Abstract
The engineering of acetylenic carbon-rich nanostructures has great potential in many applications, such as nanoelectronics, chemical sensors, energy storage, and conversion, etc. Here we show the synthesis of acetylenic carbon-rich nanofibers via copper-surface-mediated Glaser polycondensation of 1,3,5-triethynylbenzene on a variety of conducting (e.g., copper, graphite, fluorine-doped tin oxide, and titanium) and non-conducting (e.g., Kapton, glass, and silicon dioxide) substrates. The obtained nanofibers (with optical bandgap of 2.51 eV) exhibit photocatalytic activity in photoelectrochemical cells, yielding saturated cathodic photocurrent of ca. 10 µA cm−2 (0.3–0 V vs. reversible hydrogen electrode). By incorporating thieno[3,2-b]thiophene units into the nanofibers, a redshift (ca. 100 nm) of light absorption edge and twofold of the photocurrent are achieved, rivalling those of state-of-the-art metal-free photocathodes (e.g., graphitic carbon nitride of 0.1–1 µA cm−2). This work highlights the promise of utilizing acetylenic carbon-rich materials as efficient and sustainable photocathodes for water reduction
While photoelectrochemical devices combine light-absorption with fuel and electricity generation, their implementation is hampered by high costs and low output. Here, the authors synthesized acetylene-rich carbon fibers by copper-mediated polymerization for high-activity, metal-free photocathodes.
Introduction
Photoelectrochemical cells (PECs) offer the promise of producing electric energy and hydrogen through artificial photosynthesis by integrating the collection of solar energy and the electrolysis of water into a photoelectrode1. PECs are based on photochemical reactions at the junction of semiconductor and electrolyte, in which electrons and holes that generated upon solar absorption by semiconductors (p-type or n-type) are driven into electrolyte solution by applied electric field at the junction, driving a redox reaction, e.g., the reduction of H+ to H2 for p-type semiconductor2. To enable their practical use in the field of environmental and clean energy, semiconductor materials need to be low-cost and prepared from abundant resources using scalable approaches1,3,4, which preclude the utilization of the most reported, efficient PECs systems, such as metal oxides2,5,6, metal chalcogenides, and transition-metal dichalcogenides7–10.
Synthetic conjugated polymers, composed of a delocalized π-electron system, present a new generation of sustainable semiconductors for solar-energy utilization1,3,11–13. They offer tunable energy levels, low-cost facile synthesis, and respectable solid-state charge-transport characteristics. These promising characteristics have motivated intense investigation into the design and synthesis of conjugated polymer semiconductors for photocatalytic H2 evolution3,11,12,14,15. The most representative material is graphitic carbon nitride (g-C3N4)11,16–19 and many analogs and composites have also been reported, such as poly(azomethine)s20, hydrazone-based covalent organic frameworks21, triazine-based frameworks22, and biopolymer-activated g-C3N423. Recently, pyrene-based conjugated polymers have shown promising performance in direct solar water reduction24–26, suggesting that carbon-rich frameworks are a new family of synthetic polymer semiconductors for solar-to-chemical conversion.
Acetylenic carbon-rich materials (e.g., graphyne, graphdiyne, and related analogs), containing diacetylenic linkages between carbon hexagons in an extended π-conjugation structure, are predicted to exhibit unique electronic, optical, and mechanical properties27–30. Recently, the great potential of acetylenic carbon-rich materials as photocatalysts was illustrated by the visible-light-driven degradation of water pollutants (i.e., phenol and methyl orange) using bulk poly(diphenylbutadiyne) nanofibers29.
In this study, we report an efficient and generic approach for scalable fabrication of acetylenic carbon-rich nanofibers through a Cu-surface mediated Glaser polycondensation. Large-area (up to 4 × 12 cm) poly(1,3,5-triethynylbenzene) (PTEB) nanofiber films (with thicknesses from several to hundreds of nanometers) can be grown on various conducting and non-conducting substrates. The obtained PTEB nanofibers are interconnected and have a broad range of visible light absorption (up to 500 nm), corresponding to an optical bandgap of 2.51 eV. We demonstrate that the nanofibers synthesized on conductive substrates can function as metal-free photocathodes for PEC devices, and a saturated photocurrent density up to ca. 10 µA cm−2 can be achieved at 0.3–0 V vs. reversible hydrogen electrode (RHE). Furthermore, we show that the PTEB photocathode, by incorporating of thieno[3,2-b]thiophene unit in the conjugated frameworks, exhibited a ca. 100 nm redshift of the absorption edge and a twofold enhancement in the photocurrent, which is superior to those of state-of-the-art metal-free photocathode materials (in the range of 0.1–1 µA cm−2; Supplementary Table 1). These results indicate that the Cu-surface-mediated synthetic approach is promising to directly fabricate various acetylenic carbon-rich nanomaterials as photocathodes for PECs.
