Abstract
3D-printing (3DP) technology has been developing rapidly. However, limited studies on the contribution of 3DP technology, especially multimaterial 3DP technology, to droplet-microfluidics have been reported. In this paper, multimaterial 3D-printed devices for the pneumatic control of emulsion generation have been reported. A 3D coaxial flexible channel with other rigid structures has been designed and printed monolithically. Numerical and experimental studies have demonstrated that this flexible channel can be excited by the air pressure and then deform in a controllable way, which can provide the active control of droplet generation. Furthermore, a novel modular microfluidic device for double emulsion generation has been designed and fabricated, which consists of three modules: function module, T-junction module, and co-flow module. The function module can be replaced by (1) Single-inlet module, (2) Pneumatic Control Unit (PCU) module and (3) Dual-inlet module. Different modules can be easily assembled for different double emulsion production. By using the PCU module, double emulsions with different number of inner droplets have been successfully produced without complicated operation of flow rates of different phases. By using single and dual inlet module, various double emulsions with different number of encapsulated droplets or encapsulated droplets with different compositions have been successfully produced, respectively.
Introduction
Droplet microfluidics has been developed for the generation and manipulation of monodisperse droplets and bubbles in a continuous flow1,2. It has attracted more and more attentions due to its extensive applications in biology3,4, chemistry5 and nanotechnology6,7. Single emulsions have been first studied for fast analytical systems8 and the synthesis of advanced materials9. More complicated multiple emulsions have been further developed for the controlled encapsulation and release of materials in cosmetic10, drug delivery11 and food applications12.
The prerequisite consideration in droplet microfluidics is the generation of emulsion droplets. One common method for fabricating emulsion generator is soft-lithography13, which has been widely applied due to its capability of fabricating different microchannels, such as T-junction14, flow-focusing15 and co-flow16, with high resolution down to 1 μm. However, it includes relatively complicated fabrication process and normally requires expensive master molds and clean room environment, which limit its wide usage17. The other popular method is using glass capillaries18–20. Glass capillaries are welcomed due to its excellent optical transparency, electrical insulativity and chemical robustness. Even higher-order multiple emulsions can be achieved through assembly of glass capillaries21. Nevertheless, glass capillary devices require manual operation, which may cause instability and inconsistency issues. In addition, it is difficult to manufacture complex three-dimensional (3D) structures using these methods. To make extensive applications of droplet-microfluidics, it is essential to develop new techniques for emulsion generation in a simple, low-cost and reliable manner.
In recent years, typical 3D-printing (3DP) technologies such as fused deposition modeling (FDM) and stereolithography (SL) have been applied in bioengineering22–26 and starts to find a place in the microfluidic field27–29. Micromixer30,31, droplet generator32,33, reactionware34,35, helical channel36, check valve37,38 and pump39 manufactured via such typical 3DP technologies have been reported. Besides typical 3DP technologies, novel 3DP methods are still continuously emerged to create more possibilities for the academic community40,41. More recently, novel multimaterial 3DP has been developed and benefits various fields such as synthesis of novel functional materials42,43, creation of heterogeneous organ-on-a-chip44 and fabrication of novel microfluidic devices. One of the greatest advantages of multimaterial 3DP is that versatile materials can be simultaneously used for building a single object, which is ideal for fabrication of microfluidic control components consisting of different parts such as interconnects45, membranes46,47, valves and pumps48–50, and multi-flow controllers51. Compared to traditional methods, 3DP technology has distinct advantages and disadvantages for microfluidics. It offers a new rapid-prototyping and 3D-digital manufacturing method which can fabricate microfluidic devices in a simple, fast, customized and monolithic manner. On the other hand, the resolution of printing, the biocompatibility of printed materials, as well as the optical property and surface property of printed materials are still unsatisfying for the microfluidic community. Potentials of 3DP for more feature-rich microfluidic devices still need to be exploited.
Following the mechanical control methods for emulsion generation52,53, pneumatic control of generation of single and double emulsions in modular 3D-printed devices has been reported in the present study. A novel pneumatic control unit (PCU) has been designed and fabricated monolithically by multimaterial 3DP technology. This PCU has also been numerically and experimentally studied for its effects on single emulsion generation, and then we extend its capacity in active control of the generation of double emulsions with different number of encapsulated droplets without complicated operation of flow rates of different phases. Furthermore, modular 3D-printed devices for generation of various double emulsions and microspheres have been reported, which has demonstrated that 3D-printed devices can be used for the complicated operation of multiple emulsions. Potentials of 3DP technology in droplet-microfluidic fields, especially for generation of multiple emulsions, have been exploited.
Materials and Methods
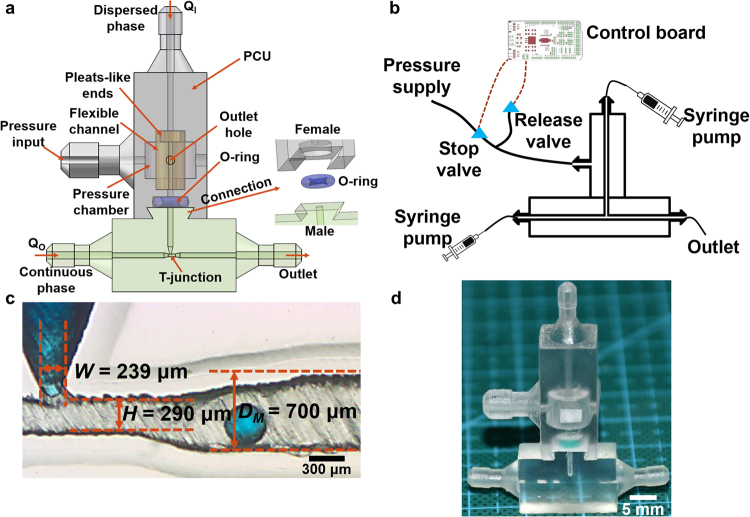
All printed files were designed using Solidworks (Dassault Systèmes). As shown in Fig. 1a, a single emulsion generator mainly includes two parts, PCU and droplet generator (T-junction). The PCU was printed with a multimaterial 3D-printer, Objet 350 Connex 3 (Stratasys, Ltd.). Veroclear, a transparent PolyJet photopolymer provided by the same company, was used to print the rigid part (grey). A rubber-like material, TangoPlus FLX930 was used to print the 3D coaxial flexible channel (orange). The flexible channel has pleat-like ends (See supplementary Fig. S1), which makes adhesion to the rigid part with larger areas to increase the structure strength. The flexible channel is located in a pressure chamber, which is connected with a pneumatic pump (AT60/25, Jiangsu dynamic medical technology Ltd.) to excite this flexible channel. Water solution containing 2% NaOH (w/w) and 1% Na2SiO3 (w/w) was used for dissolution of support materials, and then the printed PCU was put in an ultrasonic device for 2 hours to fully remove the support materials. Since our PCU structure is relatively simple and small, the required printing time is about 20 minutes, and 2 hours for removal of supports are enough. It will take longer time to print and remove supports if the structure to print is complicated.
