the total drug loaded was recovered. This suggests that the amount of drug transformed or degraded is slightly higher in Brij 58 than in Brij 35 and Tween 20, which confirm that though solubility was similar, the stability of SRL is higher at higher concentrations of surfactant. Hence, at higher surfactant concentrations (1.0% Tween 20 and 1.0% Brij 35), a greater amount of surfactant is incorporated into the matrix, which results in greater wetting of the SRL as compared to the lower concentration (0.5% Brij 58), consequently increasing the drug release rate from the biodegradable polymer matrix. The f2 profile comparison was performed to evaluate the similarity between drug release profiles obtained in all the three media. The calculated similarity factor f2 value obtained for Brij 35 and Tween 20 is 84, for Brij 35 and Brij 58 is 72 while for Brij 58 and Tween 20 is 66. These results indicate that the similarity factor f2 values between all three profiles are more than 50, suggesting similarity of the release of SRL at the predetermined time interval in all three media containing nonionic surfactants. But highest similarity factor values (84) obtained by 1.0% Tween 20% and 1.0% Brij 35 in PB (pH 4.0) system indicated that both the surfactants in similar experimental conditions have a similar influence on the SRL release rate from the biodegradable polymeric matrix. This can be explained by the molecular characteristics of both the surfactants. Tween 20 is slightly more hydrophobic than Brij 35, which helps to increase the stability of SRL in the aqueous media, leading to the higher drug release. The decrease of SRL release in 0.5% Brij 58 might be attributed to the promotion of hydrophobic-hydrophilic interactions at the comparatively higher hydrophilic part in its molecule at slightly lower concentration of surfactant. Judging from the cumulative percent release profile, approximately 25%–70% of the drug is released at a relatively rapid rate during the first five time intervals up to 5 h, followed by slower or no release over the next 19 h (up to 24 h). There was no increase observed in the rate of SRL release after 24 h (up to 48 h) with all the tested release media. Overall, the initial fast release rate is commonly ascribed to the drug detachment from the biodegradable polymer surface, while the later slow release results from the sustained drug release from the inner layer. The release media compositions of 1.0% Tween 20 (in PB pH 4.0) at predetermined time intervals gave more than 80% of SRL release at the last time point, which is recommended as a specification for the accelerated release method [30]. While the drug release media with 1.0% Brij 35% and 0.5% Brij 58 fail to achieve the recommended specification for the accelerated release profile (<80% release at last time interval). These results strongly substantiate that the optimized media and the methodology with 1.0% Tween 20 at predetermined six time intervals are most suitable for assessing the release kinetics of SRL in aqueous media from the DES coated with the blend of biodegradable polymers.
