Abstract
Introduction
We conducted a prospective, non-interventional, multicenter study to examine the effect of a fixed-dose combination of perindopril/amlodipine in patients with arterial hypertension.
Methods
Patients who were previously untreated or required a change in medication were treated with a fixed combination of perindopril/amlodipine (3.5/2.5 or 7.0/5.0 mg) for 12 weeks. Changes in office, home and ambulatory blood pressure (BP) were recorded. Adherence was assessed by the Hill-Bone medication adherence scale.
Results
Overall, 1814 patients (mean age 60.0 ± 13.4 years) were included in 614 German practices, and data of 1770 patients were analyzed. At study entry, 97.7% of patients received perindopril/amlodipine at a daily dose of 3.5 mg/2.5 mg, and 47.9% of patients remained on this dose during the study period. Treatment with perindopril/amlodipine decreased mean office BP from 163.7/95.4 to 133.6/80.3 mmHg (p < 0.0001), resulting in a hypertension control rate of 69.1%. Blood pressure control was comparable in previously untreated and treated patients (70.3 vs. 68.1%), and in younger and older patients (70.6 < 65 vs. 66.3% ≥ 65 years). Ambulatory BP measurements were available in a subgroup of patients (n = 167), and mean 24 h ambulatory BP decreased from 150.6 ± 12.6/88.9 ± 8.8 to 132.4 ± 11.9/79.4 ± 8.5 mmHg (p < 0.0001). Furthermore, the proportion of patients with severe hypertension European Society of Hypertension/European Society of Cardiology (ESH/ESC) grade II or III decreased from 64.4 to 3.9%, and patients with pre-existing isolated systolic hypertension (n = 284) converted to normal BP in 67.6% of cases. Nearly half of the patients (47.2%) were perfectly adherent during the study. In previously treated patients, the percentage of patients with perfect adherence increased from 20.6% prior to study to 43.5% at final visit (p < 0.0001). Adverse drug reactions were documented for 4.9% of patients.
Conclusion
A fixed-dose combination of perindopril/amlodipine shows significant blood pressure reduction and improvement in medication adherence in a primary care setting.
Trial Registration
ISRCTN26323538.
Funding
Servier Deutschland GmbH.
Keywords: Adherence, Amlodipine, Cardiology, Hypertension, Non-interventional study, Perindopril, Prospective, Single-pill combination
Introduction
High blood pressure is very common and the leading cause of cardiovascular morbidity and mortality worldwide. In Germany, about 30% of females and 33% of males aged 18–79 years are estimated to have arterial hypertension [1]. In the age range of 70–79 years, prevalence increases up to 75% in both genders [1]. With respect to the potential health implications and the epidemiological significance of uncontrolled blood pressure, the current European Society of Hypertension/European Society of Cardiology (ESH/ESC) hypertension guidelines recommend early intervention to normalize elevated blood pressure to < 140/90 mmHg [2]. Despite the improvements in awareness and therapeutic options attained in recent decades, only 72% of the patients treated with antihypertensive drugs reach blood pressure values < 140/90 mmHg, indicating a high medical need for further improvement in treatment strategy [3, 4].
The majority of hypertensive patients require a combination of at least two antihypertensive drugs to achieve timely blood pressure control and avoid the occurrence of early events. Unfortunately, adherence to treatment is low [4]. To improve adherence, ESH/ESC guidelines recommend single pill combination (SPC) therapy, since reducing the number of pills to be taken daily may simplify treatment and enhance adherence to prescribed therapeutic regimes [2, 4–6]. Better adherence to cardioprotective treatments, in turn, has been shown to translate into reduced morbidity and mortality [7, 8]. In addition, combining two drugs with complementary mechanisms of action can increase the rate of blood pressure control [4, 5] and exert a blood pressure-lowering effect approximately five times greater than doubling the dose of an antihypertensive monotherapy [9]. Furthermore, there is evidence that initiating antihypertensive therapy with two drugs results in a more rapid achievement of target blood pressure and a reduced risk of cardiovascular events or death in comparison to a delayed onset of combination treatment [10].
As one of the best antihypertensive treatment strategies available, the guidelines suggest the combination of a calcium channel blocker (CCB) and an angiotensin-converting enzyme (ACE) inhibitor [2]. Addition of an ACE inhibitor to a dihydropyridine CCB may also reduce the risk of CCB-associated peripheral edema in comparison to a high-dose CCB monotherapy, thereby improving tolerability of the antihypertensive treatment [11].
The beneficial effect of the combination of the ACE inhibitor perindopril and the CCB amlodipine is well established. As demonstrated in the international randomized controlled phase 4 study ASCOT-BPLA (Anglo-Scandinavian Cardiac Outcome Trial–Blood Pressure Lowering Arm), patients treated with a therapy regime based on amlodipine and perindopril reached better outcomes as compared with a beta-blocker/diuretic strategy [12]. More recently, a randomized, controlled phase 2 study showed that the fixed-dose combination of 2.5 mg amlodipine and 3.5 mg perindopril (Viacoram®) was superior to monotherapy with either 5 mg amlodipine or 5 mg perindopril in terms of blood pressure-lowering efficacy and onset [13]. Furthermore, in direct comparison with a valsartan-based strategy, the perindopril/amlodipine 2.5/3.5 mg combination exerts greater reductions in blood pressure and better control rates [14].
To further strengthen the available data on blood pressure control in a broad spectrum of patients with essential hypertension, we decided to perform the non-interventional study Viacoram–BPT (Viacoram–focus on Blood Pressure Target). The objective of the study was to assess the effectiveness of the fixed-dose single-pill combination perindopril/amlodipine as well as safety and tolerability in a heterogeneous population of patients who were either newly diagnosed or previously treated with antihypertensive drugs. Of particular interest was the potential influence of the combination therapy on blood pressure control, concomitant medication, and on patient’s adherence to medication.
Methods
This prospective, 12-week observational, open-label study was carried out in practices of 614 cardiologists, general practitioners and internists in Germany between September 2015 and June 2016. The study included patients with essential hypertension with or without concomitant disease who were either untreated or previously treated with antihypertensive drugs, but required a change in medication. Patients were eligible for this study if the decision for treatment with perindopril/amlodipine had been made prior to study inclusion. Enrollment of patients into the non-interventional study was based on physician assessment on medical usefulness and necessity.