Figure 1.
(a) Schematic of the pneumatic device for droplet generation. (b) Schematic of the experimental setup. (c) Photograph showing T-junction channel. (d) A printed pneumatic device for emulsion generation.
The T-junction structure (green) was printed using a stereolithography printer, Form-2 (Formlabs, Inc.) with Clear FLGPCL04 resin, which was provided by the same company and can provide excellent optical property. The uncured resin was removed by flushing Isopropyl Alcohol and the printed modules were post-cured under a UV light for 30 minutes. These modules are also relatively simple and small which can be printed and post-cured at a time. The whole period required for preparing all of these modules for use in our experiments is about 3 hours. The basic geometry of the T-junction structure is illustrated in Fig. 1c. The vertical conical channel with nozzle diameter W = 239 µm introduces the dispersed phase QI, whereas horizontal square channel with height H = 290 µm introduces the continuous phase QO. They both flow out from the downstream square channel with height DM = 700 µm.
As shown in Fig. 1a, the PCU and droplet generator are assembled through a connection part. A notch structure designed here together with O-ring can perfectly connect two parts without any liquid leakage. The fasten angle of snap-fit joints has been optimized as 60°, to provide excellent print quality as well as enough sealing force. No liquid leakage has been found in our experiments (up to 4 bar), and this assembly process can be finished in a minute.
Silicone oils (Beijing Hagibis Technology Ltd.) of different viscosities were used as the oil phase (O), whereas water-glycerin (Sinopharm Chemical Reagent Ltd.) mixtures with different viscosities were used as the aqueous phase (W). A 3D-printed resin (Clear FLGPCL04) was used as the solidification phase for microsphere fabrication. RSN-749 resin (CosBond Ltd.), as a surfactant, was used in the oil phase with 0.25% (v/v), and Tween-20 (Beijing Huabo Ltd.), as a surfactant, was used in the aqueous phase with 0.25% (w/v). Liquids viscosities were measured using a viscometer (NDJ-5S, YOKE INSTRUMENT Ltd.), and interfacial tension (σ = 20 mN m−1.) was measured using a tensiometer (DCAT 11, Data Physics Corp.). All data are listed in Table 1. Soluble food dyes with different colors (PT. Gunacipta Multirasa Co.) were used in the aqueous phases for a better observation.
Table 1.
Liquid use in the present worka.
| Active W/O | Active W/O/W | Passive W/O/W | Microsphere W/O/W | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| inner | outer | inner | middle | outer | inner | middle | outer | inner | middle | outer |
| 1 | 10 | 1 | 100 | 100 | 1/20 | 100 | 100 | 20 | 3D-printed resin | 100 |
aW: water-glycerin mixture, unit: cP; O: silicone oil, unit: cSt. Interfacial tension σ = 20 mN m−1.
The flow was driven and controlled with up to four syringe pumps (LSP02-2A, Longer Precision Pump Co, Ltd). Two solenoid valves (DW10AA and VX240EA, SMC Corp.) and an OB1 pressure controller MK3 (ELVEFLOW) worked corporately under the control of an Arduino Mega 2560 board (arduino.cc) to produce a stable periodic pressure wave as shown in Fig. 1b. The response time of the solenoid valves is 10 ms. The excitation frequency can be achieved up to 30 Hz. Video clips were captured with a high-speed CMOS camera, Phantom VEO 710 L (Vision Research Corp.). The droplet generation was controlled by altering the flow rates of different phases. After reaching the steady state, the generation frequency was monitored and about 50 droplets were analyzed with ImageJ. All experiments were conducted at room temperature (22 °C).
Results and Discussion
Effects of the deformation of the flexible channel on the inner flow rate
The droplet generation can be controlled by the variation of the flow rates of different liquid phases, which can be achieved via the flexible channel deformation where liquid phases pass through. In our study, the channel deformation is achieved by the pneumatic control in a simple and controllable manner. Therefore, we need to first investigate the effects of the deformation of the flexible channel on the variation of flow rates. Since quite limited studies regarding the multimaterial 3DP technology have been reported so far, some basic printed-material properties are still unknown. We have conducted experiments to measure the Young’s modulus of TangoPlus material for the fabrication of flexible channels, and obtain E = 0.504 MPa. The Poisson’s ratio ν = 0.495–0.499 is supplied by the provider. Then a numerical model has been built to analyze the deformation of the flexible channel under different applied air pressures. Finally, we can build a relation between the variation of the flow rates and the pneumatic excitation:
| 1 |
The variation of the flow rate Q(t) over time t can be expressed as the sum of the fixed inner flow rate supply QI and the additional flow rate introduced by the excitation. fF is the excitation frequency of the pressure wave. QA is the excitation amplitude of the flow rate, expressed as QA = 2πKP/B, where K and B are the coefficients determined by the experiments and P represents the peak value of the applied pressure wave. All details can be found in the SI (“Experimental and numerical analysis on the deformation of flexible channels” and “Flow rate estimation”). According to equation (1), we can conclude that the total flow rate Q(t) depends on the fixed flow rate input QI and the flow rate amplitude QA. Meanwhile, as shown in Fig. S2d, an increase of the applied pressure can increase the additional flow rate. On the other hand, with the constant excitation pressure, the amplitude of the additional flow rate keeps constant while the frequency increases with increasing fF. Furthermore, the positive Q(t) corresponds that the liquid is pushed out due to the channel deformation, while the minus Q(t) corresponds that the liquid retracts due to the channel back to the normal position. If QI is supplied in a small amount, the liquid can be sucked back to the channel. This excitation property can have influence on the droplet generation, which will be studied later.