3.3.2. Correlation between accelerated and real time in-vitro drug release profiles
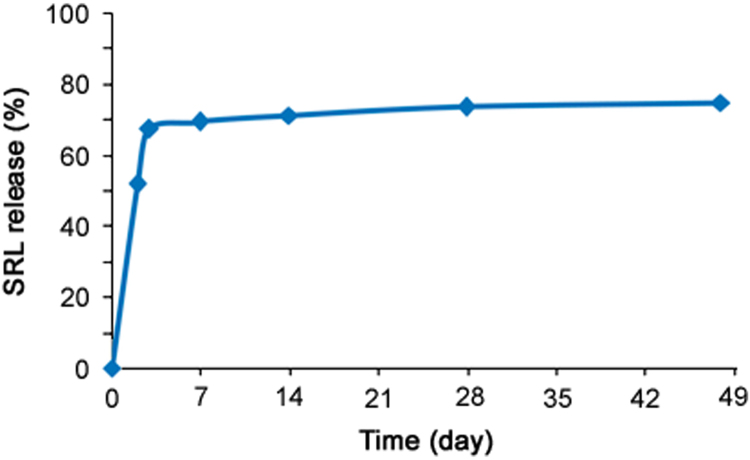
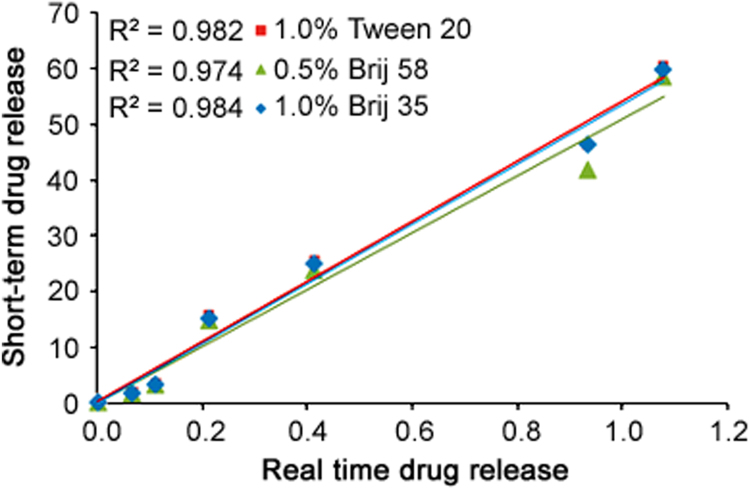
As per the methodology given in Section 2.2.5, the real time in-vitro drug release was evaluated in PB at pH 6.4 and found to be average 70%–75% drug release in 48 days period (Fig. 5). While with the newly developed accelerated method using nonionic surfactants, about 80% of SRL was released within 48 h (Fig. 4). The correlation between the accelerated and real time releases was established by plotting different levels of release in days vs. hours, as shown in Fig. 6 for all the three drug release profiles obtained using nonionic surfactants. The correlation coefficients (R2) obtained were 0.982, 0.974 and 0.984 for DRM with 1.0% Tween 20, 0.5% Brij 58% and 1.0% Brij 35, respectively. The correlation coefficients (R2) of ≥0.9 indicate that the developed accelerated release method correlates well with the real-time release and allows for a prediction of the long-time release from the accelerated release profile [39]. Hence accelerated drug release method with 1.0% Tween 20 and 1.0% Brij 35 having R2 0.9822 and 0.984, respectively, correlates well with the long-time release data and can be used in future for the SRL eluting stent with biodegradable coating for the evaluation of drug release profile.
Fig. 5.
The real time in-vitro release profile of SRL from DES in aqueous media PB (pH 7.4).
Fig. 6.
The accelerated in-vitro release profile of SRL from DES into aqueous media containing 1.0% Tween 20(■), 0.5% Brij 58(▲) and 1.0% Brij 35(♦) compared with real time release profile in PB (pH 7.4).
From the results, it can be observed that the incorporation of nonionic surfactant in the release medium results in an improved drug release rate, which is attributed to the increase in the porosity of the matrices due to the swelling. The drug can then easily migrate to the media with surfactant as compared to the media without surfactant and the acidic pH of the media with surfactant also increase the stability of SRL, leading to the accurately quantifying drug release.
3.3.3. SEM, GPC and gravimetric analysis
Alongside drug release studies, the SEM, gel permeation chromatography (GPC) and gravimetric mass studies were performed to get further insight into the possible drug release mechanisms. Changes of the surface morphology of the SRL eluting stents coated with the blend of biodegradable polymers (PLGA/PVP) upon immersion in the optimized drug release media (1.% Tween 20 in PB pH 4.0) at the end of each time interval were examined.