The conduction of this trial was performed according to §4 (23) sentence 3 AMG (Arzneimittelgesetz, German Medicinal Product Act). Ethical approval was granted by the independent ethics commission in Freiburg/Germany. This trial is registered at controlled-trials.com with registration number ISRCTN26323538. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Patients were observed over a treatment period of 3 months. There were four scheduled examinations, one at baseline (visit 1), two intermediate examinations after 1 month (visit 2) and 2 months (visit 3), and one final examination after 3 months (visit 4). All data were documented using a standardized case report form. At the initial examination, the investigators documented the demographic data (age, gender, weight, body mass index), history of hypertension, risk factors, concomitant diseases and concomitant medications. Effectiveness was assessed by the measurement of office blood pressure at each visit. In addition, patients could undergo 24-h ambulatory blood pressure monitoring and home blood pressure monitoring at the discretion of the treating physician. All measurements were taken with the techniques and devices used in each individual practice. Treatment control was defined as achieving blood pressure values < 140/90 mmHg at the final examination (visit 4) or last observation. Both at baseline and at final visit, patient’s adherence to medication was measured by means of the validated Hill-Bone questionnaire comprised of Likert-like 14 items in three subscales [15].
To assess safety and tolerability, adverse events (AE) and adverse drug reactions (ADR) were documented. Data processing and statistical analysis were performed with the SAS™ program system. Due to the non-interventional design of the study, the statistical analysis was performed in a descriptive and explorative way. AE and ADR were coded in accordance with the Medical Dictionary for Regulatory Activities v.19.0. Data are presented as mean values ± standard deviations (SD) for continuous variables and numbers of patients and/or percentages for categorical variables. Analysis of effectiveness data was performed with data imputation according to the last observation carried forward method (LOCF). The p values reported are two-tailed and an alpha level of 0.05 was used to assess statistical significance. Wilcoxon’s signed-rank test and Fisher’s exact test were applied for assessment of changes between baseline and follow-up visits. McNemar’s test was used for the assessment of changes in therapy adherence. All study data were evaluated by an independent statistical institute (ANFOMED, Möhrendorf, Germany). All statistical analyses have been performed by means of the SAS® software system (v.9.4 for Microsoft Windows 7™; SAS Institute, Cary, NC, USA).
Results
Study Population Characteristics
A total of 1814 patients with essential hypertension were enrolled at 614 study sites in Germany. The median enrollment was three patients per study center (489 centers, 79.6% of centers) and the amount ranged from 1 to 12 patients per center.
Mean duration of observation was 3.3 months (± 0.9, median 3.2, maximum up to 12), the most frequent duration of observation was 3–4 months (901 patients, 51.5% of all patients with data on duration of observation).
Eleven patients only came to the enrollment visit and were never followed up, while another 33 patients were lost to follow-up or have incomplete data, so, in total, data of 44 patients (2.4%) were not available for analysis. Complete data on all visits, baseline, control and final examinations, were available for 1720 patients (94.8% of all patients) for statistical analysis; using the LOCF-method, where–when baseline values exist and at least one follow-up measurement—missing values in the course of the study are replaced by the last actual observation), we have complete data for analysis of baseline and end point measurements in 1770 patients (97.6% of total enrolled).
Patient Characteristics
Of the total study population, 54% were male (see also Table 1). Patients’ mean age was 60 (± 13.4, median 60) years. Patients were predominantly between 50 and 60 years (28% of patients) and between 60 and 70 years (26.8%) old. More than 80% of all patients where either overweight (BMI > 25 and < 30 kg/m2, 46.8%) or obese (BMI > 30 kg/m2, 33.8% of patients), whereas 18.7% were of normal weight (and 0.7% underweight, BMI < 18.5 kg/m2). Laboratory values at baseline visit are shown in Table 2.
Table 1.
Patient baseline characteristics
| Total population (n = 1814) | Treatment naïve patients (n = 834) | Patients with previous antihypertensive treatment (n = 980) | |
|---|---|---|---|
| Gender | Male: 973 (54.0%) | Male: 453 (54.7%) | Male: 520 (53.3%) |
| Age (mean ± SD) | 60.0 ± 13.4 years | 56.6 ± 13.3 years | 62.8 ± 12.8 years |
| Body mass index (mean ± SD) | 28.9 ± 5.0 kg/m2 | 28.8 ± 5.2 kg/m2 | 28.9 ± 5.0 kg/m2 |
| Hypertension history (n, %) | |||
| Newly diagnosed | 702 (39.0%) | 682 (82.7%) | 20 (2.1%) |
| < 1 year known | 117 (6.5%) | 33 (4.0%) | 84 (8.6%) |
| 1–5 years known | 479 (26.6%) | 60 (7.3%) | 419 (43.0%) |
| 6–10 years known | 278 (15.4%) | 31 (3.8%) | 247 (25.3%) |
| > 10 years known | 224 (12.4%) | 19 (2.3%) | 205 (21.0%) |
| Risk factors and concomitant diseases (n, % of patients) | |||
| Dyslipidemia | 840 (52.8%) | 321 (46.5%) | 519 (57.6%) |
| Tobacco use | 526 (33.1%) | 273 (39.5%) | 253 (28.1%) |
| Central obesity | 515 (32.4%) | 236 (34.2%) | 279 (31.0%) |
| Diabetes mellitus | 326 (20.8%) | 92 (13.3%) | 234 (26.0%) |
| Coronary artery disease | 157 (9.9%) | 28 (4.1%) | 129 (14.3%) |
| COPD | 113 (7.1%) | 37 (4.4%) | 76 (8.4%) |
| Chronic kidney disease | 82 (5.2%) | 17 (2.5%) | 65 (7.2%) |
| Office blood pressure and heart rate (mean ± SD) | |||
| Systolic (mmHg) | 163.7 ± 14.8 (n = 1770) | 165.7 ± 15.1 (n = 803) | 161.9 ± 14.4 (n = 967) |
| Diastolic (mmHg) | 95.4 ± 9.4 (n = 1770) | 96.8 ± 9.4 (n = 803) | 94.2 ± 9.2 (n = 967) |
| Heart rate (bpm) | 77.3 ± 10.1 (n = 1680) | 78.0 ± 10.1 (n = 760) | 76.7 ± 10.0 (n = 920) |
Table 2.
