Abstract
S-Nitrosothiols or thionitrites with the general formula RSNO are formally composed of the nitrosyl cation (NO+) and a thiolate (RS−), the base of the corresponding acids RSH. The smallest S-nitrosothiol is HSNO and derives from hydrogen sulfide (HSH, H2S). The most common physiological S-nitrosothiols are derived from the amino acid L-cysteine (CysSH). Thus, the simplest S-nitrosothiol is S-nitroso-L-cysteine (CysSNO). CysSNO is a spontaneous potent donor of nitric oxide (NO) which activates soluble guanylyl cyclase to form cyclic guanosine monophosphate (cGMP). This activation is associated with multiple biological actions that include relaxation of smooth muscle cells and inhibition of platelet aggregation. Like NO, CysSNO is a short-lived species and occurs physiologically at concentrations around 1 nM in human blood. CysSNO can be formed from CysSH and higher oxides of NO including nitrous acid (HONO) and its anhydride (N2O3). The most characteristic feature of RSNO is the S-transnitrosation reaction by which the NO+ group is reversibly transferred to another thiolate. By this way numerous RSNO can be formed such as the low-molecular-mass S-nitroso-N-acetyl-L-cysteine (SNAC) and S-nitroso-glutathione (GSNO), and the high-molecular-mass S-nitrosol-L-cysteine hemoglobin (HbCysSNO) present in erythrocytes and S-nitrosol-L-cysteine albumin (AlbCysSNO) present in plasma at concentrations of the order of 200 nM. All above mentioned RSNO exert NO-related biological activity, but they must be administered intravenously. This important drawback can be overcome by lipophilic charge-free RSNO. Thus, we prepared the ethyl ester of SNAC, the S-nitroso-N-acetyl-L-cysteine ethyl ester (SNACET), from synthetic N-acetyl-L-cysteine ethyl ester (NACET). Both NACET and SNACET have improved pharmacological features over N-acetyl-L-cysteine (NAC) and S-nitroso-N-acetyl-L-cysteine (SNAC), respectively, including higher oral bioavailability. SNACET exerts NO-related activities which can be utilized in the urogenital tract and in the cardiovascular system. NACET, with high oral bioavailability, is a strong antioxidant and abundant precursor of GSH, unlike its free acid N-acetyl-L-cysteine (NAC). Here, we review the chemical and pharmacological properties of SNACET and NACET as well as their analytical chemistry. We also report new results from the ingestion of S-[15N]nitroso-N-acetyl-L-cysteine ethyl ester (S15NACET) demonstrating the favorable pharmacological profile of SNACET.
Keywords: CGMP, Nitric oxide, S-Nitrosothiols, Oxidative stress, Pharmaceuticals
1. Introduction of NO and S-nitrosothiols
Nitric oxide (NO) is one of the strongest known activators of soluble guanylyl cyclase (sGC) which catalyzes the synthesis of cyclic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP). cGMP is involved in many biological processes including relaxation of smooth cells and inhibition of platelet aggregation. These functions are of particular interest in the urogenital tract and in the cardiovascular system [1], [2]. NO is produced enzymatically from L-arginine by the catalytical action of constitutive and inducible NO synthase (NOS) isoforms virtually in all types of cell. By this reaction one of the terminal guanidine nitrogen (NG) atoms is oxidized to NO via NG-hydroxy-L-arginine. The second final reaction product is L-citrulline [3]. The physiological NG-methylated L-arginine analogs, NG-monomethyl-L-arginine, NG,NG-dimethyl-L-arginine (asymmetric dimethylarginine, ADMA) and NG,N‘G-dimethyl-L-arginine (symmetric dimethylarginine, SDMA), are all inhibitors of all NOS isoforms, albeit with in part remarkable differences in strength and type of inhibition [4], [5]. In human blood, NO is very short-lived; its half-life is considered to be less than 0.1 s [3]. Basally produced NO cannot be detected in blood. Its basal concentration in the blood is estimated to be below 1 nM. NO and its autoxidation product nitrite, the base of the nitrous acid (HONO, pKa 3.4), are oxidized in erythrocytes to nitrate [3]. Nitrite and nitrate circulate in blood and are excreted in the urine. Urinary nitrate can serve under certain conditions as biomarkers of whole-body NO production, while circulating nitrite is considered to derive mainly from NO produced in endothelial cells [6], [7], [8]. Nitrite excretion in the urine is at least in part dependent upon the activity of carbonic anhydrase (CA) in the proximal tubule [9], [10]. In the mouth and gut flora, nitrate is reduced to nitrite by nitrate reductases. In turn, in certain conditions hypoxia nitrite can be converted to NO by several proteins/enzymes including hemoglobin (Hb), xanthine oxidoreductase (XOR) and CA.
Food is an abundant contributor to circulating nitrite and nitrate. Currently, supplementation of inorganic nitrate is investigated as a pharmacological option in various conditions to enhance NO-related effects [11], [12], [13], [14], [15], [16], [17], [18]. Yet, for this ingestion of high amounts of nitrate is required and bears the potential for adverse effects especially in chronic use [19], [20], [21]. Organic nitrates (RONO2) such as glycerol trinitrate have been used for long time as NO donors for the treatment diseases associated with impaired NO synthesis or diminished NO bioavailability due to elevated oxidative stress, notably superoxide productions, independent of or dependent on NOS. A clinically relevant shortcoming of the chronic use of organic nitrates is the potential for tolerance development. Yet, the mechanisms by which organic nitrates exert NO-related effects, notably vasodilation, and tolerance to organic nitrates develops, are still incompletely understood.
Organic S-nitrosothiols or thionitrites with the general formula RSNO are formally composed of the nitrosyl cation (NO+) and the thiolate (RS−), the base of the corresponding acids RSH. Thereby, R is the organic moiety, mainly free L-cysteine (CysSH) or CysSH residues in proteins and peptides such as glutathione (GSH) [3], [22], [23], [24], [25], [26], [27]. The smallest inorganic S-nitrosothiol is HSNO and derives from hydrogen sulfide (HSH, H2S). To our knowledge, HSNO has not been detected in nature thus far [27]. The most common physiological S-nitrosothiols are derived from CysSH. The simplest organic S-nitrosothiol is S-nitroso-L-cysteine (CysSNO). CysSNO is a spontaneous donor of NO which activates sGC to form cGMP. This activation results in multiple biological actions such as relaxation of smooth muscle cells and inhibition of platelet aggregation and monocytes adhesion. Like NO, CysSNO is a short-lived species and occurs physiologically at concentrations around 1 nM in human blood. The origin of endogenous CysSNO is unclear. CysSNO can be formed from CysSH and higher oxides of NO including nitrous acid (HONO) and its anhydride (N2O3) [28], [29].
The most characteristic feature of RSNO is not the release of NO, but the S-transnitrosation reaction by which the NO+ group of RSNO is reversibly transferred to another thiolate [23]. By this mechanism numerous RSNO can be formed such as the low-molecular-mass S-nitroso-N-acetyl-L-cysteine (SNAC) and S-nitroso-glutathione (GSNO). This reaction can also produce the high-molecular-mass S-nitroso-L-cysteine hemoglobin (HbCysSNO) which is present in erythrocytes and S-nitroso-L-cysteine albumin (AlbCysSNO) which is present in plasma, both at concentrations of the order of 200 nM [3], [22], [23]. All above mentioned RSNO exert NO-related biological activity mediated by cGMP. In contrast to NO, RSNO may exert biological activity independent of cGMP.