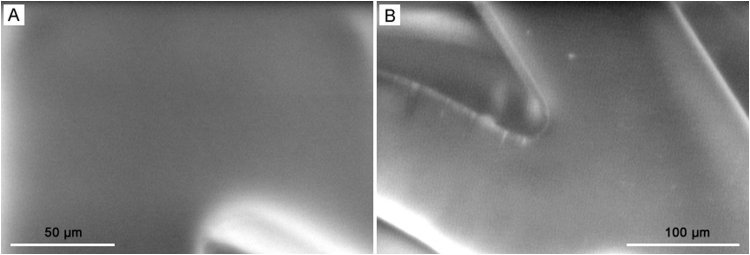
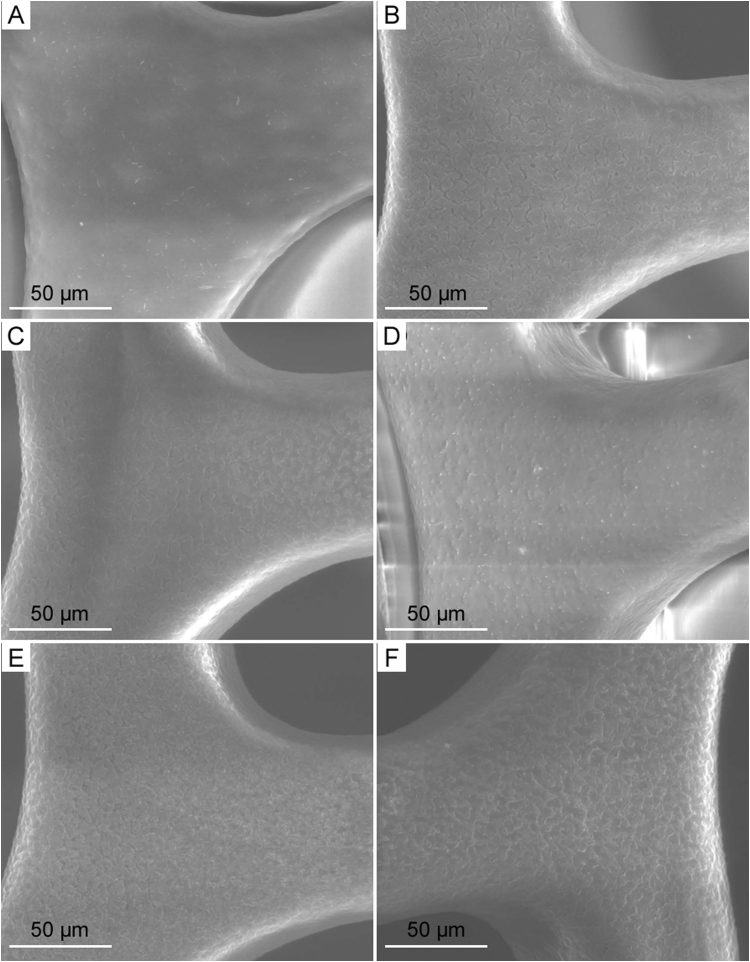
SEM images as depicted in Figs. 7 and 8 represent the surface condition of DES pre-incubation and post-incubation, respectively. The DES at 0 time interval before immersion in the drug release media as shown in Fig. 7 exhibited a smooth and nonporous surface. As the polymeric matrix gets hydrolyzed due to the aqueous release media, under the influence of concentration gradient, SRL drug diffuses out of the coating through micro-channels which can be visualized at high magnification due to their very small sizes. The hydrophilic polymer PVP gets dissolved in the media and enables release media to interact within polymeric matrix allowing SRL to get eluted. The incubation period in this study was 48 h, which is short, resulting in micro-voids from the drug release. Morphological observation of SEM in Fig. 8 reveals that the micro-channels and/or micro-voids are created at coating interface due to the diffusion of drug release during the short incubation period. Drug release has been quantified by HPLC in this research; however, surface investigation by SEM enables us to further understand the changes that occurred at the interface and thus helps us better understand the drug release mechanism.
Fig. 7.
The SEM images of the surface morphology of SRL eluting stents with biodegradable coating of PLGA/PVP before the drug release evaluation.
Fig. 8.
The SEM images of the surface morphology of SRL eluting stents with biodegradable coating of PLGA/PVP after exposure to the release medium at each time interval: (A) 0.5 h, (B) 1.5 h, (C) 3 h, (D) 5 h, (E) 24 h and (F) 48 h.