Baseline laboratory values of patient population
| Mean (± SD) | 95% KI | n | |
|---|---|---|---|
| Total cholesterol [mg/dL] | 220.7 (± 44.9) | 218.2–223.1 | 1270 |
| LDL-cholesterol [mg/dL] | 141.2 (± 37.8) | 138.8–143.6 | 958 |
| HDL-cholesterol [mg/dL] | 53.4 (± 18.8) | 52.2–54.7 | 903 |
| Triglycerides [mg/dL] | 179.5 (± 106.7) | 172.8–186.2 | 975 |
| Glucose [mg/dL] | 104.4 (± 32.9) | 102.5–106.3 | 1162 |
| HbA1c [%] | 6.2 (± 1.1) | 6.2–6.3 | 728 |
| Creatinine [mg/dL] | 0.93 (± 0.24) | 0.92–0.94 | 1145 |
| Sodium (Na) [mmol/L] | 140.2 (± 3.9) | 139.9–140.6 | 499 |
| Potassium (K) [mmol/L] | 4.5 (± 0.5) | 4.4–4.5 | 659 |
| Uric acid [mg/dL] | 5.9 (± 1.4) | 5.8–6.0 | 842 |
In the total study population, 88.0% of patients had concomitant risk factors and/or disease, the most common being dyslipidemia (52.8%), obesity (33.9%), tobacco use (33.0%) and diabetes mellitus (20.8%). At study entry, most patients had uncontrolled hypertension ESH/ESC Grades 1–3 (96.5%). Independent of the ESH/ESC grade, 16.1% of all patients had isolated systolic hypertension (ISH).
Patients’ Treatment Status at Baseline
A major focus of the present study was to evaluate the effects of perindopril/amlodipine in untreated patients versus previously treated patients who required a change in antihypertensive medication. Therefore, patients were also stratified according to treatment status at baseline.
Table 1 shows the baseline characteristics of these subgroups. Of the 1770 patients, 803 (45.4%) were previously untreated, whereas 967 (54.6%) were previously on antihypertensive treatment. The mean age was 62.8 ± 12.8 years in the subgroup of previously treated patients and 56.6 ± 13.3 years in the untreated population. Baseline values of blood pressure and heart rate in patients with and without previous antihypertensive therapy were in a similar range—the previously treated group had a systolic blood pressure (SBP) of 161.9 ± 14.4 mmHg, diastolic blood pressure (DBP) of 94.2 ± 9.2 mmHg and a heart rate of 76.7/min ± 10.0; treatment-naive patients had a SBP of 165.7 ± 15.1 mmHg, a DBP of 96.8 ± 9.4 mmHg and a heart rate of 78/min ± 10.1 (differences not significant; Table 2).
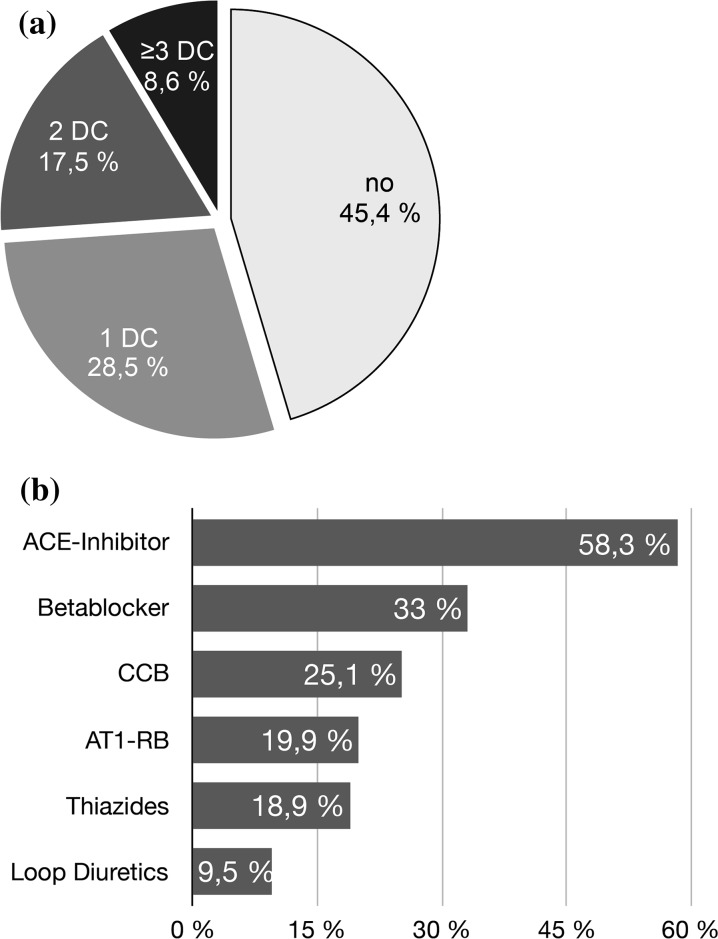
In the previously treated subgroup (n = 980 patients), most patients received one antihypertensive drug at study entry (n = 511; 52.1%), while 32.0% received two drugs (n = 314) and 11.6% received three drugs (n = 114) (Fig. 1a). The most frequently preexisting medication consisted of ACE inhibitors (58.3%), betablockers (33.0%), calcium channel blockers (25.1%), AT1 receptor blockers (19.9%), and thiazides/thiazide analogues (18.9%) (Fig. 1b).
Fig. 1.
Antihypertensive treatment status at baseline. a Treatment status at baseline according to number of antihypertensive drugs (DC drug class) (n = 1814). b Prevalence of antihypertensive drug class (n = 980) at baseline
Dosing of Perindopril/Amlodipine in the Outpatient Setting: Most Patients Received 3.5 mg/2.5 mg Fixed Combination
At study entry, 97.7% of patients received perindopril/amlodipine at a daily dose of 3.5/2.5 mg, and 47.9% of patients remained on this dose during the course of the study. Dose increases to 7/5 mg perindopril/amlodipine occurred in 28.5% of patients after the first examination, remaining unchanged until the final visit. The mean time until dose increase was 32.9 ± 11.8 days.