Results
Synthesis and structure characterization
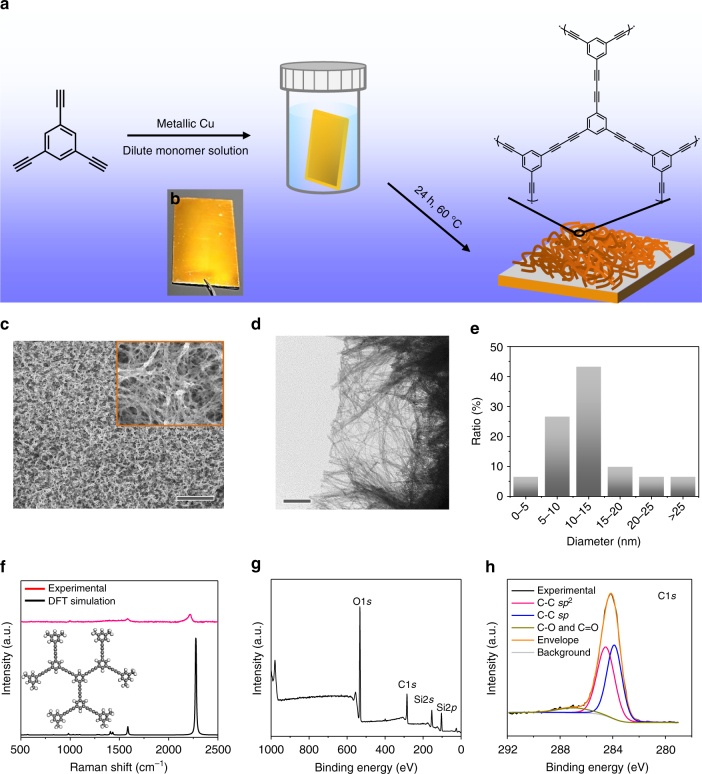
The impetus for the current synthetic strategy is derived from the observation that metallic copper is able to produce CuI/II species in polar liquids or alkaline solutions31,32. As both CuI and CuII salts have been widely used as catalysts for Glaser coupling reaction33,34, we expected that the Cu species generated from the metallic copper surface would be able to catalyze the C–C coupling reaction, which could afford acetylenic frameworks on a solid substrate (Supplementary Fig. 1). As illustrated in Fig. 1a, a clean Cu wafer was immersed in a mixture of 1,3,5-triethynylbenzene (TEB, 0.5 mg mL−1) and ligand (piperidine, 1 µL mL−1) in pyridine at 60 °C for 24 h. The C–C coupling of TEB occurred at the Cu–liquid interface where various Cu species were dissolved, yielding a yellowish PTEB framework deposited directly on the Cu wafer surface (Fig. 1b). In this process, the copper wafer is not only the catalyst (i.e., CuI and CuII) source for Glaser coupling reaction but also the substrate for the growth of PTEB nanofibers.
Fig. 1.
Synthesis and characterization of PTEB nanofibers. a Reaction scheme and employed molecules. b Photograph of the PTEB layer synthesized on a Cu wafer. The coupling reaction occurs only at the Cu–liquid interface, resulting in the selective formation of PTEB netwrok on Cu surface. c Scanning electron microscopy (SEM) images of PTEB nanofibers at Cu surface, scale bar: 10 µm. Inset: magnification of c. d Transmission electron microscopy (TEM) image of PTEB grown on a Cu grid, scale bar: 200 nm. e Histogram of the diameter of the PTEB nanofibers measured from d. The agreement between the Raman spectra obtained from f the experiment and density functional theory (DFT) calculations confirm the generation of diacetylenic linkages in PTEB. g XPS survey spectrum and h high-resolution C1s core level spectrum of PTEB on a SiO2/Si wafer. The fitting in h was performed with a set of Voigt peaks
The scanning electron microscopy (SEM) images in Fig. 1c demonstrate that the obtained PTEB nanofibers are distributed uniformly over the entire Cu surface. However, some nanofibers tended to adhere together, resulting in larger bundles ranging from 20 to 80 nm in diameter (Fig. 1c, inset). Individual nanofibers ranging from 5 to 15 nm in diameter could be clearly observed on PTEB (grown on a Cu grid) using transmission electron microscopy (TEM) (Fig. 1d and e). We found that PTEB was grown on the Cu surface at an constant rate of δd = 10 nm h−1 within 72 h (Supplementary Fig. 2a); therefore, the thickness of the PTEB nanofiber film is controllable in a quite broad range from several (e.g., 6.7 nm) to hundreds of nanometers (e.g., 750 nm) by varying the reaction time (Supplementary Figs. 2b-d). For instance, a 230 nm-thick film of PTEB nanofibers was obtained after 24 h of reaction on the Cu surface (Supplementary Fig. 3a) and further energy dispersive X-ray (EDX) elemental mapping images provide a clear contrast of different layers (PTEB, Cu, and Si) on the cross-section (Supplementary Figs. 3b-g). The film was rather robust and capable of handling and transferring onto arbitrary substrates (e.g., aluminum foil; Supplementary Fig. 4) after etching away Cu with aqueous ammonium persulfate solution (0.1 g mL−1) using the standard poly(methyl methacrylate) (PMMA) method (see Methods). The scalability was demonstrated by the preparation of a large-area PTEB film (4 × 12 cm; Supplementary Fig. 5) using only 10 mL of the dilute reaction solution, as indicated above. The resulting nanofiber film was uniform over the entire surface.