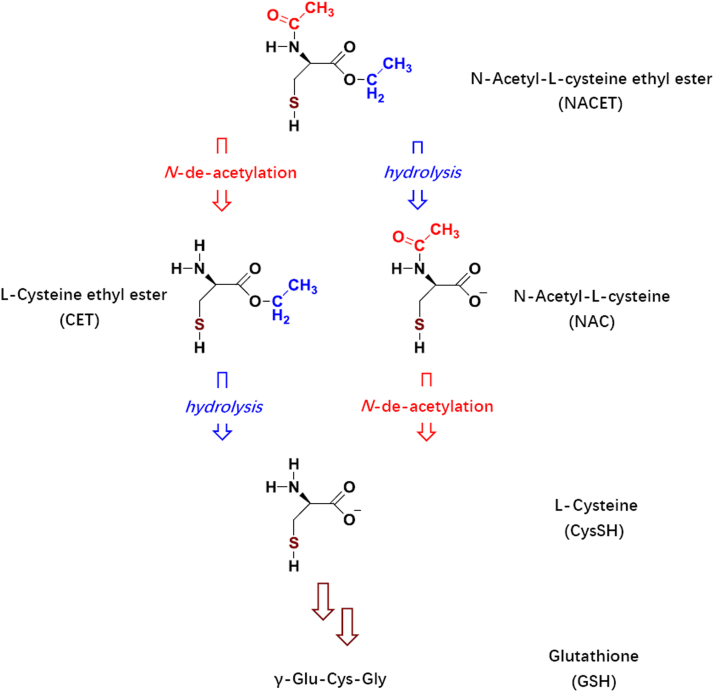
Low-molecular-mass RSNO can be easily prepared by chemical means, but they must be administered intravenously due to instability and very low oral bioavailability. This pharmacologically important drawback of physiological CysSH-based RSNO (RCysSNO) can be overcome by lipophilic charge-free RCysSNO. Indeed, we prepared the ethyl ester of SNAC, the S-nitroso-N-acetyl-L-cysteine ethyl ester (SNACET), after chemical preparation of N-acetyl-L-cysteine ethyl ester (NACET) (Fig. 1). Both NACET and SNACET are freely soluble both in water and in common organic solvents that are immiscible with water, including chloroform. SNACET can easily be prepared by slight acidification of mixtures containing NACET and nitrite in a stoichiometry of 1:1. One major pharmacological advantage of NACET and SNACET is that they can be administered orally. NACET, with high oral bioavailability, is a strong antioxidant and abundant precursor of GSH. SNACET exerts NO-related activities.
Fig. 1.
Chemical structures of NACET and its major metabolites in biological systems. Formal substitution of the H (proton) of the SH group by NO (nitrosyl) yields the corresponding S-nitrosothiols.
In the present article, we review the literature reporting on chemical, analytical and pharmacological features of SNACET and NACET. We also report new results from the ingestion of S-[15N]nitroso-N-acetyl-L-cysteine ethyl ester (S15NACET) by rabbits and humans. On the basis of the data available so far, both SNACET and NACET have favorable pharmacological feature for use as NO donors (SNACET), H2S and GSH suppliers, and an antioxidant (NACET).
2. Synthesis and analysis of NACET and SNACET
Synthesis, purification and structural characterization of NACET (C7H13NO3S, MW 191.2; melting point, 44.1–44.5 °C) by electron ionization mass spectrometry, 1H NMR, infrared spectrometry, and polarimetry have been reported [30], [31]. NACET can easily be prepared under argon atmosphere by N-acetylation of the commercially available L-cysteine ethyl ester in dichloromethane with equimolar amounts of acetic anhydride in high yield and chemical purity [30]. Alternatively, NACET can be synthesized from NAC using acetyl chloride (for the production of anhydrous HCl) and absolute ethanol [31]. At room temperature, NACET is a white powder freely soluble in water and organic solvents. The octanol/water distribution coefficient (logD) is −5.4 for NAC and 0.85 for NACET. The almost six orders of magnitude higher distribution coefficient of NACET underlines its higher lipophilicity compared to NAC. The reactivity of the SH group of NACET is comparable to that of cysteine, but is about 10 times higher than that of NAC towards Ellman's reagent in aqueous buffer of neutral pH value [32]. The strong nucleophilicity and reduction potential of NACET have also been demonstrated in human erythrocytes [33].
SNACET can be easily and instantaneously prepared in quantitative yield from stoichiometric amounts of NACET and sodium nitrite in aqueous solutions in diluted hydrochloric acid (final concentration 50 mM HCl) [30]. Such lightly acifidied SNACET solutions are redish-colored at concentrations below 50 mM and are stable for several hours when stored in an ice bath in the dark. SNACET is highly lipophilic (pKL, 0.78±0.18; water/octanol), charge-free and freely soluble in water. SNACET is highly miscible both with water-miscible and most water-immiscible organic solvents including chloroform. Both NACET and SNACET are quantitatively extracted from aqueous buffered solutions into ethyl acetate and chloroform.
NACET and its metabolites can be analyzed in biological samples by high-performance liquid chromatography (HPLC) after derivatization [32]. In pharmaceutical preparations NACET can be analyzed by kinetic spectrophotometry, generating chromogenic copper(I)Ln complexes with different ligands [34]. Being an electrically neutral molecule, SNACET does not require any derivatization for analysis by gas chromatography–mass spectrometry (GC–MS). Yet, given the thermal lability of the S-nitroso group, intact SNACET cannot be detected by GC–MS despite its high volatility. Nevertheless, SNACET can be quantitatively analyzed by GC–MS using singly or doubly stable-isotope labeled internal standards such as S15NACET, [acetylo-2H3]-SNACET or [acetylo-2H3]-S15NACET after their extraction from aqueous buffered solutions and biological samples with ethyl acetate [30].
3. The pharmacology of SNACET and NACET
3.1. Urogenital system
In the past years, NO has been identified as an important non-adrenergic and non-cholinergic (NANC) neurotransmitter in the lower urinary tract (LUT) as well as in genital/reproductive organs, acting in conjunction with the adrenergic and cholinergic systems [1], [2]. Signals mediated by NO play a central role in inducing relaxation responses of the smooth musculature of the urogenital system. The penis, prostate, bladder neck and the urethra are densely supplied by NOS-containing nerves that, upon activation, can cause relaxation of agonist-mediated or spontaneous contractions of preparations from the respective regions [35], [36], [37], [38]. To date, the pivotal role of NO in the induction and maintenance of penile erection, i.e., relaxation of male penile erectile tissue that is the corpus cavernosum penis, has been established and detailed information has been accumulated on the physiological significance of the NO system in the control of the function of the prostate in both mammals and men, including prostate smooth muscle tone and local blood flow [39], [40], [41], [42], [43]. Although a clinical meaningful role for NO and cGMP in the micturition process, for example, by inhibiting adrenergic neurotransmission and modulating afferent nerves innervating the urinary bladder, bladder neck and proximal urethra, has not been proven, the involvement of this pathway is a strong possibility because various investigators have demonstrated the occurrence of nerve-mediated relaxant responses that attenuated by inhibition of the synthesis of NO and restored by the addition of L-arginine, the substrate of NOS [44].
Drugs specifically interacting with the NO system and its key enzymes (i.e., nNOS, eNOS, sGC, cGMP-specific phosphodiesterase enzymes and cGMP-binding protein kinases) mainly by stimulating the endogenous production of NO and/or cGMP or by inhibiting/retarding the breakdown of cGMP are of particular pharmacological importance. Use of such drugs offers great opportunities in the management of dysfunctions of the male and female urogenital system and persists as an important topic among the community of pharmacologists. For example, selective inhibitors of the phosphodiesterase type 5 (PDE5), an enzyme known to degrade cGMP by hydrolytic cleavage of the phosphodiester moiety, such as sildenafil (VIAGRA™), vardenafil (LEVITRA™), tadalafil (CIALIS™), avanafil (SPEDRA™) and udenafil (ZYDENA™), have been successfully used to treat male erectile dysfunction (ED) in large populations of patients with different organogenic etiologies of the disease [45]. In addition, the chronic use of tadalafil (5 mg, once daily) to treat signs and symptoms of lower urinary tract symptomatology (LUTS ) and benign prostatic hyperplasia (BPH) (referred to as LUTS suggestive of BPH) has also been approved. Clinical studies have shown that tadalafil can effectively improve both storage and irritative symptoms in the patients, as assessed by means of the International Prostate Symptom Score (IPSS) and Quality of Life (IPSS-QOL), without any side effects on sexual function seen with other BPH/LUTS treatments (e.g. 5-alpha reductase inhibitors) [46].
Since the NO signaling has become an attractive target in drug development in the field of urology, NO donating drugs with advanced pharmacological properties have been investigated with regard to their potential to treat dysfunctions of the lower urinary and male genital tract. These preclinical research efforts did also include SNACET. In experiments using isolated human penile erectile tissue, SNACET enhanced the relaxation response mediated by the activation of nitrinergic nerves of the isolated tissue induced by means of transmural electrical field stimulation (EFS) by 44% (median increase in relaxation amplitude) in the presence of 10 µM of the drug. This effect was accompanied by a several-fold increase (> 100-fold) in the production of the second messenger cGMP. Hence, it was assumed that there might be a future significance for S-nitrosothiols and related compounds (such as NCX 911, sildenafil nitrate), mimicking the activity of physiological vascular, endothelium-derived relaxing factors, in the oral pharmacotherapy of male erectile dysfunction (ED)[47].