Effects of excitation frequency on droplet generation
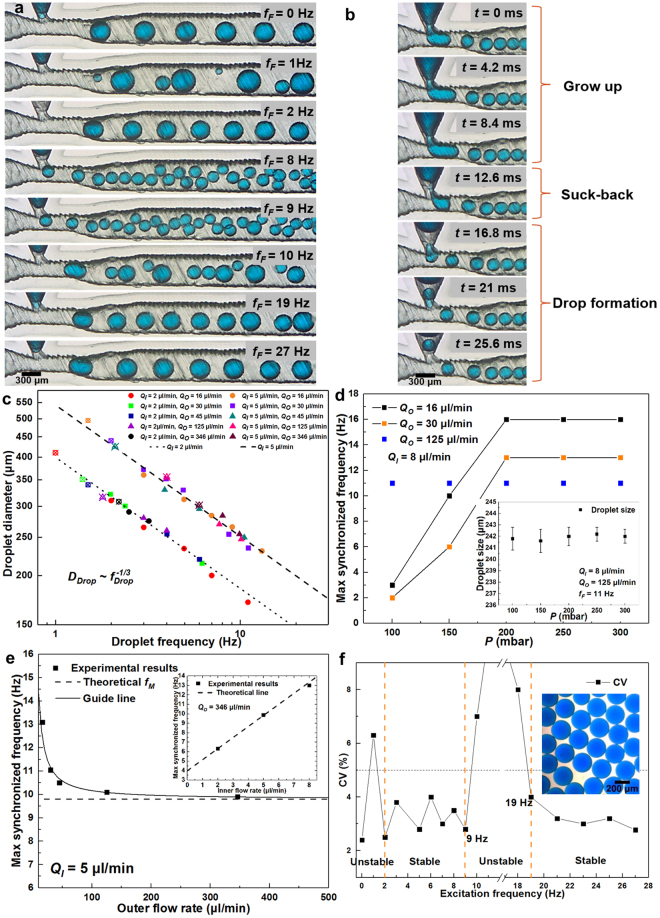
The dimensions of the channel for droplet emulsion are depicted in the Fig. 1c. QI was kept as 5 μl/min. QO was kept constant as 16 μl/min. The excitation frequency fF was varied and the applied pressure was kept constant as 150 mbar. The natural droplet generation frequency without excitation (f0) is 2 Hz. When the excitation is applied, four regimes have been discovered as shown in Fig. 2a: (1) unstable droplet generation when fF < f0 (<2 Hz); (2) stable droplet generation, synchronized with the excitation frequency (2–9 Hz); (3) unstable droplet generation (10–19 Hz); (4) stable droplet generation with the natural generation frequency (>19 Hz). The value of coefficient of variation (CV) of droplet sizes has been calculated in these regimes as depicted in Fig. 2f. The high CV value demonstrates the unstable generation of droplets, while the CV value smaller than 5% is shown in the stable regime. In regime (1), both instability (capillary and excitation instability) are superpositioned and the unstable droplet generation occurs. In regime (2), the excitation effects dominate the droplet generation and monodisperse droplets are produced. In regime (3), the excitation impacts become weak and polydisperse droplets are generated. In regime (4), excitation has negligible influence on the droplet generation, and the droplet generation frequency goes back to the natural generation frequency with narrow size distribution.
Figure 2.
(a) High-speed video frames showing the droplet generation with different excitation frequencies. fF = 0 Hz is the natural state without excitation, fF = 1 Hz is the unstable state, fF = 2–9 Hz is the stable synchronized regime, fF = 10–19 Hz shows the unstable output, and fF > 19 Hz shows the output of droplets unaffected by the excitation. (b) A cycle of the suction-generation process with fF > 9 Hz. (c) Droplet diameter versus droplet generation frequency. P = 200 mbar. Points with cross relate to the natural states without active control. (d) Plot of the variation of maximum synchronized frequency with different applied pressures, and the inset showing the droplet size generated in the synchronized regime with different applied pressures. (e) Plot of the variation of maximum synchronized frequency with the outer flow rate change, and the inset showing variation of maximum synchronized frequency with the inner flow rate change. P = 200 mbar in both figures. (f) Plot of CV value as a function of the excitation frequency.
Additionally, a new suction-generation regime has been discovered in the synchronized regime. A complete cycle of suction-generation process has been depicted in Fig. 2b. A certain volume of the dispersed phase is first pushed to the junction, and then it retracts in that the flexible channel returns to its normal position and pulls back some of the dispersed phase. This reverse flow pinches off the dispersed phase at the junction which is already surrounded by the continuous oil flow. The droplet size in this regime is always comparable to the junction size, and the drop size distribution is small. This regime can only happen when the excitation frequency fF is slightly below the maximum synchronized frequency (e.g. 8–9 Hz in Fig. 2a).
In the synchronized regime, the average inner flow rate of Q(t) is constant QI. Based on the conservation law, we have , where DDrop is the droplet size and fDrop is the droplet generation frequency. Then we can obtain
| 2 |
For a fixed QI, DDrop scales with fDrop as . As shown in Fig. 2c, experimental results agree well with the theoretical prediction given by equation (2). We can see that the droplet generation frequency mainly affects the droplet size. Therefore, wider range of droplet size can be produced. The excitation can bring faster pinch-off of the dispersed phase, and even smaller droplet, smaller than the junction size, can be generated with the low flow rate of the dispersed phase, which can benefit for various applications.