Reasons for Medication Change to Perindopril/Amlodipine SPC
The most common reasons given by treating physicians for switching patients on antihypertensive medication to perindopril/amlodipine SPC were insufficient blood pressure control (81.0%), intolerability (15.2%) or non-adherence to the previous medication (12.1%).
Coexisting Medications
Co-medications were checked at each visit. During the course of the non-interventional study, most of the patients had no other antihypertensive medication (68.5%; n = 1242/1814). The most common concomitant antihypertensive treatments were betablockers (n = 403, 22.2%), loop diuretics (n = 137, 7.6%), thiazides (n = 108, 6.0%), AT1-receptor antagonists (n = 35, 1.9%), alpha blockers (n = 34, 1.9%), potassium-sparing diuretics (n = 24, 1.3%) and calcium channel blockers (n = 24, 1.3%). A betablocker was newly commenced during our observation in 42 patients (2.3%), a loop diuretic in 10 patients (0.6%), a thiazide in 23 patients (1.3%).
Other co-medications were statins (n = 482, 26.6%), antiplatelet drugs (n = 212, 11.7%), oral antidiabetics (n = 206, 11.4%), Uricosurics/antigout preparations (n = 79, 4.3%), insulin (n = 63, 3.5%) and other anticoagulants (n = 53, 2.9%). A statin was initiated during our observation period in 24 patients (1.3%) and a dose increase occurred in 12 patients (0.7%).
Blood Pressure Effects of Perindopril/Amlodipine
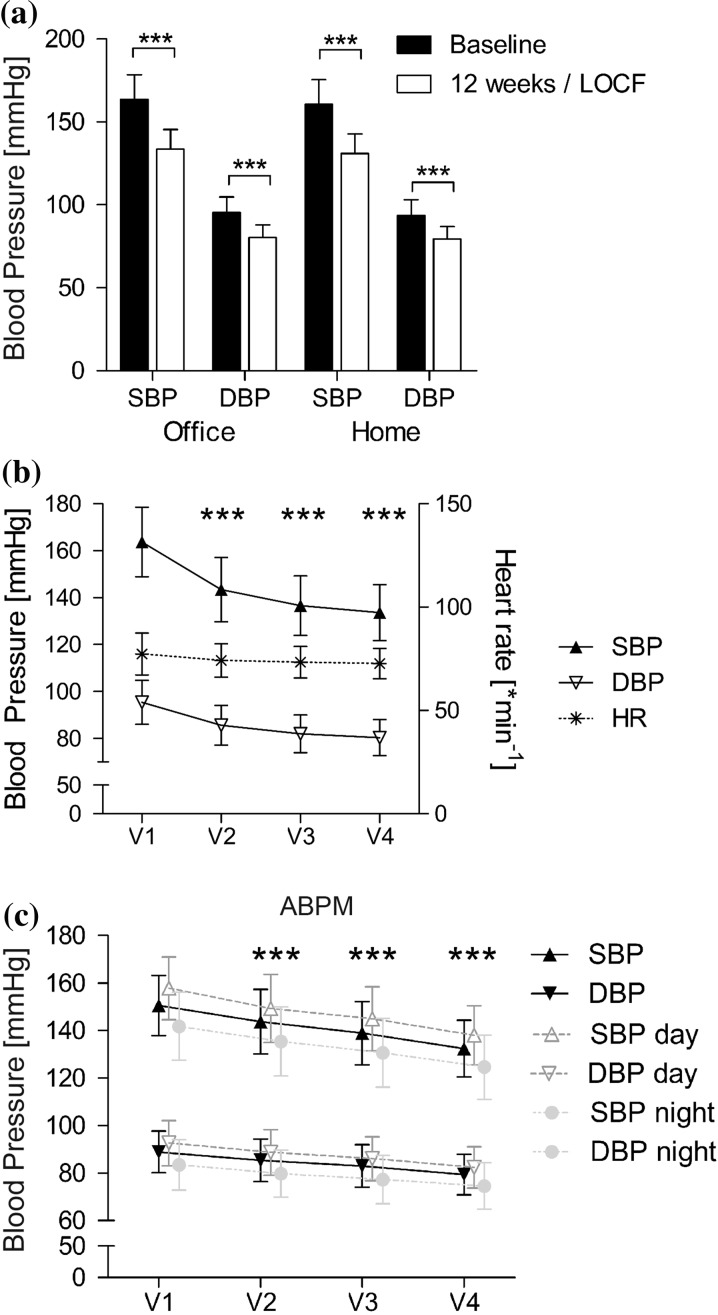
In the total study population, 1720 patients completed the 3 months follow-up and an additional 50 patients were seen at least for one additional control visit. Overall, office SBP decreased from a mean value of 163.7 ± 14.8 mmHg at baseline to 133.6 ± 11.6 mmHg at final examination (LOCF, n = 1770). Office DBP decreased from 95.4 ± 9.4 to 80.3 ± 7.7 mmHg. The mean difference was 30.1 ± 16.3/15.1 ± 10.3 mmHg (p < 0.0001 vs. baseline for SBP and DBP). Similar reductions in blood pressure occured in home blood pressure measurements in a subset of patients (Fig. 2a).
Fig. 2.
Change in systolic and diastolic blood pressure. a Total population, office vs. home measurements (n = 1770/1282, LOCF). b Systolic and diastolic office blood pressure and heart rate (HR) in the LOCF study population over time (n = 1770 for BP, n = 1680 for HR). c 24 h-ABPM measurements available in subset of patients (n = 187 for day, 175 for night, 167 for all measurements, LOCF). ***p < 0.0001 vs. V1 (baseline)
A statistically significant reduction in office blood pressure was documented as early as visit 2 (− 20.3 ± 14.8/− 9.8 ± 9.7 mmHg; p < 0.0001). In fact, the highest reduction in blood pressure occurred within the first 4 weeks of treatment (Fig. 2b). The effects on blood pressure were accompanied by a significant decrease in heart rate from 77.3 ± 10.1 to 72.7 ± 7.4 bpm (p < 0.0001) (Fig. 2b).