Raman spectroscopy has been shown to be one of the best techniques to study the structure of carbon-rich materials and identify diacetylenic moieties35. The presence of the Raman line at 2209 cm−1 in the PTEB nanofibers (Fig. 1f), assigned to the C≡C stretching, is a strong evidence for the formation of conjugated diacetylenic linkages due to the reaction of the terminal alkyne (which exhibits a C≡C stretch at 2106 cm−1; Supplementary Fig. 6)36,37. The Raman peaks at 989 and 1581 cm−1 can be assigned respectively to the ring breathing and ring stretching of aromatic moieties38. The observed peaks match well with the simulated Raman signals obtained from density functional theory (DFT) calculations, which supports the proposed assignments (Methods, Fig. 1f, and Supplementary Figs. 7-9). It should be noted that the slight mismatch of the simulated C≡C stretching peak is expected, partly because of the much higher polymer chain length of the as-prepared PTEB nanofibers (the PTEB model used in DFT calculation)39, and partly because of intermolecular interactions not included in the model, but operating in the bulk material. X-ray photoelectron spectroscopy (XPS) (Fig. 1g) reveals that the PTEB film contains only elemental carbon. Deconvolution of the C 1 s core level spectra (at 284.1 eV) displays the major fractions of sp1 and sp2 hybridized carbons with binding energies at 283.8 and 284.5 eV, respectively (Fig. 1h)40–42. The structure of PTEB was further confirmed by additional characterizations using Fourier transform infrared spectroscopy (FTIR; Supplementary Fig. 10).
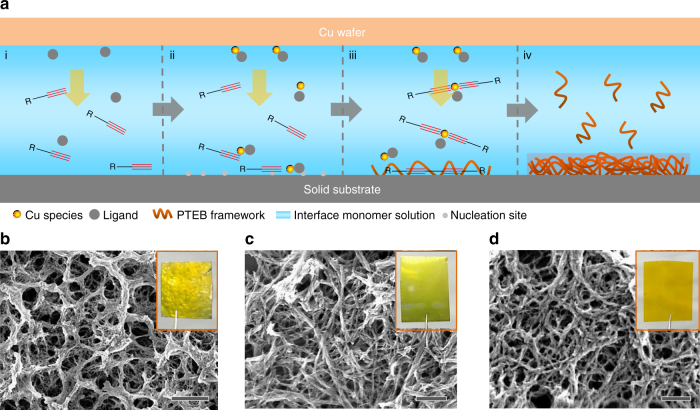
Although we demonstrated that PTEB nanofibers fabricated on Cu are able to be transferred onto arbitrary substrates (Supplementary Fig. 4), the direct growth of nanofibers on a target substrate enables high structural stability and excellent mechanical adhesion, which are required for the fabrication of high-performance devices with long-term stability43. It was reported that Cu species (i.e., CuI and CuII) generated from a metallic Cu surface could out-diffuse to initiate controlled radical polymerizations on the surface of a facing substrate (Fig. 2a)31,32. These results led us to believe that the Glaser coupling reaction, which is catalyzed by similar Cu species, is likely to be achieved on other substrates with the assistance of a Cu wafer. To this end, a bare and planar substrate (e.g., graphite foil) was sandwiched with a Cu wafer and immersed in the reaction solution as above. After an indicated time interval, the Cu wafer was separated and a uniform PTEB layer was observed on the graphite after thoroughly cleaning with various solvents (e.g., pyridine, dichloromethane, and methanol, sequentially). Finally, a wide variety of substrates, including conducting (e.g., graphite foil, nickel, titanium, Si wafer, and fluorine-doped tin oxide (FTO) glass (Fig. 2b, c and Supplementary Fig. 11a and d) and non-conducting substrates (e.g., Kapton foil, glass, and SiO2 wafer) (Fig. 2d and Supplementary Fig. 11c), were coated with PTEB films by this approach. More interestingly, the morphologies of the PTEB frameworks vary with the type of substrate. For instance, isolated PTEB nanofibers were observed on the surface of nickel (ca. 8 nm), FTO (ca. 9 nm), Kapton (ca. 10 nm) and glass (ca. 13 nm), whereas the nanofibers obtained on graphite (ca. 40 nm) and titanium surfaces (ca. 15 nm) tended to form large bundles. This result implies that the surface properties of the substrate had a crucial role in the formation of PTEB nanofibers of distinct morphologies, due to heterogeneous nucleation and polymerization process at each substrate (Fig. 2a). Control experiments showed that no such nanofibers were formed when Cu salts (used in classical Glaser coupling) or high concentrations of monomer (i.e., 5 mg mL−1) were applied.
Fig. 2.
Synthesis of PTEB nanofibers on various substrates. a Illustration of the synthetic strategy: (i) catalytic Cu species, generated on the surface of the Cu wafer, out-diffuse at the interface with the assistance of a ligand; (ii) the solubilized Cu species react with alkyne terminal monomers (i.e., TEB) and catalyze Glaser polycondensation at the confined interface; (iii) the facing substrate offers nucleation sites to attach oligomers and polymers forming nanofibers; (iv) PTEB nanofibers grow continuously until complete consumption of the monomer at the confined interface. The distance between the Cu wafer and substrate is ca. 0.2 mm. SEM images of the PTEB nanofibers grown on different substrates: b graphite foil, c nickel plate, and d Kapton foil. Insets: photographs of each sample. Scale bar: b 1 µm, c and d 100 nm
Formation mechanism
Based on these results, we propose a mechanism according to a previous model describing the synthesis of polyaniline nanofibers on a solid substrate via stepwise electrochemical polymerization44,45. Typically, two plausible nucleation sites are proposed, i.e., bulk solution and solid substrate, in the synthesis of PTEB through the Cu-surface-mediated approach. These two nucleation sites compete with each other. For example, concentrated monomer (or catalyst) leads to identical or even faster polymerization of TEB in solution than that of on solid substrates. However, in a dilute monomer (or catalyst) solution, heterogeneous nucleation and polymerization preferably occurs on solid substrate. As such, many reactive nucleation centers can be formed on the solid substrate at a faster rate than in solution at the beginning of the reaction (Fig. 2a). These initial nucleation sites minimize the energy barrier at the interface for the formation of PTEB nanofibers on various substrates. Therefore, the reason for the varied PTEB morphologies on different substrates could be put forward as: different substrates have different surface energy and roughness that resulted in varied nucleation rates, which not only affected the reaction kinetics of Glaser coupling at the interface, but also the approximation of monomer to the surface of substrate.