Effects of excitation amplitude and flow rates on the synchronized regime
By integrating Q(t) over half of the excitation period [−1/4fF, 1/4fF] during which the dispersed phase protrudes, we can estimate the maximum volume of inner fluid tip VTip. Using equation (1), we have
| 3 |
To form the droplet, VTip must be larger than the critical volume VC. Then we have
| 4 |
As a result, , which means the maximum synchronized frequency fM is
| 5 |
According to equation (5), we can conclude that if QI is increased, the synchronized regime can be extended. The increase of the applied pressure P can also increase the range of synchronized regime. It is a little complicated for VC. If VC is too large (VTip << VC), the dispersed phase cannot be pinched off during one excitation cycle and droplets will be formed with several excitation cycles, which results in an unstable regime. If VC is too small (VTip >> VC), the dispersed phase can be pinched off to several droplets during on cycle, which also results in an unstable regime. VC mainly depends on the Capillary number (: dynamic viscosity, u: characteristic velocity), flow rate ratio and channel geometry14,54. Increasing Ca and φ can reduce the value of VC. The minimum VC is limited by the nozzle diameter W, and can be expressed as .
Now we conduct experiments to verify our analysis. The excitation amplitude is firstly studied. A fixed inner flow rate is given as QI = 8 μl/min. The outer phase flow rate QO is varied. As shown in the inset of Fig. 2d, the applied pressure has little influence on the droplet size. However, the increase of the applied pressure can extend the maximum synchronized frequency fM, which agrees with our analysis. With the low and moderate flow rate of the outer phase, the influence of applied pressure on fM is significant. But above 200 mbar, fM tends to a constant value. The applied pressure over 300mbar may lead to the generation of unpredictable small satellite droplets. With a high outer phase flow rate (125 μl/min), fM is constant and the applied pressure has no influence. It is obvious that the flow rate ratio has a significant influence on the fM. The flow rate effects will be studied later.
The applied pressure is kept as 200 mbar to investigate the flow rate effects on the fM. First, we keep QO = 346 μl/min as constant and vary QI. In this large outer phase flow rate, VC should approach its minimum value, . Therefore, based on equation (5), we have fM = 1.17QI + 3.97. As shown in the inset of Fig. 2e, the experimental results fit very well with our theoretical prediction without fitting parameters. Increasing QI can linearly increase the fM. Next, we keep QI = 5 μl/min as constant and vary QO. With the increase of QO, fM decreases and tends to a constant value. Again, based on equation (5), we can get fM = 9.8 Hz, which agrees well with the experimental results.
Multiple emulsion generation with 3D-printed modular modules
Modular design strategy
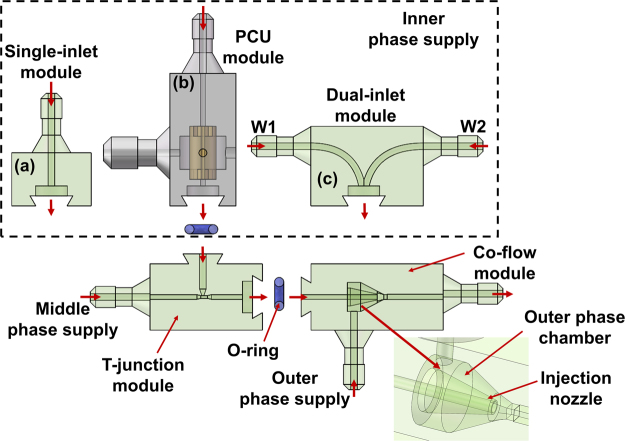
Due to the complexity of generation of multiple emulsions which normally include complicated operation of flow rates of different phases, complicated wetting conditions of channel walls and complicated structures, only a couple of studies33 have demonstrated that the 3DP technology can be applied for the generation of multiple emulsions. In this section, we are trying to extend 3DP technology applications in both passive and active control of the double emulsion production. One of the advantages of the 3DP technology lies in its capability of modular design and manufacture. Therefore, we design and fabricate the double emulsion generator in a modular way. Modular designs can lower the complexity of the microfluidic chips and also allow for their easy exchange.
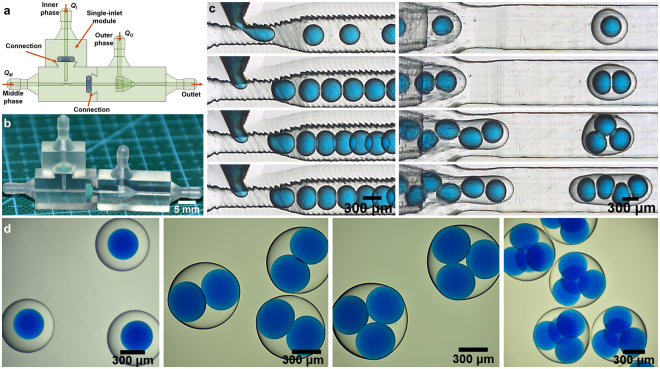
As shown in Fig. 3, the emulsion generator includes function module, T-junction module, and co-flow module. Different modules are assembled to each other with snap-fit joints which has been introduced previously. According to different requirements, function module can be chosen as: (1) Single-inlet module for passive generation of double emulsions with different number of the inner droplets. (2) PCU module for active generation of double emulsions with different number of inner droplets. (3) Dual-inlet module for passive generation of double emulsions with different compositions of inner droplets. The single-inlet module, dual-inlet module, T-junction module and co-flow module were printed using the Form-2 printer as mentioned before.
Figure 3.
Schematic of the 3D-printed modular device. It consists of three parts: function module, T-junction module, and co-flow module. The function module includes (a) Single-inlet module, (b) PCU module, (c) Dual-inlet module.
T-junction module is used for the inner droplet generation at upstream, and co-flow module is used for the outer droplet generation at downstream. Co-flow structure was firstly creatively introduced by Weitz’s group through assembly of glass capillaries18, but the fragile capillary nozzle and manual operation limit its applications for non-professions. Here we have designed and printed a monolithic co-flow structure. As shown in Fig. 3, a cone structure with a channel inside is printed. The cone chamber is used for outer phase input and also blocking the outer phase to meet the middle phase, and the middle phase reaches the nozzle from the inside channel. If the chip is printed as a whole, this nozzle is difficult to shape due to the difficulty to remove uncured resin through the whole chip. But for a modular design, the uncured resin can be removed through this small co-flow module. Furthermore, modular design and assembly strategy can bring another benefit for the surface treatment. The generation of multiple emulsions usually requires different wetting conditions of channel wall. It is still complicated to obtain different wetting conditions in a single chip, although a couple of methods to solve this issue have been proposed55–57. Here, we can easily make different surface treatments for different modules, to achieve the requirements for the generation of multiple emulsions. Different microdevices with different assembled modules (T-junction module and co-flow module are fixed, only function module needs to be changed.) have been demonstrated in the following sections.