Results of ABPM were available in some patients at the discretion of the treating physician (Fig. 2c). Mean daytime SBP/DBP decreased significantly from 157.8 ± 13.2/92.7 ± 9.5 to 138.0 ± 12.4/82.4 ± 8.8 mmHg at the last observation (− 19.8/− 10.3 mmHg; p < 0.0001 vs. baseline for SBP and DBP, n = 178). Mean nighttime 24 h blood pressure (n = 175) was reduced by 17.2/9.0 mmHg (p < 0.0001 vs. baseline) from 141.8 ± 14.3/83.5 ± 10.6 to 124.6 ± 13.6/74.5 ± 9.7 mmHg. Mean 24 h BP (n = 167) was reduced from 150.6 ± 12.6/88.9 ± 8.8 to 132.4 ± 11.9/79.4 ± 8.5 mmHg (p < 0.0001).
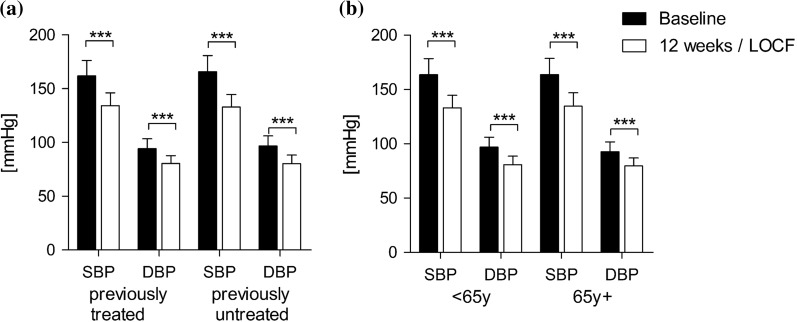
In the subgroup of patients with previous antihypertensive treatment, systolic blood pressure decreased significantly from 161.9 ± 14.4 to 134.1 ± 11.9 mmHg and diastolic blood pressure from 94.2 ± 9.2 to 80.4 ± 7.4 mmHg at the final examination (− 27.8/− 13.9; p < 0.0001 vs. baseline) (Fig. 3a). This was comparable to the blood pressure reduction in previously untreated patients (mean difference: − 32.8/− 13.9 mmHg; p < 0.0001 vs. baseline, Fig. 3a).
Fig. 3.
Changes in blood pressure in patient subgroups. a Patients with previous antihypertensive treatment (n = 967, LOCF) versus treatment naïve patients (n = 803, LOCF). b Patients younger than 65 years (n = 1133, LOCF) vs. patients older than 65 years (n = 636, LOCF)
Furthermore, patient age did not influence blood pressure response. In the subgroup of patients < 65 years of age, systolic blood pressure decreased significantly (mean difference: − 30.6/− 16.2 mmHg; p < 0.0001 vs. baseline, n = 1133) (Fig. 3b). A comparable decrease was observed in patients 65 years and older (mean difference: − 29.1/− 13.0 mmHg; p < 0.0001 vs. baseline, n = 636) (Fig. 3b).
Blood Pressure Control with Perindopril/Amlodipine
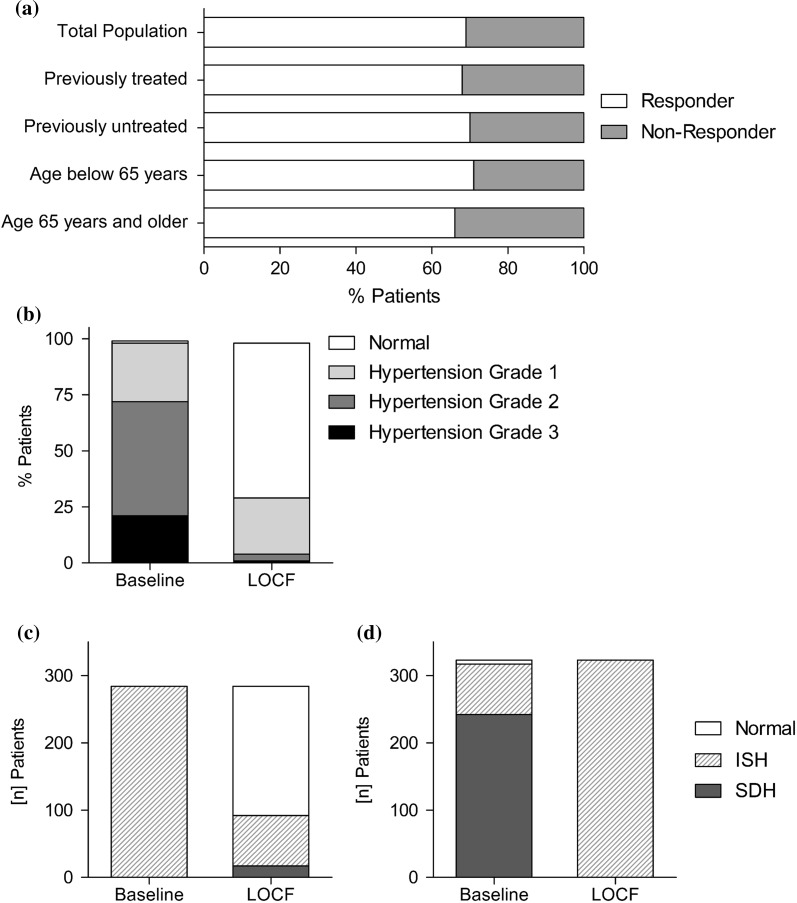
Patients whose blood pressure values were > 140/90 mmHg at the beginning of the study, and who reached the office blood pressure target of < 140/90 mmHg, were classified as controlled. In the total study population, more than two-thirds of the patients reached this goal (69.1%) (Fig. 4a). This effect remained stable in subgroup analyses: The control rate in previously treated hypertensive patients was 68.1% (n = 645/947), comparable to the effect in previously untreated hypertensive patients (70.3%; n = 560/797). Comparable results were also documented in patients with hypertension aged below 65 years (n = 1120) and patients ≥ 65 years (n = 623). At final examination, 791 patients < 65 years (70.6%) and 413 patients ≥ 65 years (66.3%) achieved blood pressure values < 140/90 mmHg (Fig. 4a).