Following this scenario, micro-patterned PTEB structures were prepared using a Cu grid closely attached to the substrate as both a catalyst source and a stencil mask (see Methods). Glaser polycondensation can be initiated in the mesh region of the grid, at which the monomer solution and solubilized Cu species are able to interact. In the end, well-defined hexagonal arrays of PTEB frameworks with diameters of ca. 40 μm were fabricated on the SiO2 substrate (Supplementary Figs. 12a-c). Furthermore, the carbon elemental mapping based on EDX spectroscopy confirmed the PTEB patterning (Supplementary Fig. 12d). The corresponding EDX spectra show that the sample contains carbon, oxygen, and silicon, where oxygen and silicon originate from the substrate. Such stencil lithography allows Glaser polycondensation on planar substrates to be spatially patterned over a large area without the use of disruptive materials (e.g., photoresists or chemical etchants), demonstrating the wide potential of this technique46,47.
Optical and electronic structure characterization
The ultraviolet-visible (UV-vis) absorption spectrum of PTEB shows several transition modes in visible wavelengths with an absorption edge of 500 nm (Fig. 3a), and the film has a goldish color on a transparent PET substrate (Fig. 3a, inset). The transmittance spectrum (Supplementary Fig. 13) reveals a ca. 80% transmittance at λ = 550 nm48,49; meanwhile, a maximum ca. 70% absorption is observed for the PTEB layer at λ = 490 nm, corresponding to an average thickness of ca. 230 nm. We note that there is no obvious difference in the absorption spectra of the PTEB nanofibers grown on different substrates (Supplementary Fig. 14).
Fig. 3.
Optical and band structure of PTEB nanofibers. a UV-vis absorption spectrum. Inset: digital photograph of the PTEB nanofiber film (ca. 230 nm) transferred to a PET substrate. b (hvα)2 vs. hv curve (black curve). The value at the intersection of two dashed red lines of baseline and the tangent of the curve is the bandgap: Ebg = 2.51 eV. c UPS spectrum (black curve). The dashed red lines mark the baseline and the tangents of the curve. The edges of the UPS spectrum are given by the intersections of two dashed red lines of the tangents and the baseline, from which the UPS width is determined. d Band structure diagram of the PTEB compared with g-C3N4. In all panels, the average thickness of the PTEB films is ca. 230 nm
The optical bandgap (Ebg) estimated from the Tauc plot [i.e., plotting (αhν)r vs. hν, where α is absorption coefficient, h Planck constant, ν photon frequency, and r = 2 for a direct bandgap material] is ~ 2.51 eV (Fig. 3b)3. Such bandgap is sufficient to overcome the theoretical endothermic-change in the process of water-splitting (i.e., 1.23 eV). In addition to an appropriate bandgap, the conduction band of a material need to match the donating energy level of water and the valence band match the electron accepting water level that are important to water splitting in PECs. Thus, ultraviolet photoelectron spectroscopy (UPS) was used to determine the energy level of valence band (i.e., Evb) of PTEB. The Evb 6.63 eV was calculated by subtracting the UPS width (Fig. 3c) from excitation energy (HeI, 21.22 eV). Furthermore, the conduction band energy Ecb was determined to be 4.12 eV from Evb – Ebg. These values (in vacuum level) were converted to electrochemical potentials according to standard reference electrode, e.g., 0 V vs. RHE is equal to − 4.44 eV vs. vacuum level3. We can see from Fig. 3d that the reduction energy level for H2O to H2 is located below the Ecb of PTEB and the oxidation energy level for H2O to O2 is above the Evb of PTEB, which agrees with DFT calculation results (Supplementary Fig. 15). The electronic band structures of PTEB are consistent with the theory of 1,3,5-graphdiyne reported by Barth et al.28 Thus, the proper position of the band structures of PTEB permit the efficient transfer of photo-generated electrons and holes, respectively, and promise the PTEB nanofibers as photoelectrodes for PECs for hydrogen production. Such optical properties are analogous to those of the most representative metal-free photoelectrode material, g-C3N411.