Active control of double emulsion generation
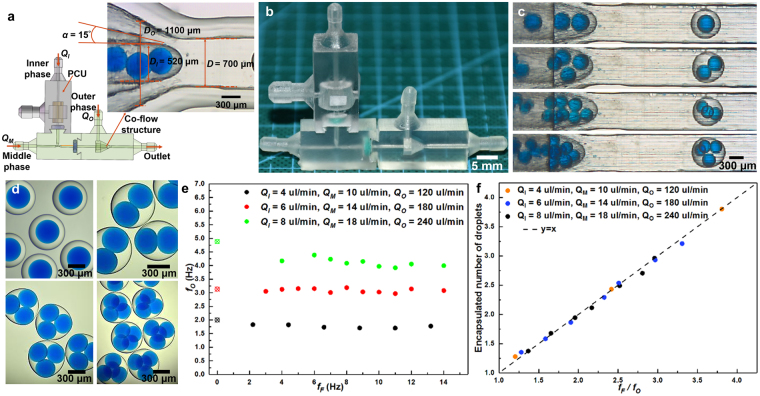
Here PCU module is chosen to be assembled with T-junction and co-flow modules as shown in Fig. 4a,b. QI, QM and QO are the inner, middle and outer phase flow rates, respectively. The inner droplets were generated at different frequencies via our pneumatic control method which has been fully discussed in the previous sections, and the outer droplets are generated through co-flow structure at downstream. The synchronized regime is used here for double emulsions production. The T-junction channel wall was surface treated as hydrophobic and the co-flow channel wall was surface treated as hydrophilic. W/O/W double emulsions can be produced under this wetting condition. The liquids used for generation of double emulsions are listed in the Table 1. A relatively viscous middle phase used here can reduce the influence of the upstream oscillation on the downstream droplet generation. Alternatively, a longer channel can be used here instead of using viscous middle phase to reduce the upstream influence. As shown in Fig. 4e, different excitation frequency applied at upstream has little influence on the droplet generation at downstream. The number of encapsulated droplets n can be simply expressed as:
| 6 |
where fI and fO represent the droplet generation frequencies of inner and outer droplets, respectively. fF is the excitation frequency as described previously. Based on the equation (6), double emulsions can be produced with controllable inner droplet number when the inner droplets are generated in the synchronized regime with variation of the excitation frequency. As shown in Fig. 4f, the experimental results have shown an excellent accordance with equation (6). High-speed video frames shown in Fig. 4c have demonstrated that double emulsions with 1–4 of inner droplets are generated with excitation frequency: 3 Hz, 6 Hz, 9 Hz and 12 Hz, respectively. The flow condition is QI = 6 μl/min, QM = 14 μl/min, and QO = 180 μl/min (Also see supplementary movie S1). Microscope photographs shown in Fig. 4d have depicted different double emulsions with different number of inner droplets. We can conclude that generation of double emulsions with controllable inner droplet number can be easily achieved by only altering the excitation frequencies, which avoids the complicated operation of flow rates for different phases to produce different double emulsions.
Figure 4.
(a) Schematic of assembled active control device with PCU module and the illustration of the co-flow structure. (b) A printed sample of the assembled active control device. (c) High-speed video frames showing active control of W/O/W double emulsions with different encapsulated number of inner droplets. (d) Photographs of the different W/O/W double emulsions. (e) Plot of the generation frequency of the outer droplet at downstream versus the excitation frequency. Points with cross inside represent natural frequencies. (f) Plot of the encapsulated droplet number corresponding to the ratio of the excitation frequency and the generation frequency of outer droplets.
Passive generation of double emulsions
Single-inlet module for double emulsions
Here single-inlet module is chosen to be assembled with T-junction and co-flow modules as shown in Fig. 5a,b. The same surface treatment was made as described previously, and therefore W/O/W double emulsions can be produced. The liquids used are listed in Table 1. By tuning the flow rates of different phases, double emulsions with different number of encapsulated droplets can be achieved, which is depicted in Fig. 5c (Also see supplementary movie S2). Microscope photographs shown in Fig. 5d have depicted different double emulsions with different number of inner droplets.
Figure 5.
(a) Schematic and (b) the printed sample of assembled passive double emulsion generation device with single inlet module, respectively. (c) High-speed video frames showing the passive generation of W/O/W double emulsions with different number of inner droplets. (d) Photographs of different W/O/W double emulsions.
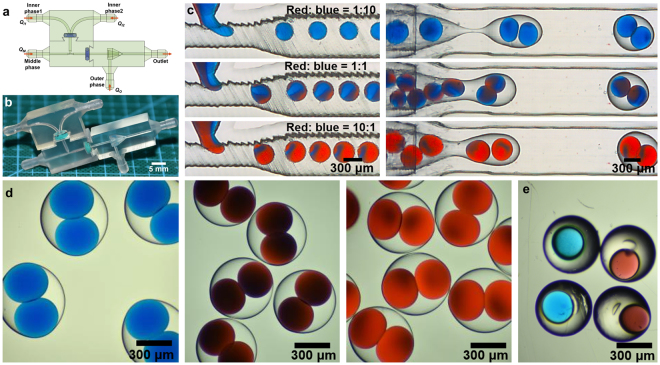
Dual-inlet module for double emulsions
Here dual-inlet module is chosen to assemble with T-junction and co-flow modules as shown in Fig. 6a,b. QI1, QI2, QM and QO are the flow rates of the two inner aqueous phases, middle phase and outer phase, respectively. This chip is used for the passive generation of double emulsions with different compositions of inner droplets. The same surface treatment was made as described previously, and therefore W/O/W double emulsions can be produced. Due to the characteristic of the laminar flow in microfluidics, the ratio of two phase volume coming into the droplet depends linearly on the flow rate ratio of two phases as shown in Fig. 6c. Therefore, different compositions of droplets can be acquired accurately after these two phases mix or react inside the droplet by tuning the flow rate ratio. Here we use two inner aqueous phases with different soluble food dyes (red and blue) as a prototyping demonstration. By tuning the flow rate ratio of these two aqueous phases, different compositions of the inner droplets (different mixed colors represent different compositions here) can be achieved, and then the double emulsions encapsulated these droplets are formed at downstream as shown in Fig. 6c (Also see supplementary movie S3). Microscope photographs shown in Fig. 6d have depicted different double emulsions with different compositions. Finally, we use the same device and replace the middle phase with commercial photopolymer as the solidification phase for fabricating functional microspheres containing different compositions which are shown in Fig. 6e. The liquids used are listed in Table 1. Due to the excellent optical properties, transparent microspheres containing droplets with different color can be further applied in optics fields. The structure reported here is painful for other fabrication methods and also these double emulsions with different compositions we produce are difficult to achieve using other methods.