It has been assessed in vitro (tissue bath technique, mechanical recording of force generation), using substances known to release NO at physiological pH, that sodium nitroprusside (SNP) and S-nitroso-N-acetyl-penicillamine (SNAP) evoke cGMP-related responses as shown by the inhibition of the sGC by methylene blue in human detrusor strip preparations challenged by the cholinergic agonist carbachol. In bladder neck smooth muscle isolated from sheep, CysSNO mimics the relaxation produced by the electrical stimulation of non-cholinergic nerves. Neither of the experimental designs applied SNACET. Although it was concluded from these findings that the nerve-evoked relaxation of the bladder neck may involve the local release of NO, it still remains unclear whether NO is one of the main inhibitory factors keeping the bladder relaxed during the filling phase and promoting relaxation via the modulation of parasympathetic excitation-contraction coupling [48], [49].
While SNACET has not yet been applied to in vitro testings using prostate smooth musculature, it has been shown that GSNO and CysSNO can reduce the generation of contractile force in response to stimulation by both the alpha-adrenergic agonist norepinephrine or vasoconstrictor peptide ET-1 of tissue strips isolated from the transition zone [50], [51]. The relaxing effects of the drugs were paralleled by a 5-fold to 17-fold (time- and dose-dependent) increase in tissue levels of cGMP. The production of cyclic AMP was also enhanced significantly; however, this increase was not dose-dependent. Experiments conducted on isolated sheep urethra (challenged by norepinephrine) have suggested that the metabolic activation of GSNO, CysSNO and SNAP to NO is feasible in target tissues in the outflow region of mammals, resulting in an accumulation of cGMP and, subsequently, smooth muscle relaxation (and a putative reduction in outflow resistance). The median reversion of tension was measured in an interval ranging from 56% (SNAP) to 80% (SNAC). Interestingly, no correlation was seen between the amount of NO released by the drugs (their potential to generate NO in aqueous solutions) and the efficacy to reverse the tonic contraction or stimulate the production of cGMP [52]. In anesthetized female rats, the intra-urethral infusion of SNAP (2 mM, infused at 0.075 mL/min, with a catheter assembly inserted through the bladder dome) led to a decrease in urethral pressure (by 37%) and also in the frequency of spontaneous bladder contractions (mean: 60%), while no effects on the amplitude of the contractions were registered [53]. Nevertheless, it remains to be established whether NO/cGMP-mediated smooth muscle relaxation in this region of the LUT does have a pivotal role to reduce resistance in the bladder outlet and urethra during the micturition phase. Studies using a cell culture set-up and smooth muscle cells (PSMC) isolated by means of the explant culture technique from the transition zone of the human prostate reported no effects of SNACET on the tonic contraction of the PSMC brought about by the vasoconstrictor peptide ET-1 (1 nM), while the number of contracted cells was significantly reduced by GSNO and SNP [54].
Normal ejaculatory function is a multifunctional process involving coordinated contraction and relaxation of smooth musculature of the seminal vesicles (SV) and ductus deferens. In this context, disturbances on the level of neuromuscular control may result in ejaculatory disturbances, such as anejaculation or premature ejaculation (ejaculatio praecox). Some findings have led to the hypothesis that the NO pathway might play a role in the control of human SV smooth muscle tension (by counteracting the sympathetic neuronal input to the ductus ejaculatius) and glandular secretory activity [55], [56]. In tissue bath studies, the tension of isolated human SV smooth musculature induced by means of the alpha-adrenergic agonist norepinephrine was not significantly affected by increasing concentrations of SNACET (0.01–100 µM), whereas the contraction was attenuated to a degree of 50% in the presence of 10 µM GSNO or SNAC. The frequency of spontaneous contraction amplitudes of the tissue preparations was also not diminished by SNACET. In contrast, cumulative addition of the compound (1 nM to 10 µM) attenuated the phasic contraction mediated via the activation of sympathetic nerves (by EFS) by 40%. This was paralleled by a 2-fold to 5-fold elevation in cGMP levels, while no or only very minor effects on the production of cAMP were registered [57], [58]. These findings suggest that there might be a potential for drugs interfering with the NO-mediated signaling, e.g., S-nitrosothiols such as SNACET, or other compounds that can elevate intracellular levels of NO and cGMP in the pharmacotherapy of hyper-excitatory disturbances of ejaculation.
In conclusion, NO-releasing (pro)drugs, including SNACET, might represent a putative option for the pharmacological modification of disease-related alterations of the NO/cGMP pathway in the out-flow region (LUT) and in male reproductive organs [59]. Because the normal function of the male LUT is, to a certain degree, dependent on the activity of the smooth musculature in the bladder, prostate and urethra, targeting these tissues might help to restore unimpaired storage and voiding of urine. Further studies may prove whether effective pharmacological treatment strategies based on this knowledge are likely to emerge in the future.
3.2. Cardiovascular system
3.2.1. In vitro effects of NACET and SNACET
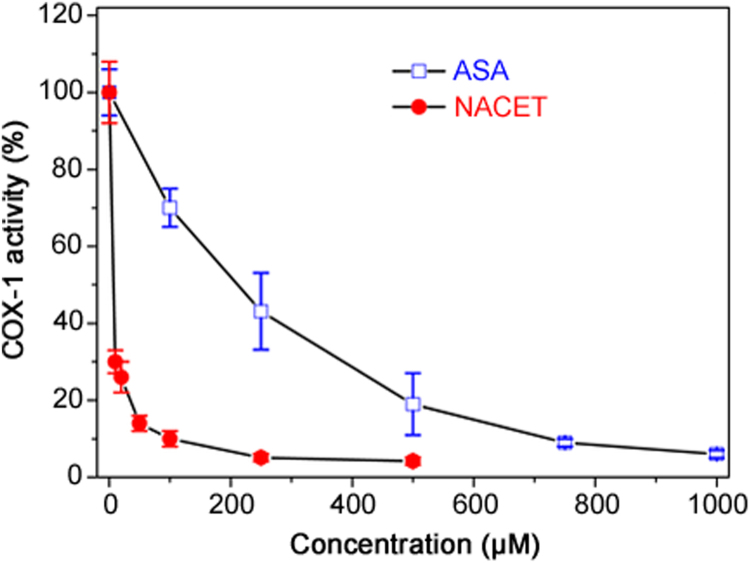
RCysSNO and RCysSH were found to inhibit recombinant COX-1 and COX-2 activity when measured as prostaglandin E2 (PGE2) formation rate [60]. At a molar basis (100 µM each), SNACET (50%) was a stronger inhibitor than SNAC (20%), CysSNO (20%) and GSNO (10%) for both COX isoforms [60]. Given the very high inhibitory SNACET concentrations, such effects of SNACET and other RCysSNO are unlikely to occur in vivo. At a molar basis (100 µM each), NACET (90%) was a stronger inhibitor than NAC (60%), CysSH (60%) and GSH (40%) for both COX isoforms [60]. NACET turned out to be about 200 times stronger as an inhibitor of recombinant COX-1 activity with regard to PGE2 formation than acetylsalicylic acid (ASA) (Fig. 2). We also synthesized ASA derivatives of L-cysteine ethyl ester (CET), i.e., ASA-CET and di-ASA-CET, and found that they inhibit the activity of recombinant COX-1 with regard to PGE2 formation. ASA-CET was found to be a stronger inhibitor of COX-1 activity than di-ASA-CET, suggesting that COX-1 is inhibited by the SH group of ASA-CET [61].
Fig. 2.
Time-dependent inhibition of the activity (prostaglandin E2 formation from 10 µM arachidonic acid) of recombinant COX-1 (5 U) by NACET and acetylsalicylic acid (ASA) at the indicated concentrations. Data are indicated as mean±SEM from three analyses. The experimental conditions and the measurement of prostaglandin E2 were described previously [60]. Under the same conditions and concentrations, SNACET was found to increase COX-1 activity up to 125%, while salicylic acid did not alter COX-1 activity at all (data not shown).
In platelet-rich human plasma, NACET (500 µM) was found to inhibit arachidonic acid-induced TxA2 synthesis, while GSH (500 µM) was found even to increase arachidonic acid-induced TxA2 synthesis [60]. Based on these data, one may expect some inhibitory action of NACET against both COX isoforms in vivo.