PEC characterization
PEC experiments were conducted with the PTEB nanofibers on a titanium substrate as photocathode in a solution of 0.01 M Na2SO4 (pH 6.8) (Fig. 4a). The PEC characterization was performed in a 3-electrode setup with applied bias relative to the reference electrode (Ag/AgCl). The PTEB photocathode gave an apparent photoresponse to light on/off switching at an applied bias under chopped irradiation (100 mW cm−2) (Fig. 4b and Supplementary Fig. 16). The appearance of the cathodic photocurrent suggests that PTEB has typical p-type semiconductor behavior50. In addition, a saturated cathodic photocurrent density of ca. 10 µA cm−2 was obtained on ca. 230 nm-thick PTEB below 0.3 V vs. RHE (after subtracting the dark current). This value is superior to most reported metal-free photoelectrodes, such as g-C3N417–19,23,51 and analogs23,52, red phosphorus53,54, and silicon carbide55, which are typically in the range of 0.1–1 µA cm−2 (Supplementary Table 1). In our own experiments, the g-C3N4-based photocathode, prepared according to a previously reported method56, yielded a much lower photocurrent of ca. 2 µA cm−2 than the PTEB photocathode (Fig. 4c). We can confirm that the observed photocurrent at PTEB electrodes is due to the light absorption of PTEB nanofibers, as the incident-photon-to-current (IPCE) spectrum matches well with the UV-Vis absorption spectrum (Supplementary Fig. 17). Moreover, we found that the photocurrent strongly related to the thickness of PTEB layer, and both thinner and thicker films gave lower photocurrent density (Supplementary Fig. 18). Therefore, the optimization of PTEB film thickness to reach equilibrium between the light adsorption capability and charge transfer efficiency is necessary to achieve the optimized PEC performance. When a consistent bias voltage of 0.5–0 V vs. RHE (i.e., − 0.1 to − 0.6 V vs. Ag/AgCl) was applied to the PTEB photoelectrode (Fig. 4d), respectively, the transient photocurrents exhibited good switching behavior at all applied bias voltages. A reduced charge-transport resistance under irradiation was detected for the PTEB photocathode, as the arc radius with light irradiation was lower than that in dark (Fig. 4e). These results verify that photoelectrons and holes were generated over the PTEB nanofibers under light irradiation. Given that PTEB is an organic polymer, the stability of the photocathode was further studied. An insignificant change in the photocurrent was observed over 2 h of constant irradiation (Fig. 4f), and the Raman spectrum remained unchanged after the PEC test (Supplementary Fig. 19), which suggest the good stability of the PTEB photocathodes.
Fig. 4.
PEC characterization of the PTEB nanofiber based photocathodes. a PEC cell with a PTEB photocathode under simulated sunlight irradiation (100 mW cm−2) in 0.01 M Na2SO4 aqueous solution. b Current density-potential curves vs. bias of PTEB under intermittent irradiation. c Transient current density vs. time at 0.3 V vs. RHE under intermittent light irradiation for PTEB (red curve) and g-C3N4 (black curve). d Photocurrent densities vs. time for PTEB with varied applied potentials (from 0.5 to 0 V vs. RHE) under intermittent irradiation; scale bar: 10 µA cm−2. e EIS Nyquist plots of PTEB at a voltage of 0.3 V vs. RHE under dark and light irradiation. f Current density and efficiencies vs. time of the PTEB electrode under illumination for 7500 s. Inset: magnification of 50–350 s, where light irradiation started at 100 s
PTEB-co-PDET copolymer photocathode
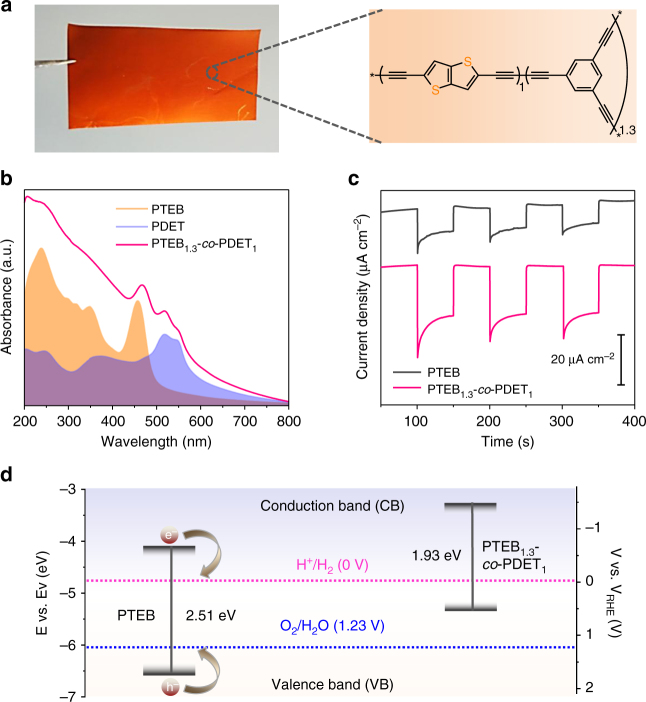
For polymeric photocathodes, one of the most important advantages is their rich synthetic modularity, allowing to tailor their structural, optical, and electronic properties. In general, the optical properties of a photocathode material can significantly affect its PEC performance, because photogenerated carriers under light irradiation are prerequisites for PEC reactions57. As one example, we show the tuning of the absorption spectrum, and hence the optical bandgap of the PTEB photocathode via the copolymerization of TEB with 2,5-diethynylthieno[3,2-b]thiophene (DET), which has been used extensively in the preparation of high-performance polymers for organic solar cells because of its wide absorption in visible-light region58. It can be clearly seen that the incorporation of DET monomer into the PTEB backbone greatly changes its color appearance (i.e., from yellow to red) (Fig. 5a), which results in a > 100 nm redshift of the absorption edge in the UV-vis spectrum (Fig. 5b). The relative loading of DET in the final copolymer structure was determined by EDX spectrum, which gave a structure with subunits PTEB1.3-co-PDET1 (Supplementary Fig. 20a). Although, SEM image reveals that the PTEB1.3-co-PDET1 copolymer showing larger nanofiber (bundle) morphology (Supplementary Fig. 20b), we found that the photocurrent density of the copolymer (PTEB1.3-co-PDET1) was obviously improved (more than twofolds) from ca. 10 µA cm−2 to ca. 21 µA cm−2 at 0 V vs. RHE (i.e., − 0.6 V vs. Ag/AgCl; Fig. 5c). This value is comparable to that of the B13C2 (ca. 16 µA cm−2 at – -0.76 V vs. Ag/AgCl, i.e., − 0.16 V vs. RHE) (Supplementary Table 1)50 and even inorganic 2D WSe2 thin films (ca. 40 µA cm−2 at 0 V vs. RHE, i.e., − 0.2 V vs. Ag/AgCl)9. The PTEB1.3-co-PDET1 has a conduction band minimum of − 1.47 V vs. NHE (Fig. 5d and Supplementary Fig. 21), which is much higher than the pristine PTEB (− 0.68 V) as well as theoretical value of H2 evolution (0 V vs. NHE). In addition, the conductivity, σ, of the PTEB1.3-co-PDET1 copolymer film was found to be of 1.9 × 10−5 S cm–1, which is an order of magnitude greater than pristine PTEB film (i.e., 3.0 × 10−6 S cm–1) (Supplementary Fig. 22). Thereby, it is reasonable to conclude that by introduction of PDET segment in the PTEB structure, not only the light absorption range was enlarged but also the electronic band structure and charge transfer capability for water reduction were improved for the PTEB1.3-co-PDET1 photocathode.