Figure 6.
(a) and (b) is schematic and the printed sample of assembled double emulsion generation device with dual inlet module, respectively. (c) High-speed video frames showing generation of W/O/W double emulsions with different compositions of inner droplets. (d) Photographs of the W/O/W double emulsion with different compositions of inner droplets. (e) Photographs of the microspheres containing different compositions.
Conclusions
A PCU device has been designed and fabricated monolithically based on multimaterial 3DP. A 3D coaxial flexible channel is printed by a rubber-like material (TangoPlus FLX930) and other rigid structures are printed by a photopolymer (Veroclear). The numerical results and experiments have demonstrated that this flexible channel can be deformed by the air pressure excitation in a controllable manner. Such deformation can result in additional variation of fluid flow rate when liquids flow through this channel. Therefore, the droplet generation can be actively controlled by tuning the flow rate of the dispersed phase which flow through this flexible channel excited by the air pressure, and then different regimes have been discovered and studied in detail by changing excitation frequency, excitation amplitude and flow rates of both phases. In the synchronized regime, droplet generation frequency fully follows the excitation frequency, and highly monodisperse emulsion droplets can be produced.
Traditional studies58–60 usually use polydimethylsiloxane (PDMS) with actuation sources to actively control droplet generation, which requires complicated fabrication process. A monolithic device with different parts can be one-step achieved by multimaterial 3DP technology, which avoids the complicated fabrication process. On the other hand, due to the rigidity of PDMS, a thin PDMS membrane is always required to better translate the actuation. According to our measurement above, the TangoPlus material is softer and therefore thicker flexible channels can be printed, which can ensure excellent translation of actuation and also provide more stability and robustness. Furthermore, a fully 3D coaxial structure can be achieved using multimaterial 3DP technology, which can provide 3D homogeneous deformation for the droplet generation
Furthermore, 3D-printed modular microfluidic devices for multiple emulsion production have been developed, which consist of three modules: function module, T-junction module and co-flow module. The T-junction module is used for the generation of inner droplets, and the co-flow module is used for the generation of outer droplets. The function module can be replaced by single-inlet module, dual-inlet module and PCU module. With assembly of different modules, versatile double emulsions can be produced. By utilizing the synchronized regime abovementioned, double emulsions with different inner number of droplets have been successfully generated without complicated operation of flow rates of different phases. By utilizing the dual-inlet module, double emulsions with inner droplets with different compositions can also be produced successfully. Additionally, transparent microspheres containing different color droplets have been produced, which can be used in the optics field.
We highlight the importance and advantage of our work proposing a modular strategy and combining different 3DP technologies, which can achieve production of versatile complicated multiple emulsions in both active and passive manner by assembly of different modules. This concept and strategy can be further applied in other fields where 3DP is popular such as material engineering and bioengineering. In future, we believe that more complicated devices for microfluidics and more controllable methods for emulsion generation can be developed based on rapidly developing 3DP technologies, especially for multimaterial 3DP.
Electronic supplementary material
Acknowledgements
The financial support from the Beijing Natural Science Foundation under Grants No. L172002, National Natural Science Foundation of China under Grants No. 11632001, No. 11521202 and No. 11702003 are gratefully acknowledged.
Author Contributions
J.Z. conceived the project. Q.J. and Y.L. conducted the experiments and performed the numerical analysis. Q.J., J. Z., X.L., P.L. and D.J. analyzed the results. Q.J. and J.Z. wrote the manuscript. H.D. supervised the study. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Qinglei Ji and Jia Ming Zhang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22756-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 2.Seemann R, Brinkmann M, Pfohl T, Herminghaus S. Droplet based microfluidics. Reports on progress in physics. 2011;75:016601. doi: 10.1088/0034-4885/75/1/016601. [DOI] [PubMed] [Google Scholar]
- 3.Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–189. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 4.Deng, Y. et al. An integrated microfluidic chip system for single-cell secretion profiling of rare circulating tumor cells. Scientific reports4 (2014). [DOI] [PMC free article] [PubMed]
- 5.Zhu Y, Fang Q. Analytical detection techniques for droplet microfluidics—A review. Analytica chimica acta. 2013;787:24–35. doi: 10.1016/j.aca.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, et al. Droplet microfluidics for producing functional microparticles. Langmuir. 2014;30:1473–1488. doi: 10.1021/la403220p. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, et al. Controllable microfluidic strategies for fabricating microparticles using emulsions as templates. Particuology. 2016;24:18–31. doi: 10.1016/j.partic.2015.08.001. [DOI] [Google Scholar]