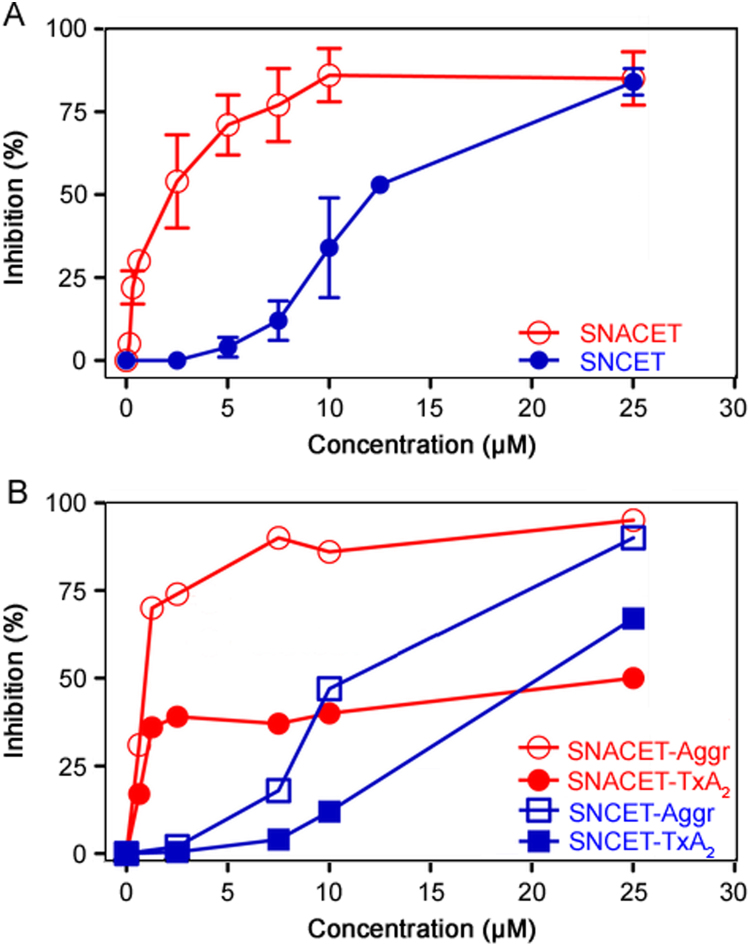
SNACET and SNCET were found to inhibit collagen-induced washed human platelet aggregation (IC50 ≈ 2 µM and ≈10 µM, respectively) and TxA2 synthesis (IC50 ≈ 1 µM and approximately 15 µM, respectively) (Fig. 3). SNACET was also found to inhibit ADP-induced (4 µM) platelet aggregation in rabbit platelet-rich plasma (IC50≈0.5 µM) (data not shown). Thus, SNACET is much more potent than SNCET with respect to platelet aggregation and TxA2 synthesis, most likely because SNACET is charge-free at neutral pH and can reach the cytosolic COX-1 in higher amounts than the positively charged and presumably cell-impermeable SNCET. That SNCET inhibited platelet-aggregation and TxA2 synthesis could be due to partial hydrolysis of SNCET to CysSNO, which is a potent NO donor and inhibitor of platelet aggregation and is actively transported in cells including platelets [62,63] and erythrocytes [64].
Fig. 3.
(A) Inhibition of collagen-induced aggregation of washed human platelets by SNACET and SNCET at the indicated concentrations. Data are indicated as mean±SD from three independent measurements. (B) Inhibition of collagen-induced aggregation of washed human platelets and thromboxane A2 (TxA2) synthesis by SNACET and SNCET at the indicated concentrations. Data are indicated as mean±SD from four independent measurements. The collagen concentration was 2 µg/µL in both experiments. Human platelets were isolated as described elsewhere. Platelet aggregation and the measurement of thromboxane B2 (TxB2), the stable reaction product of TxA2, were performed as described previously [62], [63].
Dimethylarginine dimethylaminohydrolase (DDAH) catalyzes the hydrolysis of the NOS inhibitor ADMA to L-citrulline and dimethylamine (DMA). In the catalytic reaction, the SH group of a certain cysteine moiety in the active-site of DDAH is involved, as has been demonstrated by using the SH-blocking substance HgCl2. We confirmed this in rat liver homogenate [65] and found, moreover, that SNACET and other RCysSNO may also inhibit DDAH activity, most likely by reversibly nitrosating the SH group of the CysSH residue in the active site of DDAH. The order of inhibition was determined to be S-nitroso-homocysteine > SNACET > CysSNO > GSNO. However, the inhibitory potency (IC50 about 500 µM) of SNACET and the other RCysSNO is negligible from a pharmacological perspective [65]. Indeed, the in vivo study in two healthy subjects showed that oral administration of S15NACET (1 µmol/kg bodyweight) resulted in contemporary elevation of urinary [15N]nitrate and [15N]nitrite excretion, yet without inhibiting endogenous DMA formation when measured as urinary excretion of DMA [65].
Being esters, NACET and SNACET are rapidly hydrolyzed by plasma esterases to their free acids NAC and SNAC, respectively. PMSF, a non-specific esterase inhibitor, was found to inhibit SNACET hydrolysis in aqueous buffer that contained purified esterase in a concentration-dependent manner [30]. Yet, in human plasma PMSF failed to inhibit SNACET hydrolysis, except when used at very high concentrations (> 1 mM). In contrast, the chelator DTPA was able to abolish SNACET hydrolysis in human plasma in a concentration-dependent manner. As carbonic anhydrases exert esterase activity as well, it is possible that plasma carbonic anhydrases hydrolyze NACET and SNACET. Yet, the mechanism(s) by which DPTA inhibits esterase and presumably carbonic anhydrase activity in human plasma is still unknown. It could involve chelation of transition free metal ions as well as the coordinatively bound Zn2+ ion in their active side.
In human blood in vitro, nitrite induces oxidation to methemoglobin and S-glutathionylation of oxyhemoglobin (HbO2) [66]. Na2S and many cysteinyl thiols inhibit the nitrite-induced oxidation of HbO2. The order of reactivity was determined to be Na2S > CysSH > NACET > GSH = NAC [27], [66]. Interestingly, NACET was found to become oxidized to its disulfide in human hemolysate, with nitrite enhancing disulfide formation (data not shown). NACET has a much stronger potential than NAC as an antioxidant against nitrite-induced methemoglobinemia and other strong oxidants such as NAPQI, the toxic metabolite of paracetamol. SNACET undergoes S-transnitrosation reactions with thiols including hydrogen sulfide supplied as Na2S. The reactivity order for the reaction of hydrogen sulfide with RCysSNO in aqueous buffer of pH 7.4 is CysSNO > SNACET > GSNO > SNAC [27], indicating that SNACET is more reactive than SNAC towards RCysSNO.
3.2.2. Pharmacokinetics and pharmacodynamics of NACET in the rat
Large clinical studies did not confirm the beneficial effects of NAC and other antioxidants in the prevention of oxidative stress-related diseases as suggested by in vitro and in vivo experiments [67]. This is likely due to the low oral bioavailability of NAC and its limited cell membrane permeability. We found that orally administered NACET is rapidly absorbed in rats but reaches very low concentrations in plasma [32]. NACET can freely penetrate the erythrocyte cell membrane and supply in the cytosol CysSH which is further utilized for the synthesis of GSH [32], the major endogenous intracellular antioxidant. NACET rapidly enters the cells where it is trapped being transformed by esterase-catalyzed hydrolysis to NAC and its subsequent deacetylation to CysSH. In the rat, oral administered NACET increased considerably the tissue GSH content and protected from paracetamol intoxication [32]. NACET was found to accumulate in human erythrocytes where it potently protects from H2O2-induced oxidative damage. In the rat, NACET increased circulating hydrogen sulfide (H2S) to a higher extent than NAC [32]. NACET may be a more appropriate candidate than NAC for the use as an oral H2S donor in vivo.
3.2.3. Pharmacokinetics and blood pressure lowering effects of S15NACET in the rat
Intravenous infusion of S15NACET (600 µM in physiological saline) in a male 280g SPRD rat resulted in decreases of the mean arterial pressure (MAP) in a manner depending upon the infusion rate. A 10min infusion of 3.2 µmol S15NACET/h decreased the MAP from 107 mmHg to 90 mmHg. Subsequently, a 30min infusion of 6.4 µmol S15NACET/h decreased the MAP to 70 mmHg. The MAP did not further change upon infusion of 3.2 µmol S15NACET/h for additional 80 min (data not shown). In this experiment, blood samples were taken at different time points and [15N]nitrite and [15N]nitrate were measured in the plasma samples. [15N]Nitrite and [15N]nitrate were identified as the major S15NACET metabolites. The plasma [15N]nitrite curve reached a plateau between 40 and 90 min, whereas the plasma [15N]nitrate curve increased almost linearly over time (data not shown).
S15NACET (37 µmol in 1.2 mL of a 10 % (m/v) D-glucose solutions) was administered orally to a male 260g SPRD rat. In urine samples collected over 25 h [15N]nitrite and [15N]nitrate were identified. No unchanged S15NACET was found in the urine. Maximum [15N]nitrite (about 3.5 µM) and [15N]nitrate (about 1000 µM) excretion was observed after about 75 min. In the last collected urine sample no S15NACET-derived [15N]nitrite and [15N]nitrate were detected.