Fig. 5.
PTEB incorporated with poly[2,5-diethynylthieno[3,2-b]thiophene] (PDET) as photocathode. a Photograph of PTEB1.3-co-PDET1 grown on a Cu surface (left) and its chemical structure (right). b UV-vis absorption spectra of PTEB, PDET and PTEB1.3-co-PDET1, respectively. c Transient photocurrent density vs. time at a bias of 0 V vs. RHE under intermittent irradiation for PTEB1.3-co-PDET1 (red curve) and pristine PTEB (black curve). d Band structure diagram of the PTEB1.3-co-PDET1 compared with PTEB. In all panels, the average thickness of the polymer films is ca. 230 nm
Discussion
To gain insights on the PEC activity of PTEB nanofiber for water reduction, we evaluated the effects of the sacrificial reagents on photocurrent density of PTEB photocathode. In this respect, the transient photocurrent density of PTEB photocathode was examined in 0.01 M Na2SO4 electrolyte (pH 6.8) in the presence of electron scavenger (10−5 M Cu2+, it reacts with electron to yield Cu+)59. The reduction of Cu2+ is thermodynamically and kinetically more facile than the reduction of water. As such, the cathodic photocurrent density at PTEB nanofibers is noticeably accelerated (to ca. 18 µA cm−2 at 0.3 V vs. RHE) in the presence of Cu2+ (Supplementary Fig. 23). The significant enhancement of photocurrent implies that the electron-hole recombination rate decreased due to the reaction of Cu2+ with photogenerated electron, leaving excess holes at the photoelectrode. We analyzed the gaseous product from the PEC cell after irradiation using a gas chromatograph (GC) and a moderate amount of H2 production (2.53 µmol in 10 h at 0 V vs. RHE) was detected on PTEB cathode (Supplementary Fig. 24). The amount of H2 production is close to the value from theoretical calculation, suggesting the photocurrent of PTEB cathode mainly attributing to the PEC water reduction (Supplementary Fig. 24). Furthermore, in a PTEB nanofibers film-based photocatalytic cell60,61, a total amount of 11.4 µmol H2 gas was produced after 10 h reaction without noticeable deterioration of the activity within 30 h (Supplementary Fig. 25). The average H2 evolution rate of the PTEB nanofibers was about 1.14 µmol h−1, with an apparent quantum efficiency of 1.83% at 420 nm (see Methods). Notably, a particularly high rate of > 11,400 µmol h−1 g−1 for photocatalytic H2 evolution was obtained, if the mass weight (< 0.1 mg) of PTEB nanofibers film was considered. To gain more insights on the active sites of PTEB for H2 evolution reaction, the reaction process of proton adsorption–reduction–hydrogen adsorption was simulated using DFT calculations62 and the free-energy changes were calculated regarding to four different carbon atoms of PTEB (Fig. 6a). The reaction pathways for both single and dual sites H2 evolution from water reduction were studied, and corresponding free-energy variations indicate that site 1 and site 3 are favorable for single-site H2 evolution (Fig. 6b), and the sites 1 and 2 are favorable for dual-site H2 evolution (Fig. 6c). The DFT results imply that carbon atoms of benzene ring (in PTEB) are dominant active sites for photocatalytic H2 evolution, which agrees with the results from Cooper et al.24 proving that carbon-rich polymers (based on phenylenes and pyrenes) are able to catalyse photocatalytic H2 production from water.
Fig. 6.