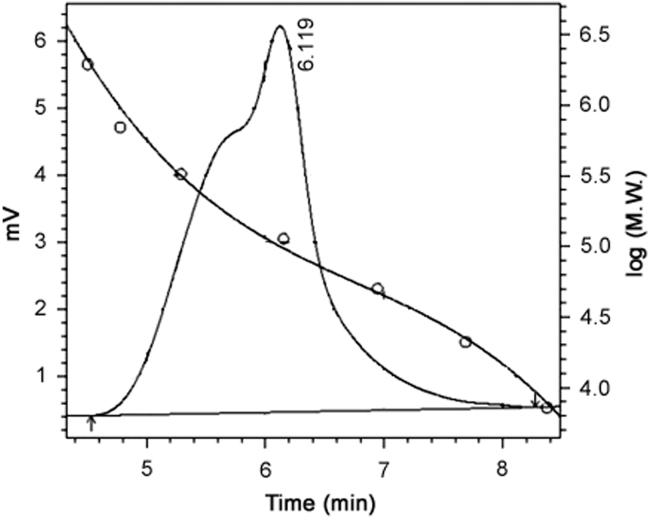
Blend of biodegradable polymers 50:50 PLGA (Mw 135 kDa) and PVP (Mw of 1300 kDa) as per the formulation proportion was used for the coating of the SRL eluting stents. From the earlier studies it is well understood that PLGA does not undergo degradation in short-time interval [45], [46], [47]; hence GPC and gravimetric analysis were performed before (0 h) and after the drug release time intervals (48 h) to get some idea about the possible drug release phenomena. By the GPC method used, we could not separately analyze both the polymers from the blend of polymers (PLGA/PVP). As we could evaluate the combined molecular weight, the representative chromatogram of GPC blend is shown in Fig. 9.
Fig. 9.
Representative GPC chromatogram for the blend of polymers (PLGA/PVP).
At the end of drug release time intervals of 48 h, an average of 6% decrease in the molecular weight for the blend of biodegradable polymers (PLGA+PVP) was observed by GPC. From the results, it was observed that the major part of biodegradable polymeric (PLGA/PVP) coating remained because the PLGA did not undergo degradation or hydrolysis till the completion of release (48 h). While due to the hydrophilic nature of the PVP, it got dissolved in the media, giving about 6% molecular weight loss by GPC. As the PVP got dissolved rapidly after exposure into media, it would also take the drug particles along from the stent. The molecular weight of the polymeric coating remained almost unchanged with just 6% decrease during the release period, suggesting that the SRL release occurred mainly because of the diffusion process through the matrix and the major part of the coating did not undergo degradation due to the hydrolysis within short period of study. During the gravimetric weight analysis of DES, an average of 32% decrease in the total weight of DES was observed after the completion of drug release (48 h). The presence of nonionic surfactant in the drug release media can readily solubilize the diffused SRL which creates pores as observed in SEM. Hence dissolution of PVP and SRL together in the media leads to about 32% decrease in the gravimetric weight of DES. The GPC and gravimetric weight loss data indicate that the polymer does not undergo degradation (hydrolytic or biodegradation) through cleavage of its backbone linkages.