Fig. 4.
Treatment response, blood pressure classification according to ESH/ESC and tracking of patients with isolated systolic hypertension (ISH). a Overall Responder rates (RR < 140/90 mmHg at last observation) in patients with hypertension grade 1–3 at baseline in the total population (n = 1744) as well as in the subgroups of patients with previous antihypertensive medication (n = 947), without previous antihypertensive treatment (n = 797), patients younger than 65 years (n = 1120) and patients age 65 years and older (n = 623). b Classification of blood pressure according to the ESH/ESC-guidelines at admission and at the last observation: while initially 72.4% of patients had hypertension grade 2 or worse, this fraction is reduced to 5.2% at the last observation. c Reanalysis to track patients with ISH. Patients with ISH at baseline show good blood pressure control in > 67% at the last observation (n = 284). d Patients with ISH at the last observation showed mainly systolic/diastolic hypertension (SDH) at baseline (n = 323)
Treatment Response According to Hypertension Severity
At study entry, 21.3% of patients (n = 377) had grade 3 hypertension, 51.1% (n = 905) grade 2 hypertension and 26.1% (n = 462) grade 1 hypertension according to the ESH/ESC guidelines [2] (Fig. 4b). At the final examination, 69.1% (n = 1223) of 1770 patients had controlled hypertension with normal or high normal blood pressure values, while 25.7% (n = 455) patients ended the study with grade 1 hypertension and only 3.8% (n = 68) and 1.4% (n = 24) had grade 2 and 3 hypertension at the end of the study, respectively (Fig. 4b).
When we followed-up the ESC/ESH grade subgroups, of the 426 patients entering the trial with grade 1 hypertension, 86.2% (n = 367) had controlled blood pressure at the end of the trial period. The majority of patients entered the study with grade 2 or 3 hypertension (n = 1282); of these, 65.4% (n = 838) had controlled hypertension at the end of the study period and an additional 28.6% (n = 367) had grade 1 hypertension, showing an improvement in blood pressure (as by the lower hypertension group at study end) in 94% of patients (n = 873) in this group.
Treatment Response in Patients with Isolated Systolic Hypertension
Isolated systolic hypertension (ISH) is associated with increased morbidity and mortality [16]. In our study, 16.1% of all patients (n = 284) entered it with ISH, of whom most patients had ESH/ESC grade 1 (n = 142) or grade 2 (n = 116) (Fig. 4c). At the end of the study, 18.3% (n = 323) of patients had ISH, of whom most had grade 1 hypertension (n = 301) (Fig. 4d). At first glance, ISH may look like a stable population in our dataset. However, analysis of hypertension subgroup tracking showed that the hypertension subtype changed: among the 284 patients entering the study with isolated systolic hypertension, 67.6% (n = 192) had controlled blood pressure at the final examination (Fig. 4c). In contrast, among the 323 patients with ISH at the end of the study, 74.9% had initially presented with systolic/diastolic hypertension (Fig. 4d).
Treatment Adherence Before and After Perindopril/Amlodipine SPC
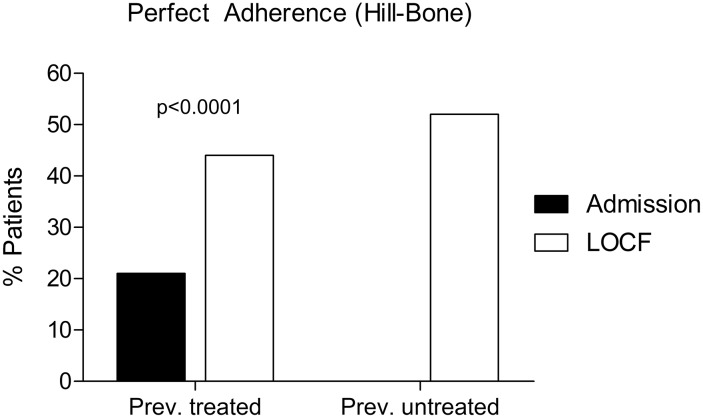
Adherence to antihypertensive medication, as assessed by the Hill-Bone Medication Adherence scale, was measured at baseline in previously treated patients and in all patients at the final visit (Fig. 5). At baseline, 939 of 980 patients answered the questionnaire; perfect adherence was defined as answering all nine items of the Hill-Bone scale [15] with “none of the time”. Most of the patients with uncontrolled hypertension under treatment showed no perfect adherence (745/939, 79.3%), and only 20.6% were assessed to be perfectly adherent to previous antihypertensive medication [15] (Fig. 5, black bar).
Fig. 5.
Treatment adherence before and after switching to perindopril/amlodipine. Change in adherence rate to antihypertensive treatment in patients with previous antihypertensive treatment (n = 897) after switching to perindopril/amlodipine as well as in patients with newly treated hypertension. Perfect adherence rate is defined as percentage of patients who answer all items of the Hill-Bone Medications Adherence Scale with “none of the time”
At the final visit, 47.2% of all patients (791 out of 1676) showed perfect adherence to treatment with perindopril/amlodipine. In previously treated patients, adherence increased statistically significantly from 20.6% (n = 185/897) before the study to 43.4% (n = 391/897) at the final visit (p < 0.0001; Fig. 5). In the subgroup of patients without prior antihypertensive treatment, 51.7% (n = 395/764) showed perfect adherence to the study medication.