- 8.Erkal JL, et al. 3D printed microfluidic devices with integrated versatile and reusable electrodes. Lab on a Chip. 2014;14:2023–2032. doi: 10.1039/C4LC00171K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nisisako T, Torii T, Higuchi T. Novel microreactors for functional polymer beads. Chemical Engineering Journal. 2004;101:23–29. doi: 10.1016/j.cej.2003.11.019. [DOI] [Google Scholar]
- 10.Lee M-H, Oh S-G, Moon S-K, Bae S-Y. Preparation of silica particles encapsulating retinol using O/W/O multiple emulsions. Journal of colloid and interface science. 2001;240:83–89. doi: 10.1006/jcis.2001.7699. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Chan HF, Leong KW. Advanced materials and processing for drug delivery: the past and the future. Advanced drug delivery reviews. 2013;65:104–120. doi: 10.1016/j.addr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muschiolik G. Multiple emulsions for food use. Current Opinion in Colloid & Interface Science. 2007;12:213–220. doi: 10.1016/j.cocis.2007.07.006. [DOI] [Google Scholar]
- 13.Xia Y, Whitesides GM. Soft lithography. Annual review of materials science. 1998;28:153–184. doi: 10.1146/annurev.matsci.28.1.153. [DOI] [Google Scholar]
- 14.Garstecki P, Fuerstman MJ, Stone HA, Whitesides GM. Formation of droplets and bubbles in a microfluidic T-junction—scaling and mechanism of break-up. Lab on a Chip. 2006;6:437–446. doi: 10.1039/b510841a. [DOI] [PubMed] [Google Scholar]
- 15.Anna SL, Bontoux N, Stone HA. Formation of dispersions using “flow focusing” in microchannels. Applied physics letters. 2003;82:364–366. doi: 10.1063/1.1537519. [DOI] [Google Scholar]
- 16.Nisisako T, Torii T, Takahashi T, Takizawa Y. Synthesis of monodisperse bicolored janus particles with electrical anisotropy using a microfluidic Co‐Flow system. Advanced Materials. 2006;18:1152–1156. doi: 10.1002/adma.200502431. [DOI] [Google Scholar]
- 17.Waheed S, et al. 3D printed microfluidic devices: enablers and barriers. Lab on a Chip. 2016;16:1993–2013. doi: 10.1039/C6LC00284F. [DOI] [PubMed] [Google Scholar]
- 18.Utada A, et al. Monodisperse double emulsions generated from a microcapillary device. Science. 2005;308:537–541. doi: 10.1126/science.1109164. [DOI] [PubMed] [Google Scholar]
- 19.Li EQ, Zhang JM, Thoroddsen ST. Simple and inexpensive microfluidic devices for the generation of monodisperse multiple emulsions. Journal of Micromechanics and Microengineering. 2013;24:015019. doi: 10.1088/0960-1317/24/1/015019. [DOI] [Google Scholar]
- 20.Zhang JM, Li EQ, Thoroddsen ST. A co-flow-focusing monodisperse microbubble generator. Journal of Micromechanics and Microengineering. 2014;24:035008. doi: 10.1088/0960-1317/24/3/035008. [DOI] [Google Scholar]
- 21.Chu LY, Utada AS, Shah RK, Kim JW, Weitz DA. Controllable monodisperse multiple emulsions. Angewandte Chemie International Edition. 2007;46:8970–8974. doi: 10.1002/anie.200701358. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H, et al. Printing@ clinic: from medical models to organ implants. ACS Biomaterials Science & Engineering. 2017;3:3083–3097. doi: 10.1021/acsbiomaterials.7b00542. [DOI] [PubMed] [Google Scholar]
- 23.Liu A, et al. 3D printing surgical implants at the clinic: a experimental study on anterior cruciate ligament reconstruction. Scientific reports. 2016;6:21704. doi: 10.1038/srep21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, et al. Fabrication of cerebral aneurysm simulator with a desktop 3D printer. Scientific Reports. 2017;7:44301. doi: 10.1038/srep44301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Y, et al. Research on the printability of hydrogels in 3D bioprinting. Scientific reports. 2016;6:29977. doi: 10.1038/srep29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun M, et al. Systematical evaluation of mechanically strong 3D printed diluted magnesium doping wollastonite scaffolds on osteogenic capacity in rabbit calvarial defects. Scientific reports. 2016;6:34029. doi: 10.1038/srep34029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Au AK, Huynh W, Horowitz LF, Folch A. 3D‐printed microfluidics. Angewandte Chemie International Edition. 2016;55:3862–3881. doi: 10.1002/anie.201504382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharjee N, Urrios A, Kang S, Folch A. The upcoming 3D-printing revolution in microfluidics. Lab on a Chip. 2016;16:1720–1742. doi: 10.1039/C6LC00163G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He Y, Wu Y, Fu Jz, Gao Q, Qiu Jj. Developments of 3D printing microfluidics and applications in chemistry and biology: a review. Electroanalysis. 2016;28:1658–1678. doi: 10.1002/elan.201600043. [DOI] [Google Scholar]
- 30.Shallan AI, Smejkal P, Corban M, Guijt RM, Breadmore MC. Cost-effective three-dimensional printing of visibly transparent microchips within minutes. Analytical chemistry. 2014;86:3124–3130. doi: 10.1021/ac4041857. [DOI] [PubMed] [Google Scholar]
- 31.Li F, Macdonald NP, Guijt RM, Breadmore MC. Using Printing Orientation for Tuning Fluidic Behavior in Microfluidic Chips Made by Fused Deposition Modeling 3D Printing. Analytical chemistry. 2017;89:12805–12811. doi: 10.1021/acs.analchem.7b03228. [DOI] [PubMed] [Google Scholar]
- 32.Zhang JM, Aguirre-Pablo AA, Li EQ, Buttner U, Thoroddsen ST. Droplet generation in cross-flow for cost-effective 3D-printed “plug-and-play” microfluidic devices. RSC Advances. 2016;6:81120–81129. doi: 10.1039/C6RA11724D. [DOI] [Google Scholar]
- 33.Zhang JM, Li EQ, Aguirre-Pablo AA, Thoroddsen ST. A simple and low-cost fully 3D-printed non-planar emulsion generator. RSC Advances. 2016;6:2793–2799. doi: 10.1039/C5RA23129A. [DOI] [Google Scholar]
- 34.Symes MD, et al. Integrated 3D-printed reactionware for chemical synthesis and analysis. Nature Chemistry. 2012;4:349–354. doi: 10.1038/nchem.1313. [DOI] [PubMed] [Google Scholar]