4. Conclusion
In this research work, we developed an aqueous drug release medium in which SRL is stable for a desired time interval (48 h) with required adequate solubility. The data generated using different media suggested that in phosphate buffer with pH 4.0 containing 1.0% Tween 20 we obtained about 80% drug release during the period of 48 h and hence it is the most appropriate release medium to assess the accelerated in-vitro release of SRL from the biodegradable polymeric matrix (PLGA/ PVP) coated stents. Also, Tween 20 and Brij 35 in the same concentration and experimental conditions affect the drug release rate differently due to their specific molecular characteristics in drug release media. The current approach may be applied to evaluate drug release from a biodegradable polymeric matrix and in the selection of an appropriate release medium containing nonionic surfactants for in-vitro drug release method development of the specific formulation of SRL. The obtained accelerated in-vitro drug release profile (1.0% Tween 20 in PB at pH 4.0) is correlated well with the real time in-vitro drug release data (in PB at pH 7.4). This newly developed accelerated in-vitro drug release method can be used during pharmaceutical formulation development of DES and as a rapid quality control tool during commercial manufacturing of DES after careful validation as well as evaluation of its discrimination power to identify specification products.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors wish to express gratitude to Mr. Dhirajlal Kotadia, Chairman, Sahajanand Group of Companies, for providing required support, some materials and facilities for the experiments.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Betriu A., Masotti M., Serra A. Randomized comparison of coronary stent implantation and balloon angioplasty in the treatment of de novo coronary artery lesions (START): a four-year follow-up. J. Am. Coll. Cardiol. 1999;34:1498–1506. doi: 10.1016/s0735-1097(99)00366-6. [DOI] [PubMed] [Google Scholar]
- 2.Serruys P.W., de Jaegere P., Kiemeneij F. A comparison of balloon expandable stent implantation with balloon angioplasty in patients with coronary artery disease. N. Engl. J. Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 3.Morice M.-C., Serruys P.W., Sousa J.E. A randomized comparison of a sirolimus eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 4.Moses J.W., Leon M.B., Popma J.J. Sirolimus eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 5.Colombo A., Drzewiecki J., Banning A. Randomized study to assess the effectiveness of slow and moderate release polymer based paclitaxel eluting stents for coronary artery lesions. Circulation. 2003;108:788–794. doi: 10.1161/01.CIR.0000086926.62288.A6. [DOI] [PubMed] [Google Scholar]
- 6.Buszman P., Trznadel S., Milewski K. Novel paclitaxel eluting, biodegradable polymer coated stent in the treatment of de novo coronary lesions: a prospective multicenter registry. Catheter. Cardiovasc. Interv. 2008;71:51–57. doi: 10.1002/ccd.21392. [DOI] [PubMed] [Google Scholar]
- 7.Rechavia E., Litvack F., Fishbien M.C. Biocompatibility of polyurethane coated stents: tissue and vascular aspects. Cathet. Cardiovasc. Diagn. 1998;45:202–207. doi: 10.1002/(sici)1097-0304(199810)45:2<202::aid-ccd20>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Acharya G., Park K. Mechanisms of controlled drug release from drug-eluting stents. Adv. Drug Deliv. Rev. 2006;58:387–401. doi: 10.1016/j.addr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Byrne R.A., Kastrati A., Kufner S. Randomized, non-inferiority trial of three limus agent eluting stents with different polymer coatings: the intracoronary stenting and angiographic results: test efficacy of 3 limus eluting stents (ISAR-TEST-4) trial. Eur. Heart J. 2009;30:2441–2449. doi: 10.1093/eurheartj/ehp352. 〈http://eurheartj.oxfordjournals.org/content/30/20/2441.abstract〉 [DOI] [PubMed] [Google Scholar]
- 10.De Scheerder I.K., Wilczek K.L., Verbeken E.V. Biocompatibility of polymer-coated oversized metallic stents implanted in normal porcine coronary arteries. Atherosclerosis. 1995;114:105–114. doi: 10.1016/0021-9150(94)05472-u. [DOI] [PubMed] [Google Scholar]
- 11.Gunatillake P., Mayadunne R., Adhikari R. Recent developments in biodegradable synthetic polymers. Biotechnol. Annu. Rev. 2006;12:301–347. doi: 10.1016/S1387-2656(06)12009-8. [DOI] [PubMed] [Google Scholar]
- 12.O. Hnojewyj, P.J. Rivelli, T.B. Shaffer, Drug delivery polyanhydride composition and method: US, US 20080014170 A1. 〈https://www.google.co.in/patents/US20080014170〉, 2008.