Taken together, these observations suggest that S15NACET is metabolized in the rat to [15N]nitrite and [15N]nitrate, S15NACET-derived [15N]nitrite is further oxidized to [15N]nitrate, and [15N]nitrite and [15N]nitrate are excreted in the urine.
3.2.4. Pharmacokinetics and blood pressure lowering effects of S15NACET in the rabbit
Previously we reported that SNACET intravenous infusion decreases the MAP in the rabbit in a dose-dependent manner. At the constant 2-h lasting infusion rate of SNACET of 180 nmol/min/kg, the MAP was kept constant at the level of about 55 mmHg (starting MAP value 75 mmHg) [21].
We investigated the pharmacokinetics of S15NACET in chinchilla bastard rabbits (Charles River Deutschland, aged 3–4 months) using freshly prepared S15NACET in water after dilution with glucose. S15NACET 40.3, 44.6 or 62.6 nmol/kg bodyweight were administered orally. Venous blood and plasma samples were extracted immediately with ethyl acetate without any pH adjustment. Orally administered S15NACET could not be detected at all in rabbit plasma, even not at the highest applied dose of 62.6 nmol/kg bodyweight. In contrast, we found, in rabbit plasma, [15N]nitrite and [15N]nitrate as the major S15NACET metabolites and S-[15N]nitroso-albumin (CysS15NOALB) as its minor metabolite (maximal plasma concentration, 90 nM). The concentration-time curve of [15N]nitrite showed a maximum concentration of about 10 μM after 20 min of administration, whereas the [15N]nitrate concentration was relatively constant at about 20 μM after 60 min of administration. The plasma concentrations of [15N]nitrite and [15N]nitrate 60 min after oral administration of the lower doses (40.3 nmol/kg and 44.6 nmol/kg S15NACET) in two other rabbits were 1.1 μM and 1.4 μM, and 5.3 μM and 6.4 μM, respectively [21].
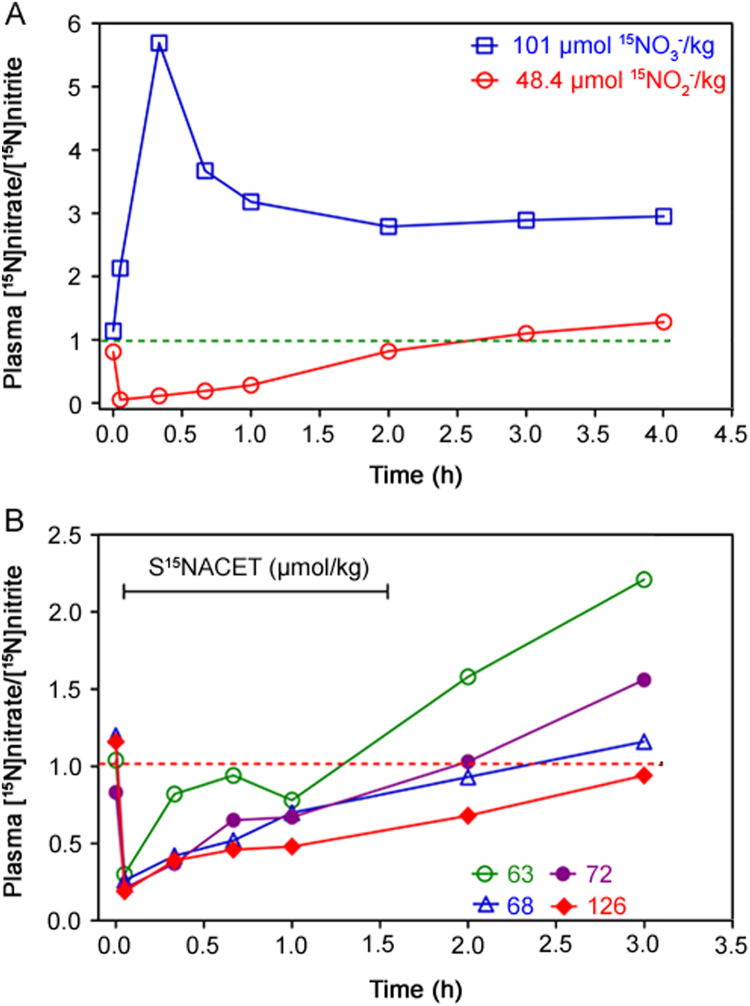
The pharmacokinetics of S15NACET was also investigated in comparison to the pharmacokinetics of its major metabolites [15N]nitrite and [15N]nitrate upon their oral administration at comparable doses to male rabbits as described above. The plasma molar ratio [15N]nitrate/[15N]nitrite, i.e., P15NOxR, was determined. The natural P15NOxR value is about 1. Administration of [15N]nitrate resulted in a maximum plasma molar ratio [15N]nitrate/[15N]nitrite, i.e., P15NOxR, of about 5.8 after 20 min, whereas P15NOxR decreased to a value of less than 0.1 already 5 min after administration indicating rapid absorption of [15N]nitrite (Fig. 4A). The pharmacokinetics of S15NACET orally administered to rabbits is dose-dependent and reproducible, closely resembling the pharmacokinetics of [15N]nitrite (Fig. 4B). The natural P15NOxR value of 1 was reached after about 1.3 h (63 µmol/kg), 2 h (68 and 72 µmol/kg) and 3 h (126 µmol/kg) for S15NACET and after about 2.5 h for [15N]nitrite (48.4 µmol/kg), whereas the P15NOxR for [15N]nitrate remained constant at the value of 3 between 1 and 4 h [15N]nitrate administration.
Fig. 4.
(A) Pharmacokinetics of [15N]nitrate (Na15NO3, 99 atom% 15N; Sigma, Germany) at the dose of 101 µmol [15N]nitrate/kg bodyweight, or [15N]nitrite (Na15NO2, 99 atom% 15N; Aldrich, Germany) at the dose of 48.4 mmol [15N]nitrite/kg bodyweight in two male rabbits. (B) Pharmacokinetics of S-[15N]nitroso-N-acetylcysteine ethyl ester (S15NACET) in four male rabbits at the doses of 63, 68, 72 and 126 µmol S15NACET/kg bodyweight. The weight of the rabbits ranged between 1.8–4.8 kg. All substances were administered together with D(+)-glucose (220 mg) in 2 mL of drinking water via a stomach probe. Administered S15NACET was free of [15N]nitrite and [15N]nitrate. Rabbits were treated once only. The first blood sample was taken immediately before drug administration. Horizontal lines at the value of 1.0 indicate the theoretical baseline molar ratio in rabbit plasma. The study was approved by the local supervisory committee for studies in animal (Hannover, Germany). The y axes indicate the molar ratio of [15N]nitrate/[15N]nitrite in the rabbit plasma relative to the unlabeled nitrate (i.e., [14N]nitrate) and unlabeled nitrite ([14N]nitrite).
3.2.5. Pharmacokinetics of S15NACET in a human subject
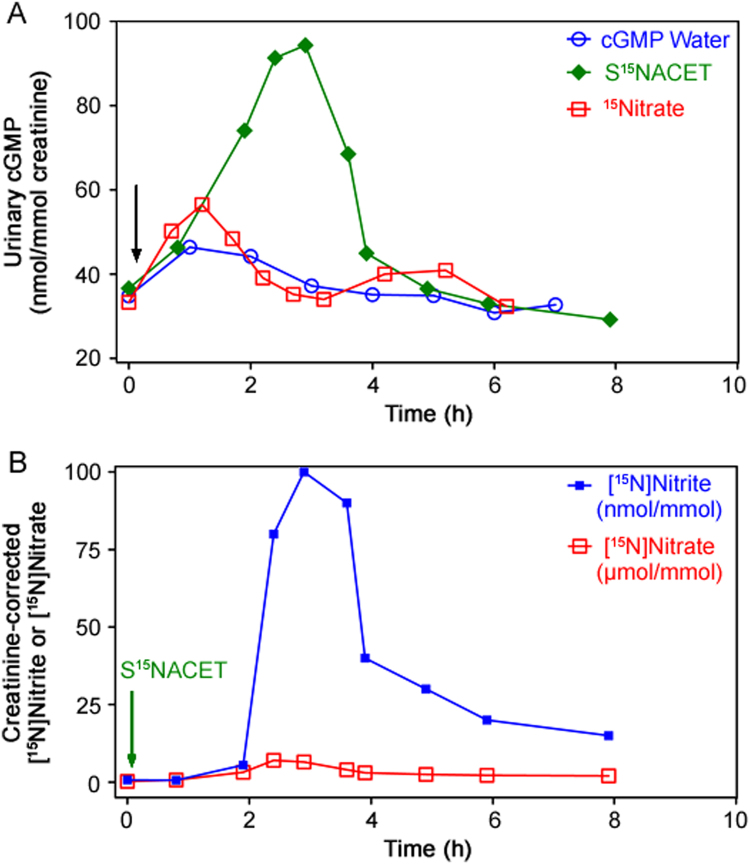
In a proof-of-concept study, the pharmacokinetics of S15NACET was investigated in healthy male subject. Orally administered S15NACET (1 µmol/kg bodyweight) diluted in drinking water (200 mL) resulted in concomitant changes in the creatinine-corrected urinary excretion rates of cGMP, [15N]nitrate and [15N]nitrite (Fig. 5). There was a close correlation between the creatinine-corrected excretion rates of [15N]nitrate and [15N]nitrite (r=0.83, P=0.005), and [15N]nitrate and cGMP (r=0.75, P=0.03). The creatinine-corrected excretion rates of [15N]nitrite and cGMP failed to correlate (r=0.45, P=0.19). These observations suggest that orally administered S15NACET is rapidly absorbed and converted to 15NO which activates sGC to form cGMP, before it is oxidized to [15N]nitrate and [15N]nitrite. cGMP, [15N]nitrate and [15N]nitrite are finally excreted in the urine. At the same dose, [15N]nitrate, diluted in drinking water (200 mL), also caused increases in urinary cGMP; however, they were less intense and showed biphasic excretion kinetics (Fig. 5).