Safety and Tolerability of Perindopril/Amlodipine SPC
Altogether, 140 ADRs were reported for 88 patients (4.9% of the total population), mostly drug ineffectiveness (n = 19; 1.1%), cough (n = 17; 0.9%), and dizziness (n = 8; 0.4%). Outcome of the ADR was reported to be recovered for 135 events in 83 patients, not recovered for 4 patients (0.2%), and fatal for one event in 1 patient with a hypertensive crisis. The majority of 130 ADR in 80 patients were assessed as non-serious. Seriousness was confirmed for ADR in 8 patients (0.4%). Premature termination of treatment with perindopril/amlodipine was documented in 120 patients (6.6%). The most common reasons for premature termination were AE/ADR in 40 patients (33.3% of patients with premature termination) and lack of compliance in 32 patients (26.7%). In nearly all patients, the tolerability of perindopril/amlodipine was rated by the treating physician as “very good” (n = 1308/1724; 75.9%) or “good” (n = 392/1724; 22.7%).
Discussion
The main conclusion of our non-interventional study is that the fixed-dose single-pill combination perindopril/amlodipine is effective and in general well tolerated in a typical outpatient population of patients with essential hypertension—independent of previous antihypertensive treatment, age and concomitant medication. It is also effective in patients with isolated systolic hypertension, a condition associated with increased morbidity and mortality that is generally considered to respond to CCB and diuretics [16].
In addition, treatment with perindopril/amlodipine was associated with a significant increase in patients’ adherence as compared to previous antihypertensive treatment. The combination therapy also showed a beneficial safety and tolerability profile. Taken together, the present results indicate that perindopril/amlodipine is a suitable treatment option for hypertensive patients in routine medical practice.
The objective of this prospective, non-interventional study was to assess whether the combination perindopril/amlodipine at fixed doses in a single pill provides blood pressure control and sufficient adherence in a typical mixed population with essential hypertension in routine practical care, with a focus on potential treatment effects on blood pressure, adherence, and tolerability in both newly diagnosed patients and patients previously insufficiently treated with one or more antihypertensive drugs.
Our study population may be representative for ambulatory patients with essential hypertension who are treated by general practitioners, internists and cardiologists, due to the clinically and demographically diverse cohort with 1814 unselected patients from different German outpatient practices enrolled in the present trial. Most patients (87.8%) had additional cardiovascular risk factors and/or diseases. Of note, the average baseline cholesterol and LDL-values were above the therapeutic aims stated by the ESC/EAS, and hyperlipidemia is reported as comorbidity in 840 study participants (46.3%). However, only 482 patients (26.5%) receive a statin comedication during the course of the study, which is similar to the reported prevalence of statin use in Germany in patients aged 65 years or older [17]. During the observation time, the statin dose was increased in 12 patients (0.66%) and a statin was commenced in 24 patients (1.32%). These medication changes are reflected in a slight decrease in total cholesterol and LDL (− 11 mg/dL) at visit 4. The rate of patients at high risk for cardiovascular events, e.g., with coronary artery disease (8.7%, n = 157), arteriosclerosis (6.4%, n = 116) or diabetes (18.0%, n = 326), is lower than the rate of patients receiving a statin, suggesting that patients at very high cardiovascular risk are likely treated. However, cardiovascular risk control in the entire collective may be suboptimal, a common finding throughout Europe [18].
Overall, the magnitude of the blood pressure-lowering effects and improvement in hypertension class with perindopril/amlodipine observed over 3 months in the present non-interventional study are in line with results of various clinical trials with this drug combination [10, 12–14]. After about 3 months of treatment with perindopril/amlodipine, mean values of systolic and diastolic blood pressure were significantly reduced, from 163.6/95.4 to 133.6/80.3 mmHg in the total population and from 161.9/94.2 to 134.1/80.4 mmHg in previously treated patients. The decrease in blood pressure was accompanied by a decline in heart rate and by a pronounced shift in hypertension grade distribution towards lower classes. Interestingly, the highest decrease in blood pressure occurred within the first 4 weeks of treatment, and the most common time point to change treatment dose was visit 2, showing that treatment response can be judged early and therapy can be adjusted accordingly. Of 1744 patients with blood pressure values ≥ 140/90 mmHg at baseline, 1205 patients (69.1%) were classified as controlled patients, i.e. achieved target values < 140/90 mmHg.
An additional benefit of the single-pill combination with perindopril and amlodipine was an improvement in patients’ adherence to medication, as demonstrated by a significant change in the percentage of participants with perfect adherence according to the Hill-Bone Medication Adherence Scale. Single-pill combinations are known to be associated with better adherence to treatment [6] and a greater rate of blood pressure control [5]. The positive influence on adherence may be of special relevance for patients with comorbidities and co-medications as well as for previously insufficiently treated patients. Indeed, in the subgroup of patients with previous antihypertensive treatment, the number of individuals showing perfect adherence doubled after switching to the fixed-dose single-pill combination. When we compared the percentage of perfect adherence (47% of all patients) with the percentage of responders (69%), or with the even higher group of patients whose hypertension grade class improved, we can speculate that the fixed combination leads to treatment response even in patients who are not perfectly adherent.
The ESC/ESH guidelines stress that great efforts should be devoted to prevent or at least to reduce drug-related side effects as one of the most important reasons for non-adherence [2]. In the present study, perindopril/amlodipine showed a favorable safety and tolerability profile across a broad range of ambulatory patients with different ages, comorbidities and co-medications. Of note, cough—a typical adverse event induced by ACE inhibitors—was reported in 0.9% of patients. This rate is comparable to the cough rate in a randomized study of the fixed-dose combination of perindopril/amlodipine, which reported cough in 0.8% of patients on perindopril 3.5/amlodipin 2.5 [13]. Thus, in a primary care setting, a low cough rate would be expected and would be a potential advantage. One potential explanation is positive selection bias, since in our study almost 60% of previously treated patients had previous ACE inhibitor treatment. However, perindopril has a relatively low cough rate even in treatment-naive patients in randomized trials, as seen in the EUROPA (3.5%), ADVANCE (4.3%) and PROGRESS (4.4%) trials [19]. This contrasts with higher cough rates for ramipril observed in the HOPE trial, which showed cough in 7.3% of patients who were ACE inhibitor treatment-naive. Furthermore, as only a minor percentage of patients discontinued medication, the present study suggests that treatment with perindopril/amlodipine was tolerated well in this patient cohort with co-morbidities. The rate of ADR (< 4.9%), especially of serious ADR (< 0.5%), was low, e.g., angioedema was reported in only 1 patient (0.06%). However, our study may suffer from under-reporting, a frequent problem in observational studies [20].