- 13.Vetrovec G.W., Rizik D., Williard C. Sirolimus PK trial: a pharmacokinetic study of the sirolimus eluting Bx Velocity stent in patients with de novo coronary lesions. Catheter. Cardiovasc. Interv. 2006;67:32–37. doi: 10.1002/ccd.20565. [DOI] [PubMed] [Google Scholar]
- 14.Patel J.K., Kobashigawa J.A. Everolimus: an immunosuppressive agent in transplantation. Expert. Opin. Pharmacother. 2006;7:1347–1355. doi: 10.1517/14656566.7.10.1347. [DOI] [PubMed] [Google Scholar]
- 15.Sehgal S.N., Baker H., Vézina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J. Antibiot. 1975;28:727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 16.Singh K., Sun S., Vézina C. Rapamycin (AY-22,989), a new antifungal antibiotic. IV. mechanism of action. J. Antibiot. 1979;32:630–645. doi: 10.7164/antibiotics.32.630. [DOI] [PubMed] [Google Scholar]
- 17.Nashan B. Review of the proliferation inhibitor everolimus. Expert. Opin. Investig. Drugs. 2002;11:1845–1857. doi: 10.1517/13543784.11.12.1845. [DOI] [PubMed] [Google Scholar]
- 18.Versaci F., Gaspardone A., Tomai F. Immunosuppressive therapy for the prevention of restenosis after coronary artery stent implantation (impress study) J. Am. Coll. Cardiol. 2002;40:1935–1942. doi: 10.1016/s0735-1097(02)02562-7. [DOI] [PubMed] [Google Scholar]
- 19.Sabatini D.M., Erdjument-Bromage H., Lui M. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 2015;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 20.Marx S.O., Jayaraman T., Go L.O. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ. Res. 1995;76:412–417. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- 21.Napoli K.L., Wang M.-E., Stepkowski S.M. Distribution of sirolimus in rat tissue. Clin. Biochem. 1997;30:135–142. doi: 10.1016/s0009-9120(96)00157-9. [DOI] [PubMed] [Google Scholar]
- 22.R.P. Waranis, T.W. Leonard, Rapamycin formulation for IV injection: EP, US 5616588 A. 〈https://www.google.co.in/patents/US5616588〉, 1997.
- 23.Sehgal S.N. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant. Proc. 2003;35:S7–S14. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 24.Ruan G., Feng S.-S. Preparation and characterization of poly(lactic acid)–poly(ethylene glycol)–poly(lactic acid) (PLA–PEG–PLA) microspheres for controlled release of paclitaxel. Biomaterials. 2003;24:5037–5044. doi: 10.1016/s0142-9612(03)00419-8. [DOI] [PubMed] [Google Scholar]
- 25.H.S. Yalkowsky, Solubility and Solubilization in Aqueous Media, American Chemical Society, Oxford University Press, Washington, D.C., New York, 1999: 236–320
- 26.Hodgdon T.K., Kaler E.W. Hydrotropic solutions. Curr. Opin. Colloid Interface Sci. 2007;12:121–128. [Google Scholar]
- 27.Chakraborty S., Shukla D., Jain A. Assessment of solubilization characteristics of different surfactants for carvedilol phosphate as a function of pH. J. Colloid Interface Sci. 2009;335:242–249. doi: 10.1016/j.jcis.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 28.Faisant N., Akiki J., Siepmann F. Effects of the type of release medium on drug release from PLGA-based microparticles: experiment and theory. Int. J. Pharm. 2006;314:189–197. doi: 10.1016/j.ijpharm.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Raval A., Parmar A., Raval A. Preparation and optimization of media using Pluronic® micelles for solubilization of sirolimus and release from the drug eluting stents. Colloids Surf. B Biointerfaces. 2012;93:180–187. doi: 10.1016/j.colsurfb.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Velikaya E.V., Kemenova V.A., Demina N.B. Determining the effective surfactant concentration for the drug dissolution test. Pharm. Chem. J. 2004;38:267–270. [Google Scholar]
- 31.Rangel-Yagui C.D.O., Pessoa A., Tavares L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005;8:147–163. [PubMed] [Google Scholar]
- 32.Simamora P., Alvarez J.M., Yalkowsky S.H. Solubilization of rapamycin. Int. J. Pharm. 2001;213:25–29. doi: 10.1016/s0378-5173(00)00617-7. [DOI] [PubMed] [Google Scholar]
- 33.Raval A., Parikh J., Engineer C. Mechanism and in vitro release kinetic study of sirolimus from a biodegradable polymeric matrix coated cardiovascular stent. Ind. Eng. Chem. Res. 2011;50:9539–9549. [Google Scholar]
- 34.Alexis F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)] Polym. Int. 2005;54:36–46. [Google Scholar]
- 35.U S Food and Drug Administration, Guidance for Industry: Coronary Drug-Eluting Stents - Nonclinical and Clinical Studies. 〈http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM228704.pdf〉, 2008.