- 35.Kitson PJ, Rosnes MH, Sans V, Dragone V, Cronin L. Configurable 3D-Printed millifluidic and microfluidic ‘lab on a chip’reactionware devices. Lab on a Chip. 2012;12:3267–3271. doi: 10.1039/c2lc40761b. [DOI] [PubMed] [Google Scholar]
- 36.Lee W, et al. 3D-printed microfluidic device for the detection of pathogenic bacteria using size-based separation in helical channel with trapezoid cross-section. Scientific reports. 2015;5:7717. doi: 10.1038/srep07717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comina G, Suska A, Filippini D. 3D printed unibody lab-on-a-chip: features survey and check-valves integration. Micromachines. 2015;6:437–451. doi: 10.3390/mi6040437. [DOI] [Google Scholar]
- 38.Rogers CI, Qaderi K, Woolley AT, Nordin GP. 3D printed microfluidic devices with integrated valves. Biomicrofluidics. 2015;9:016501. doi: 10.1063/1.4905840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Au AK, Bhattacharjee N, Horowitz LF, Chang TC, Folch A. 3D-printed microfluidic automation. Lab on a chip. 2015;15:1934–1941. doi: 10.1039/C5LC00126A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urrios A, et al. 3D-printing of transparent bio-microfluidic devices in PEG-DA. Lab on a Chip. 2016;16:2287–2294. doi: 10.1039/C6LC00153J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Y, et al. Printing 3D microfluidic chips with a 3D sugar printer. Microfluidics and Nanofluidics. 2015;19:447–456. doi: 10.1007/s10404-015-1571-7. [DOI] [Google Scholar]
- 42.Hardin JO, Ober TJ, Valentine AD, Lewis JA. Microfluidic printheads for multimaterial 3D printing of viscoelastic inks. Advanced materials. 2015;27:3279–3284. doi: 10.1002/adma.201500222. [DOI] [PubMed] [Google Scholar]
- 43.Truby RL, Lewis JA. Printing soft matter in three dimensions. Nature. 2016;540:371. doi: 10.1038/nature21003. [DOI] [PubMed] [Google Scholar]
- 44.Lee H, Cho D-W. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab on a Chip. 2016;16:2618–2625. doi: 10.1039/C6LC00450D. [DOI] [PubMed] [Google Scholar]
- 45.Paydar O, et al. Characterization of 3D-printed microfluidic chip interconnects with integrated O-rings. Sensors and Actuators A: Physical. 2014;205:199–203. doi: 10.1016/j.sna.2013.11.005. [DOI] [Google Scholar]
- 46.Anderson KB, Lockwood SY, Martin RS, Spence DM. A 3D printed fluidic device that enables integrated features. Analytical chemistry. 2013;85:5622–5626. doi: 10.1021/ac4009594. [DOI] [PubMed] [Google Scholar]
- 47.Lockwood SY, Meisel JE, Monsma FJ, Jr., Spence DM. A Diffusion-Based and dynamic 3D-Printed device that enables parallel in vitro pharmacokinetic profiling of molecules. Analytical chemistry. 2016;88:1864–1870. doi: 10.1021/acs.analchem.5b04270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Begolo S, Zhukov DV, Selck DA, Li L, Ismagilov RF. The pumping lid: investigating multi-material 3D printing for equipment-free, programmable generation of positive and negative pressures for microfluidic applications. Lab on a Chip. 2014;14:4616–4628. doi: 10.1039/C4LC00910J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor AP, Velásquez-García LF. Miniaturized diaphragm vacuum pump by multi-material additive manufacturing. Journal of Microelectromechanical Systems. 2017;26:1316–1326. doi: 10.1109/JMEMS.2017.2743020. [DOI] [Google Scholar]
- 50.Keating SJ, et al. 3D printed multimaterial microfluidic valve. PloS one. 2016;11:e0160624. doi: 10.1371/journal.pone.0160624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sochol R, et al. 3D printed microfluidic circuitry via multijet-based additive manufacturing. Lab on a Chip. 2016;16:668–678. doi: 10.1039/C5LC01389E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bransky A, Korin N, Khoury M, Levenberg S. A microfluidic droplet generator based on a piezoelectric actuator. Lab on a Chip. 2009;9:516–520. doi: 10.1039/B814810D. [DOI] [PubMed] [Google Scholar]
- 53.Zhu P, Tang X, Wang L. Droplet generation in co-flow microfluidic channels with vibration. Microfluidics and Nanofluidics. 2016;20:47. doi: 10.1007/s10404-016-1717-2. [DOI] [Google Scholar]
- 54.Zhu P, Wang L. Passive and active droplet generation with microfluidics: a review. Lab on a Chip. 2017;17:34–75. doi: 10.1039/C6LC01018K. [DOI] [PubMed] [Google Scholar]
- 55.Bauer W-AC, Fischlechner M, Abell C, Huck WT. Hydrophilic PDMS microchannels for high-throughput formation of oil-in-water microdroplets and water-in-oil-in-water double emulsions. Lab on a Chip. 2010;10:1814–1819. doi: 10.1039/c004046k. [DOI] [PubMed] [Google Scholar]
- 56.Barbier V, et al. Stable modification of PDMS surface properties by plasma polymerization: application to the formation of double emulsions in microfluidic systems. Langmuir. 2006;22:5230–5232. doi: 10.1021/la053289c. [DOI] [PubMed] [Google Scholar]
- 57.Abate AR, et al. Photoreactive coating for high-contrast spatial patterning of microfluidic device wettability. Lab on a Chip. 2008;8:2157–2160. doi: 10.1039/b813405g. [DOI] [PubMed] [Google Scholar]
- 58.Cai B, et al. Three-dimensional valve-based controllable PDMS nozzle for dynamic modulation of droplet generation. Microfluidics and Nanofluidics. 2016;20:56. doi: 10.1007/s10404-016-1725-2. [DOI] [Google Scholar]
- 59.Raj A, Halder R, Sajeesh P, Sen A. Droplet generation in a microchannel with a controllable deformable wall. Microfluidics and Nanofluidics. 2016;20:1–16. doi: 10.1007/s10404-015-1676-z. [DOI] [Google Scholar]
- 60.Wu H-W, Huang Y-C, Wu C-L, Lee G-B. Exploitation of a microfluidic device capable of generating size-tunable droplets for gene delivery. Microfluidics and nanofluidics. 2009;7:45–56. doi: 10.1007/s10404-008-0359-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.