DFT calculation to investigate the H2 evolution active sites. a Reaction cycles and active sites for single and dual sites H2 evolution from water. b Free-energy variations for H2 evolution via single site reaction pathway: 1, 2, 3, and 4 denote for different active sites as labeled in a. c Free-energy variations for H2 evolution via dual sites reaction pathway: sites 1–2, 2–3, and 3–4 denote for different active sites as labelled in a. *Catalyst (i.e., PTEB) surface
In this work, the superior PEC performance of the PTEB nanofibers can be shown in the following ways: first, the light adsorption region of a photoelectrode can significantly affect its usable light source. The PTEB nanofibers absorb light in a wide visible range from ultraviolet to blue region and show a similar profile as g-C3N4 (bandgap of 2.7 eV)11 with an absorption edge around 500 nm (bandgap of 2.51 eV) (Fig. 3a, b). This indicates that PTEB can be excited by broader regions of solar light and a large amount of electron and holes can be produced under irradiation. Second, the efficiency of electron-hole separation is another crucial factor that determines the performance of a photoelectrode. The interconnected nanofibrous structure of PTEB offers a short diffusion distance, which results in an enhanced charge transport and a high surface area for fast interfacial charge collection, largely contributing to the considerable PEC activity (Fig. 2b–d and Supplementary Figs. 11 and 26)43. More importantly, the direct growth of PTEB frameworks on conductive substrates can greatly enhance electron transfer and adhesion between the substrate and the active component and enhance the structural stability for long-term operation29,43.
In conclusion, the method described herein affords a facile and scalable approach for the synthesis of acetylenic carbon-rich nanofibers via Cu-surface mediated Glaser polycondensation. In this process, both conductive and non-conductive substrates can be uniformly coated with PTEB nanofibers; meanwhile, micro-patterned PTEB was achieved using a patterned Cu grid as a stencil mask. We demonstrated that the PTEB nanofibers fabricated on conductive substrates can be directly utilized as metal-free photocathodes in PEC for H2 production, affording a saturated photocurrent of ca. 10 µA cm−2 at 0.3–0 V vs. RHE. The achieved photocurrent is largely improved to ca. 21 µA cm−2 through the introduction of thieno[3,2-b]thiophene units in the backbone of PTEB framework. These results clearly illustrate that acetylenic PTEB frameworks can serve as a promising polymeric photocathode in PEC devices for hydrogen production. Owing to the diversity of terminal alkynes and the chemical tailorability of the C≡C triple bond (e.g., thiol-yne reaction63, cycloaddition with cyano-containing acceptor molecules64, and metal coordination65), it is feasible to further improve the PEC performance with a much broader set of acetylenic carbon-rich frameworks and composites. Therefore, this work offers opportunities in the development of metal-free photocathode materials for solar water reduction.
Methods
Materials
All the reagents were obtained from Sigma-Aldrich and used as received. Copper wafer (MicroChemicals GmbH, Germany): Prime CZ-Si wafer 4 inch, one side polished, p-type (boron), total-thickness-variation < 10 μm, 1–10 Ω cm; 10 nm titanium adhesion layer; 200 nm copper (purity > 99.9 %), root-mean-square roughness < 10 nm. Copper foil (thickness 0.25 mm, 99.98%) was purchased from Sigma-Aldrich. The copper was consecutively washed with portions of 3 M HCl (in methanol), methanol and ethanol under ultrasonication (2 min), and dried under a flow of argon. The cleaned copper wafer was immediately used for catalysis.
Synthesis of PTEB on Cu wafer or foil
Typically, TEB (5 mg, 0.033 mmol) and piperidine (10 µL, 0.1 mmol) were added in a glass bottle containing 10 mL pyridine as solvent. The freshly cleaned copper was submerged into a reaction mixture. Afterwards, the bottle was sealed and heated to 60 °C in an oven for a certain time. Repetitive series of experiments gave no significant differences in terms of the resulting PTEB layer thickness and morphology. After reaction, the samples were immediately washed with fresh pyridine, dichloromethane, and methanol sequentially. Finally, the substrates were blow-dried by a jet of dry nitrogen and a golden yellow film was obtained uniformly on the substrate.
Transfer of PTEB
To transfer the PTEB film on copper to another substrate (e.g., PET), the film was coated with PMMA resist (Allresist GmbH product number AR-P671.04, dissolved in chlorobenzene), and cured at 90 °C for 10 min. The copper substrate was etched away by a water solution of ammonium persulfate (0.25 g mL−1) in 2 h. After being rinsed thoroughly with deionized water, the PMMA/PTEB film was transferred to a target substrate. The samples were naturally dried in air for 1 h and stored in high vacuum (room temperature) for 24 h to enhance the adhesion of PTEB with targeted substrate surface. PMMA was removed by thorough rinsing in acetone and cured in isopropyl alcohol.
Synthesis of PTEB on other substrates
A planar substrate (e.g., SiO2 wafer, graphite, titanium, nickel, FTO, glass, and Kapton) piece cleaned by water and ethanol was sandwiched with a copper wafer in a distance of d = 0.1 mm adjusted by two spacers. The assembly was immersed in the reaction mixture as indicated above and the washing procedures are similar.
Patterned PTEB network
Patterned PTEB film was fabricated on SiO2 wafer by using copper grid as both a catalyst source and a stencil mask. The samples were clamped with copper TEM grids with various hole sizes (Plano, Germany) and immersed in the reaction mixture as described above. The distance between the copper grid and substrate has an important role on fabricating positive and negative patterns on the substrate, where negative patterned PTEB film was obtained by a direct attachment of copper grid to the substrate and positive pattern was achieved by close attachment assisted by the evaporation of a drop of isopropanol due to capillary force.
Synthesis of g-C3N4
Bulk g-C3N4 was synthesized according to a reported procedure with some modifications. In a typical synthesis, 5.0 g urea was heated at 550 °C in Ar atmosphere with a rate of 2 °C per min for 4 h56. The obtained sample was deposited onto as-washed (using 3 M HCl in methanol) titanium plate with controlled thickness of ca. 250 nm using spin-coating to form a film photoelectrode.