Fig. 5.
Pharmacokinetics and pharmacodynamics of synthetic S15NACET and sodium [15N]nitrate in a male healthy subject. (A) Effects of orally administered S15NACET (1 µmol/kg bodyweight), sodium [15N]nitrate (1 µmol/kg bodyweight) or drinking water free of synthetic S15NACET and [15N]nitrate on creatinine-corrected urinary excretion rate of cGMP (in nmol/mmol). (B) Effect of orally administered S15NACET (1 µmol/kg bodyweight) on creatinine-corrected urinary excretion rate of [15N]nitrate and [15N]nitrite. S15NACET and [15N]nitrate were taken orally as their dilutions in 200 mL drinking water (indicated by the arrows) immediately after preparation of their stock solutions and collection of the first urine sample. Administered S15NACET was free of [15N]nitrite and [15N]nitrate. Note the different units used for [15N]nitrate (in µmol/mmol creatinine) and [15N]nitrite (in nmol/mmol creatinine). The [15N]nitrate-to-[15N]nitrite molar ratio ranged between 44:1 and 1167:1, indicating [15N]nitrate as the major S15NACET metabolite across the entire observation time window.
3.2.6. Blood pressure lowering and platelet aggregation inhibition by organic nitrates, thionitrites (S-nitrosothiols) and inorganic nitrate
In normotensive and hypertensive human subjects, acute and short-term ingestion of salts of inorganic nitrate through supplements or vegetables lowers blood pressure, increases circulating nitrate, nitrite and cGMP levels, suggesting that nitrate's depressor action is mediated by the nitrate/nitrite/NO route [11], [12]. Another group found that short-term dietary nitrate does not modify blood pressure and cardiac output at rest and during exercise in older adults [15]. Ingestion of potassium nitrate was found to result in peak plasma nitrate and nitrite concentrations being up to 35- and 4-fold higher than their basal concentrations, respectively [11]. In the study, plasma cGMP levels were found to increase upon nitrate ingestion as well. We demonstrated that [15N]nitrate ingested by humans is reduced to [15N]nitrite [9] (see also Fig. 5B). Similar results were obtained for [15N]nitrate in male rabbits [21]. Upon oral administration of S15NACET to rabbits, [15N]nitrate and [15N]nitrite were detected in plasma, indicating conversion of S15NACET to [15N]nitrate and [15N]nitrite [21]. For comparison, oral administration of the organic nitrates isosorbide dinitrate (ISDN) (130 µmol daily) and pentaerythrityl tetranitrate (PETN) (250 µmol daily) at comparable amounts to healthy young volunteers increased moderately plasma concentration of nitrate (1.2- and 1.4-fold, respectively) and nitrite (2.2- and 1.8-fold, respectively) [68]. Organic nitrates, inorganic salts of nitrate, and organic thionitrites are blood pressure-lowering drugs, but have different pharmacokinetics. A single dose of 320–450 µmol nitrate/kg bodyweight [11] is about 60 times higher than the mean daily endogenous NO synthesis rate in healthy subjects [3], [6], [7], and 36 times higher than the therapeutic ISDN and PETN dose [68]. In mice, long-term administration of inorganic nitrate did not reduce blood pressure and did not alter atherosclerosis [14], [15].
Blood pressure lowering by orally administered inorganic nitrate requires high amounts of nitrate and results in very high and long-lasting extracellular and intracellular nitrate and nitrite concentrations which may be toxic, mutagenic, and cancerogenic [20]. As nitrate and nitrite are actively transported in various cells including the nephron [9], [10], mM-tissue concentrations of nitrate and presumably of nitrite as well may induce methemoglobinemia and acidosis in mammalian cells [19], and may also elevate GSH consumption in erythrocytes thus contributing to oxidative stress. The beneficial cardiovascular and anti-atherosclerotic effects of long-term, high-dosed inorganic nitrate administration/supplementation are still debatable [16], [17], [18], irrespective of the cancerogenic potential of nitrate-derived nitrosating species [20], which has not been considered in most recent studies.
4. Conclusions and perspectives
NAC has been used for several decades in various disorders and paracetamol intoxication. At physiological pH values, the free carboxylic group of NAC is almost entirely negatively charged. Thus, active transmembrane transport is required, which is obviously limited. This pharmacological shortcoming can be overcome by esterifying NAC to its ethyl ester. Compared to NAC, NACET has highly improved pharmacological properties. NACET has the potential to substitute pharmacological NAC not only as a mucolytic agent, but also as an antioxidant, a supplier of GSH, a paracetamol antidote, and not least a donor of the gasotransmitter H2S [32], and a protector against UV radiation [31].
Slight acidification of an aqueous solution of NACET and inorganic nitrite in stoichiometric amounts results in instantaneous S-nitrosation of the SH group of NACET to form SNACET. Analogous to NACET, SNACET exerts pleiotropic NO-related effects including muscle cell relaxation and blood pressure fall. From a pharmacological perspective, SNACET is superior to inorganic nitrate and SNAC. SNACET's chemical instability is still a problem but it is a solvable pharmaceutic problem. One interesting and practical pharmaceutical solution could be instantaneous preparation of SNACET from its stable components sodium nitrite and NACET just before use, preferably in 1.1-fold molar excess of the hydrochloride salt of NACET over sodium nitrite.