Study Limitations
The open-label, observational, non-interventional design of the present study and the missing reference group of patients without or with alternative treatment may lead to an overestimation of the effectiveness of perindopril/amlodipine, as well as an underreporting of adverse drug-related events by participating physicians. Despite the obvious advantages of randomized controlled studies, the patients who are included in those trials are not always representative of the patients in daily routine practice. In addition to the data of those studies, non-interventional trials such as the Viacoram-BPT study might result in a more realistic picture of everyday use of the drug, e.g., include previously insufficiently treated patients, elderly patients, or patients with multiple risk factors and concomitant diseases, which are usually not fully represented in controlled clinical trials. Another limitation of our non-interventional study is the observation duration of 3 months, which is rather short for the evaluation of patients’ adherence to long-term treatment. Nevertheless, it seems to be sufficient to evaluate blood pressure control, as demonstrated in other randomized, controlled and “real-life” studies [10, 12–14], and it already showed an improvement of patient adherence.
Conclusion
Taken together, the results of this prospective, non-interventional study provide further evidence about the effectiveness and safety of perindopril/amlodipine under conditions of routine medical practice in a broad cohort of patients with essential hypertension. The study population is considered representative in terms of age, gender, cardiovascular risk factors, comorbidities and concomitant medications. In patients with and without antihypertensive treatment prior to study entry, perindopril/amlodipine was effective, in general well tolerated and associated with high adherence rates. In line with the recommendations of the ESC/ESH guidelines, the results emphasize the advantage of fixed-dose single-pill combinations with an ACE inhibitor and a calcium channel blocker for treating patients with arterial hypertension.
Acknowledgements
Funding
Sponsorship for this study, article processing charges and the open access fee was funded by Servier Deutschland GmbH.
Medical Writing, Editorial, and Other Assistance
We thank Abdol A. Ameri for assistance in the preparation of the manuscript. Study data were analyzed by an independent statistical institute, ANFOMED GmbH, Möhrendorf, Germany, and both were funded by Servier Deutschland GmbH.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for this work as a whole, and have given final approval to this version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Susanne V. Fleig and Hermann Haller have nothing to disclose. Florian P. Limbourg received honoraria from Servier for scientific consultancy. Bettina Weger is an employee of Servier Deutschland GmbH, Munich (Medical Affairs Department).
Compliance with Ethics Guidelines
Ethical approval was granted by the independent ethics commission in Freiburg/Germany (FEKI). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Content
To view enhanced content for this article go to 10.6084/m9.figshare.5882350.
References
- 1.Neuhauser H, Thamm M, Ellert U. Blood pressure in Germany 2008–2011: results of the German Health Interview and Examination Survey for Adults (DEGS1) Bundesgesundheitsblatt Gesundh Gesundh. 2013;56(5–6):795–801. doi: 10.1007/s00103-013-1669-6. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, Fagard R. ESH/ESC guidelines for themanagement of arterial hypertension. J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 3.Sarganas G, et al. Trends in antihypertensive medicatrion use and blood pressure control among adults with hypertension in Germany. Am J Hypertens. 2016;29(1):104–113. doi: 10.1093/ajh/hpv067. [DOI] [PubMed] [Google Scholar]
- 4.Claxton A, Cramer J, Pierce C. A systematic review of the association between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/S0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents. Hypertension. 2010;55:399–407. doi: 10.1161/HYPERTENSIONAHA.109.139816. [DOI] [PubMed] [Google Scholar]
- 6.Bangalore S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120(8):713–719. doi: 10.1016/j.amjmed.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Corrao G, et al. Cardiovascular protection by initial and subsequent combination of antihypertensive drugs in daily life practice. Hypertension. 2011;58(4):566–572. doi: 10.1161/HYPERTENSIONAHA.111.177592. [DOI] [PubMed] [Google Scholar]
- 8.Simpson SH, Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wald DS, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122(3):290–300. doi: 10.1016/j.amjmed.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 10.Gradman AH, et al. Initial combination therapy reduces the risk of cardiovascular events in hypertensive patients. Hypertension. 2013;61:309–318. doi: 10.1161/HYPERTENSIONAHA.112.201566. [DOI] [PubMed] [Google Scholar]
- 11.Makani H, et al. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med. 2011;124(2):128–135. doi: 10.1016/j.amjmed.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Dahlof B, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 13.Laurent S, et al. Randomized evaluation of a novel, fixed-dose combination of perindopril 3.5 mg/amlodipine 2.5 mg as a first-step treatment in hypertension. J Hypertens. 2015;33(3):653–661. doi: 10.1097/HJH.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 14.Mancia G, et al. Comparison of single-pill strategies first line in hypertension: perindopril/amlodipine versus valsartan/amlodipine. J Hypertens. 2015;33(2):401–411. doi: 10.1097/HJH.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 15.Kim MT, et al. Development and testing of the Hill-Bone Compliance to High Blood Pressure Therapy Scale. Prog Cardiovasc Nurs. 2000;15(3):90–96. doi: 10.1111/j.1751-7117.2000.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 16.Bavishi C, Goel S, Messerli FH. Isolated systolic hypertension: an update after SPRINT. Am J Med. 2016;129(12):1251–1258. doi: 10.1016/j.amjmed.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 17.Knopf HC, et al. Changes in the prevalence of statin use in Germany—findings from national health interview and examination surveys 1997–1999 and 2008–2011. Z Evid Fortbild Qual Gesundhwes. 2017;122:22–31. doi: 10.1016/j.zefq.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Kotseva K, et al. EUROASPIRE IV: a European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol. 2016;23(6):636–648. doi: 10.1177/2047487315569401. [DOI] [PubMed] [Google Scholar]
- 19.Brugts JJ, et al. The incidence and clinical predictors of ACE-inhibitor induced dry cough by perindopril in 27,492 patients with vascular disease. Int J Cardiol. 2014;176(3):718–723. doi: 10.1016/j.ijcard.2014.07.108. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2009;32(1):19–31. doi: 10.2165/00002018-200932010-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.