- 36.Raval A., Parikh J., Engineer C. Dexamethasone eluting biodegradable polymeric matrix coated stent for intravascular drug delivery. Chem. Eng. Res. Des. 2010;88:1479–1484. [Google Scholar]
- 37.Raval A., Choubey A., Engineer C. Novel biodegradable polymeric matrix coated cardiovascular stent for controlled drug delivery. Trends Biomater. Artif. Organs. 2007;20:101–110. [Google Scholar]
- 38.Wang X., Venkatraman S.S., Boey F.Y. Controlled release of sirolimus from a multilayered PLGA stent matrix. Biomaterials. 2006;27:5588–5595. doi: 10.1016/j.biomaterials.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Il’ichev Y., Alquier L., Cynthia M. Degradation of rapamycin and its ring-opened isomer: role of base catalysis. Arkivoc. 2007;2007:110–131. [Google Scholar]
- 40.Moore J.W., Flanner H.H. Mathematical comparison of curves with an emphasis on in vitro dissolution profiles. Pharm. Technol. 1996;20:64–74. [Google Scholar]
- 41.Burgess D.J., Hussain A.S., Ingallinera T.S. Assuring quality and performance of sustained and controlled release parenterals: AAPS workshop report, Co-Sponsored by FDA and USP. Pharm. Res. 2002;19:1761–1768. doi: 10.1023/a:1020730102176. [DOI] [PubMed] [Google Scholar]
- 42.Zackrisson G., Östling G., Skagerberg B. Accelerated dissolution rate analysis (ACDRA) for controlled release drugs. Application to Roxiam®. J. Pharm. Biomed. Anal. 1995;13:377–383. doi: 10.1016/0731-7085(95)01293-t. [DOI] [PubMed] [Google Scholar]
- 43.Jamzad S., Fassihi R. Role of surfactant and pH on dissolution properties of fenofibrate and glipizide—A technical note. AAPS PharmSciTech. 2006;7:E33. doi: 10.1208/pt070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barzegar-Jalali M., Adibkia K., Valizadeh H. Kinetic analysis of drug release from nanoparticles. J. Pharm. Pharm. Sci. 2008;11:167–177. doi: 10.18433/j3d59t. [DOI] [PubMed] [Google Scholar]
- 45.Vey E., Roger C., Meehan L. Degradation mechanism of poly(lactic-co-glycolic) acid block copolymer cast films in phosphate buffer solution. Polym. Degrad. Stab. 2008;93:1869–1876. [Google Scholar]
- 46.Chlopek J., Morawska-Chochol A., Paluszkiewicz C. FTIR and NMR study of poly(lactide-co-glycolide) and hydroxyapatite implant degradation under in vivo conditions. Polym. Degrad. Stab. 2009;94:1479–1485. [Google Scholar]
- 47.Engineer C., Parikh J., Raval A. Effect of copolymer ratio on hydrolytic degradation of poly(lactide-co-glycolide) from drug eluting coronary stents. Chem. Eng. Res. Des. 2016;89:328–334. [Google Scholar]