The urogenital tract and the cardiovascular system are attractive and promising areas of application of NACET and SNACET. Given the pleiotropic effects of NACET and SNACET, additional pharmacologic and toxicological indications, most notably the use of NACET as a paracetamol and nitrite antidote, are conceivable.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Moncada S., Palmer R.M.J., Higgs E.A. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 2.Andersson K.E., Persson K. Nitric oxide synthase and nitric oxide-mediated effects in lower urinary tract smooth muscles. World J. Urol. 1994;12:274–280. doi: 10.1007/BF00191207. [DOI] [PubMed] [Google Scholar]
- 3.Tsikas D. A critical review and discussion of analytical methods in the L-arginine/nitric oxide area of basic and clinical research. Anal. Biochem. 2008;379:139–163. doi: 10.1016/j.ab.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Kielstein A., Tsikas D., Galloway G.P. Asymmetric dimethylarginine (ADMA)--a modulator of nociception in opiate tolerance and addiction? Nitric Oxide. 2007;17:55–59. doi: 10.1016/j.niox.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsikas D. Does the inhibitory action of asymmetric dimethylarginine (ADMA) on the endothelial nitric oxide synthase activity explain its importance in the cardiovascular system? The ADMA paradox. J. Controv. Biomed. Res. 2017;3:16–22. [Google Scholar]
- 6.Tsikas D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic. Res. 2005;39:797–815. doi: 10.1080/10715760500053651. [DOI] [PubMed] [Google Scholar]
- 7.Tsikas D., Gutzki F.M., Stichtenoth D.O. Circulating and excretory nitrite and nitrate as indicators of nitric oxide synthesis in humans: methods of analysis. Eur. J. Clin. Pharmacol. 2006;62:51–59. [Google Scholar]
- 8.Tsikas D. Circulating and excretory nitrite and nitrate: their value as measures of nitric oxide synthesis, bioavailability and activity is inherently limited. Nitric Oxide. 2015;45:1–3. doi: 10.1016/j.niox.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Tsikas D., Schwarz A., Stichtenoth D.O. Simultaneous measurement of [15N]nitrate and [15N]nitrite enrichment and concentration in urine by gas chromatography mass spectrometry as pentafluorobenzyl derivatives. Anal. Chem. 2010;82:2585–2587. doi: 10.1021/ac902970m. [DOI] [PubMed] [Google Scholar]
- 10.Chobanyan-Jürgens K., Schwarz A., Böhmer A. Renal carbonic anhydrases are involved in the reabsorption of endogenous nitrite. Nitric Oxide. 2012;26:126–131. doi: 10.1016/j.niox.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Kapil V., Milsom A.B., Okorie M. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 12.Kapil V., Khambata R.S., Robertson A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hezel M.P., Liu M., Schiffer T.A. Effects of long-term dietary nitrate supplementation in mice. Redox Biol. 2015;5:234–242. doi: 10.1016/j.redox.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsch E., Theelen T.L., Janssen B.J. The effect of prolonged dietary nitrate supplementation on atherosclerosis development. Atherosclerosis. 2016;245:212–221. doi: 10.1016/j.atherosclerosis.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Oggioni C., Jakovljevic D.G., Klonizakis M. Dietary nitrate does not modify blood pressure and cardiac output at rest and during exercise in older adults: a randomised cross-over study. Int. J. Food Sci. Nutr. 2018;69:74–83. doi: 10.1080/09637486.2017.1328666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawlak-Chaouch M., Boissière J., Gamelin F.X. Effect of dietary nitrate supplementation on metabolic rate during rest and exercise in human: a systematic review and a meta-analysis. Nitric Oxide. 2016;53:65–76. doi: 10.1016/j.niox.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 17.McMahon N.F., Leveritt M.D., Pavey T.G. The effect of dietary nitrate Supplementation on endurance exercise performance in healthy adults: a systematic review and meta-analysis. Sports Med. 2017;47:735–756. doi: 10.1007/s40279-016-0617-7. [DOI] [PubMed] [Google Scholar]
- 18.Cheng H.M., Koutsidis G., Lodge J.K. Tomato and lycopene supplementation and cardiovascular risk factors: a systematic review and meta-analysis. Atherosclerosis. 2017;257:100–108. doi: 10.1016/j.atherosclerosis.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Chow C.W., Kapus A., Romanek R R. NO3−-induced pH changes in mammalian cells. Evidence for an NO3−-H+ cotransporter. J. Gen. Physiol. 1997;110:185–200. doi: 10.1085/jgp.110.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erdman S.E., Rao V.P., Poutahidis T. Nitric oxide and TNF-α trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc. Natl. Acad. Sci. USA. 2009;106:1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsikas D., Stichtenoth D.O., Jordan J. Inorganic nitrate for blood pressure lowering? Hypertension. 2011;57:e1–2. doi: 10.1161/HYPERTENSIONAHA.110.164574. author reply e3. [DOI] [PubMed] [Google Scholar]
- 22.Giustarini D., Milzani A., Dalle-Donne I. Detection of S-nitrosothiols in biological fluids: a comparison among the most widely applied methodologies. J. Chromatogr. B. 2008;851:124–139. doi: 10.1016/j.jchromb.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Tsikas D., Sandmann J., Rossa S. Investigations of S-transnitrosylation reactions between low- and high-molecular-weight S-nitroso compounds and their thiols by high-performance liquid chromatography and gas chromatography-mass spectrometry. Anal. Biochem. 1999;270:231–241. doi: 10.1006/abio.1999.4084. [DOI] [PubMed] [Google Scholar]
- 24.Tsikas D., Sandmann J., Luessen P. S-Transnitrosylation of albumin in human plasma and blood in vitro and in vivo in the rat. Biochim. Biophys. Acta. 2001;1546:422–434. doi: 10.1016/s0167-4838(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 25.Warnecke A., Luessen P., Sandmann J. Application of a stable-isotope dilution technique to study the pharmacokinetics of human 15N-labelled S-nitrosoalbumin in the rat: possible mechanistic and biological implications. J. Chromatogr. B. 2009;877:1375–1387. doi: 10.1016/j.jchromb.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 26.Tsikas D., Schmidt M., Böhmer A. UPLC-MS/MS measurement of S-nitrosoglutathione (GSNO) in human plasma solves the S-nitrosothiol concentration enigma. J. Chromatogr. B. 2013;927:147–157. doi: 10.1016/j.jchromb.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Tsikas D., Böhmer A. S-Transnitrosation reactions of hydrogen sulfide (H2S/HS-/S2-) with S-nitrosated cysteinyl thiols in phosphate buffer of pH 7.4: results and review of the literature. Nitric Oxide. 2017;65:22–36. doi: 10.1016/j.niox.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Hanff E., Böhmer A., Zinke M. Carbonic anhydrases are producers of S-nitrosothiols from inorganic nitrite and modulators of soluble guanylyl cyclase in human platelets. Amino Acids. 2016;48:1695–1706. doi: 10.1007/s00726-016-2234-z. [DOI] [PubMed] [Google Scholar]
- 29.Zinke M., Hanff E., Böhmer A. Discovery and microassay of a nitrite-dependent carbonic anhydrase activity by stable-isotope dilution gas chromatography-mass spectrometry. Amino Acids. 2016;48:245–255. doi: 10.1007/s00726-015-2081-3. [DOI] [PubMed] [Google Scholar]
- 30.Tsikas D., Dehnert S., Urban K. GC-MS analysis of S-nitrosothiols after conversion to S-nitroso-N-acetyl cysteine ethyl ester and in-injector nitrosation of ethyl acetate. J. Chromatogr. B. 2009;877:3442–3455. doi: 10.1016/j.jchromb.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 31.Van den Broeke L.T., Beijersbergen van Henegouwen G.M. UV radiation protecting efficacy of cysteine derivatives, studies with UVA-induced binding of 8-MOP and CPZ to rat epidermal biomacromolecules in vivo. Int. J. Radiat. Biol. 1995;67:411–420. doi: 10.1080/09553009514550471. [DOI] [PubMed] [Google Scholar]
- 32.Giustarini D., Milzani A., Dalle-Donne I. N-Acetylcysteine ethyl ester (NACET): a novel lipophilic cell-permeable cysteine derivative with an unusual pharmacokinetic feature and remarkable antioxidant potential. Biochem. Pharmacol. 2012;84:1522–1533. doi: 10.1016/j.bcp.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Michaelsen J.T., Dehnert S., Giustarini D. HPLC analysis of human erythrocytic glutathione forms using OPA and N-acetyl-cysteine ethyl ester: evidence for nitrite-induced GSH oxidation to GSSG. J. Chromatogr. 2009;877:3405–3417. doi: 10.1016/j.jchromb.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 34.Kukoc-Modun L., Tsikas D., Kraljević T. Kinetic spectrophotometric determination of N-Acetyl-L-cysteine ethyl ester (NACET) generating chromogenic copper(I)Ln complexes with different ligands. Croat. Chem. Acta. 2017;90:263–271. [Google Scholar]
- 35.Andersson K.E. Neurotransmission and drug effects in urethral smooth muscle. Scand. J. Urol. Nephrol. 2001;207(Suppl):26–34. doi: 10.1080/003655901750174854. [DOI] [PubMed] [Google Scholar]
- 36.Persson K., Alm P., Johansson K. Nitric oxide synthase in pig lower urinary tract: immunohistochemistry, NADPH diaphorase histochemistry and functional effects. Br. J. Pharmacol. 1993;110:521–530. doi: 10.1111/j.1476-5381.1993.tb13842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon J.S., Jen P.Y. Development of nerves containing nitric oxide synthase in the human male urogenital organs. Br. J. Urol. 1995;76:719–725. doi: 10.1111/j.1464-410x.1995.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 38.Hedlund P., Ekstrom P., Larsson B. Heme oxygenase and NO-synthase in the human prostate - relation to adrenergic, cholinergic and peptide-containing nerves. J. Auton. Nerv. Syst. 1997;63:115–126. doi: 10.1016/s0165-1838(96)00139-7. [DOI] [PubMed] [Google Scholar]
- 39.Andersson K.E., Holmquist F. Regulation of tone in penile cavernous smooth muscle. Established concepts and new findings. World. J. Urol. 1994;12:249–261. doi: 10.1007/BF00191204. [DOI] [PubMed] [Google Scholar]
- 40.Di Iulio J.L., Li C.G., Rand M.J. Determination of nitric oxide synthase activity in rat, pig and rabbit prostate glands. Eur. J. Pharmacol. 1997;337:245–249. doi: 10.1016/s0014-2999(97)01269-7. [DOI] [PubMed] [Google Scholar]
- 41.Burnett A.L., Maguire M.P., Chamness S.L. Characterization and localization of nitric oxide synthase in the human prostate. Urology. 1995;45:435–439. doi: 10.1016/S0090-4295(99)80012-0. [DOI] [PubMed] [Google Scholar]
- 42.Takeda M., Tang R., Shapiro E. Effects of nitric oxide on human and canine prostates. Urology. 1995;45:440–446. doi: 10.1016/S0090-4295(99)80013-2. [DOI] [PubMed] [Google Scholar]
- 43.Gradini R., Realacci M., Ginepri A. Nitric oxide synthases in normal and benign hyperplastic human prostate: immunohistochemistry and molecular biology. J. Pathol. 1999;189:224–229. doi: 10.1002/(SICI)1096-9896(199910)189:2<224::AID-PATH422>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 44.Andersson K.E., Mattiasson A., Sjogren C. Electrically induced relaxation of the noradrenaline contracted isolated urethra from rabbit and man. J. Urol. 1983;129:210–214. doi: 10.1016/s0022-5347(17)51986-2. [DOI] [PubMed] [Google Scholar]
- 45.Hawksworth D.J., Burnett A.L. Pharmacotherapeutic management of erectile dysfunction. Clin. Pharmacol. Ther. 2015;98:602–610. doi: 10.1002/cpt.261. [DOI] [PubMed] [Google Scholar]
- 46.Brock G., Broderick G., Roehrborn C.G. Tadalafil once daily in the treatment of lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) in men without erectile dysfunction. BJU Int. 2013;112:990–997. doi: 10.1111/bju.12251. [DOI] [PubMed] [Google Scholar]
- 47.Seidler M., Ückert S., Waldkirch E. In vitro effects of a novel class of nitric oxide (NO)-donating compounds on isolated human erectile tissue. Eur. Urol. 2002;42:523–528. doi: 10.1016/s0302-2838(02)00397-4. [DOI] [PubMed] [Google Scholar]
- 48.Moon A. Influence of nitric oxide signaling pathways on pre-contracted human detrusor smooth muscle in vitro. BJU Int. 2002;89:942–949. doi: 10.1046/j.1464-410x.2002.02795.x. [DOI] [PubMed] [Google Scholar]
- 49.Thornbury K.D., Hollywood M.A., McHale N.G. Mediation by nitric oxide of neurogenic relaxation of the urinary bladder neck muscle in sheep. J. Physiol. 1992;451:133–144. doi: 10.1113/jphysiol.1992.sp019157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kedia G.T., Ückert S., Scheller F. In vitro functional responses of isolated normal human prostate tissue to compounds interacting with the cGMP-pathway. Urology. 2006;67:1292–1297. doi: 10.1016/j.urology.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Kedia G.T., Ückert S., Kedia M. [In vitro effects of cAMP- and cGMP-stimulating drugs on the relaxation of prostate smooth muscle tissue contraction induced by endothelin-1] Georgian Med. News. 2006;131:7–13. (Article in Russian) [PubMed] [Google Scholar]
- 52.García-Pascual A., Costa G., Labadía A. Differential mechanisms of urethral smooth muscle relaxation by several NO donors and nitric oxide. Naunyn Schmiede. Arch. Pharmacol. 1999;360:80–91. doi: 10.1007/s002109900038. [DOI] [PubMed] [Google Scholar]
- 53.Jung S.Y., Fraser M.O., Ozawa H. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress incontinence and detrusor instability. J. Urol. 1999;162:204–212. doi: 10.1097/00005392-199907000-00069. [DOI] [PubMed] [Google Scholar]
- 54.O. Heuer, S. Ückert, G. Dobler, et al., Effects of phosphodiesterase inhibitors and nitric oxide donors on cultured human prostatic smooth muscle cells, Eur. Urol. 3 (No 2, Suppl) (2004) 19.
- 55.S. Machtens, S. Ückert, A. Stanarius, et al., Functional control of the human seminal vesicles by the L-arginine/nitric oxide/cyclic GMP pathway, Eur. Urol. 35 (Suppl 2) (1999) 132 [Abstract, presented at the 14th Congress of the European Association of Urology (EAU), Stockholm, Sweden, 07-April to 11-April-].
- 56.Ückert S., Stanarius A., Stief C.G. Immunocytochemical distribution of nitric oxide synthase in the human seminal vesicle: a light- and electron microscopical study. Urol. Res. 2002;31:262–266. doi: 10.1007/s00240-003-0322-5. [DOI] [PubMed] [Google Scholar]
- 57.Heuer O., Ückert S., Machtens S. Effects of various nitric oxide donating agents on the contractility and cyclic nucleotide turnover of human seminal vesicles in vitro. Urology. 2002;59:958–962. doi: 10.1016/s0090-4295(02)01534-0. [DOI] [PubMed] [Google Scholar]
- 58.Machtens S., Ückert S., Stief C.G. Effects of various nitric oxide-donating drugs on adrenergic tension of human seminal vesicles in vitro. Urology. 2003;61:479–483. doi: 10.1016/s0090-4295(02)02165-9. [DOI] [PubMed] [Google Scholar]
- 59.Hedlund P. Nitric oxide/cGMP-mediated effects in the outflow region of the lower urinary tract - Is there a basis for pharmacological targeting of cGMP? World J. Urol. 2005;23:362–367. doi: 10.1007/s00345-005-0019-1. [DOI] [PubMed] [Google Scholar]
- 60.Tsikas D., Niemann J. Nitric oxide, peroxynitrite, S-nitrosothiols and thiols are unlikely to exert their effects on recombinant cyclooxygenase-1 and cyclooxygenase-2 activity in vitro by modifying cysteine moieties. Nitric Oxide. 2012;26:192–194. doi: 10.1016/j.niox.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Böhmer A., Niemann J., Schwedhelm K.S. Potential pitfalls with the use of acetoxy (CH3COO) drugs in studies on nitric oxide synthase in platelets. Nitric Oxide. 2013;28:14–16. doi: 10.1016/j.niox.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Tsikas D., Ikic M., Tewes K.S. Inhibition of platelet aggregation by S-nitroso-cysteine via cGMP-independent mechanisms: evidence of inhibition of thromboxane A2 synthesis in human blood platelets. FEBS Lett. 1992;442:162–166. doi: 10.1016/s0014-5793(98)01633-0. [DOI] [PubMed] [Google Scholar]
- 63.Tewes K.S., Tsikas D., Gutzki F.M. Measurement of thromboxane B2 in platelet-rich human plasma by gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry following extractive pentafluorobenzyl esterification. Anal. Biochem. 1998;261:121–124. doi: 10.1006/abio.1998.2658. [DOI] [PubMed] [Google Scholar]
- 64.Sandmann J., Schwedhelm K.S., Tsikas D. Specific transport of S-nitrosocysteine in human red blood cells: implications for formation of S-nitrosothiols and transport of NO bioactivity within the vasculature. FEBS Lett. 2005;579:4119–4124. doi: 10.1016/j.febslet.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 65.Chobanyan K., Thum T., Suchy M.T. GC-MS assay for hepatic DDAH activity in diabetic and non-diabetic rats by measuring dimethylamine (DMA) formed from asymmetric dimethylarginine (ADMA): evaluation of the importance of S-nitrosothiols as inhibitors of DDAH activity in vitro and in vivo in humans. J. Chromatogr. B. 2007;858:32–41. doi: 10.1016/j.jchromb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Böhmer A., Pich A., Schmidt M. Evidence by chromatography and mass spectrometry that inorganic nitrite induces S-glutathionylation of hemoglobin in human red blood cells. J. Chromatogr. B. 2016;1019:72–82. doi: 10.1016/j.jchromb.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 67.Giustarini D., Dalle-Donne I., Tsikas D. Oxidative stress and human diseases: origin, link, measurement, mechanisms, and biomarkers. Crit. Rev. Clin. Lab. Sci. 2009;46:241–281. doi: 10.3109/10408360903142326. [DOI] [PubMed] [Google Scholar]
- 68.Keimer R., Stutzer F.K., Tsikas D. Lack of oxidative stress during sustained therapy with isosorbide dinitrate and pentaerythrityl tetranitrate in healthy humans: a randomized, double-blind crossover study. J. Cardiovasc. Pharmacol. 2003;41:284–292. doi: 10.1097/00005344-200302000-00018. [DOI] [PubMed] [Google Scholar]