Synthesis of PTEB1.3-co-PDET1 on Cu substrate
Typically, TEB (2.5 mg, 0.017 mmol), DET (2.5 mg, 0.013 mmol), and piperidine (8.9 µL, 0.09 mmol) were added in a glass bottle containing 10 mL pyridine as solvent. The freshly cleaned copper was immersed in the reaction mixture. The following procedures are similar to the synthesis of PTEB on Cu substrate.
Synthesis of PTEB1.3-co-PDET1 on other substrates
A planar substrate (e.g., titanium and quartz glass) piece cleaned by water and ethanol was sandwiched with a copper wafer in a distance of d = 0.1 mm adjusted by two spacers. The assembly was immersed in the reaction mixture mentioned above and washing procedures are similar.
PEC measurements
The polarization curves of as-prepared PTEB nanostructured on titanium plate as photocathodes were performed using a three-electrode setup contains working electrode (PTEBs), counter electrode (Pt wire), and reference electrode (Ag/AgCl). The simulated sunlight was from a 200 W Xenon lamp (100 mW cm−2) coupled with an AM 1.5 G filter (Newport). An electrochemical analyzer (CHI 760 E) was applied to measure the LSV characteristic of the electrodes, with 1 mV s−1 scan rate, and there is no correction on data for any losses of uncompensated resistance. The electrolyte (0.01 M Na2SO4, pH = 6.8) was degassed for 30 min by flushing high purity argon at room temperature (ca. 25 °C) before the measurement. The EIS spectra were recorded by applying a 10 mV AC signal in the frequency range from 100 K to 0.01 Hz at a DC bias of 0.3 V vs. RHE (i.e. − 0.3 V vs. Ag/AgCl). Current density was calculated using the exposed geometric surface area of 1.0 cm2 of the photoelectrode
| 1 |
The applied potential vs. Ag/AgCl is converted to RHE potential using the following equation:
| 2 |
The amount of H2 evolved on the PTEB photocathode was measured by GC equipped with a thermal conductivity detector (TCD, N2 carrier) at 0 V vs. RHE of applied bias in 0.01 M Na2SO4 solution under AM 1.5 G irradiation (100 mW cm−2)
The IPCE was measured by using a Xenon lamp (100 mW cm−2, AM 1.5 G) with specific wavelength filters to select the required wavelength of light. IPCE can be expressed as:
| 3 |
where the JPh and Jlight are the real photocurrent density and light intensity at the wavelength λ.
Photocatalytic H2 evolution
The PTEB nanofibers film-based photocatalytic cell for H2 evolution test was constructed according to a reported process60,61. In brief, the PTEB nanofibers film (3 × 3 cm2, ca. 230 nm-thick) was placed at the center of a gas-closed reaction cell with 120 mL 25% triethanolamine water solution with magnetic stirring. The temperature of the reaction system was kept at around 25 °C. A 200 W Xenon lamp with a filter of λ > 420 nm was applied to execute the photocatalytic reaction. The amount of H2 produced from water was determined by GC equipped with a TCD.
The monochromatic illumination quantum yield (MIQY) for H2 evolution was characterized using a similar setup but with a 420 nm band-pass filter. The MIQY was calculated based on the equation:
| 4 |
The number of the incident photons was determined using a radiant power energy meter (Newport). The produced H2 molecules reached 1.3 μmol in 10 h, and the MIQY was calculated as 1.83 %.
DFT calculation
Raman spectra: calculations were performed at B3LYP/6-31 G(d,p) level of theory with the Gaussian09 suite of programs66 on a cluster model representing a section of PTEB (Supplementary Fig. 7). The equilibrium structure of this model, its Hessian, and polarizability derivatives were used as input to compute the simulated Raman spectrum (Fig. 1f) in presence of heavy mass (100 amu) at the peripheral atoms. As confirmed by a comparison (Supplementary Fig. 8) with the results from periodic boundary conditions calculation (Crystal1467, same functional and basis set as above), this approach allows to effectively quench the vibrations of the peripheral moieties of the cluster model and their contributions to the simulated Raman spectrum. However, the results from the Gaussian09 calculation allow to more straightforwardly analyse and assign the vibrational modes with a suite of ad hoc programs developed for graphene molecules in Milano (Supplementary Fig. 9).
Hydrogen evolution reaction: DFT calculations were carried out by using quantum ESPRESSO code68. The generalized gradient approximation of Perdew–Burke–Ernzerhof was used for exchange correlation functional in DFT69. In all the cases, spin polarization was considered in the calculation. The kinetic energy cutoffs were set to 35 Ry and 350 Ry, respectively, for the wavefunction and the charge. 3 × 3 × 1 k-points grids were used for structure optimization and total energy calculations. The adsorption energies of intermediates are calculated by using H2O (l) and H2 (g) as references. The free-energy variation is obtained by DFT total energy calculations through adding corrections to entropy, zero point energy, and solvation energy according to the method developed by Nørskov et al.70. Thus, free energies can be obtained from total energies of intermediates by adding some corrections:
| 5 |
where ETotal is DFT calculated total energy, ΔEZPE zero point energy, ΔS entropy, and ΔGs (– 0.22 eV) solvation energy for reaction intermediate. The pH effect were considered as 0.0592 pH and – 0.0592 pH for hydrogen evolution reaction (HER). We set pH 7 in all the calculations. By considering external potential U, the free energies corrected by eUred and – eUox for HER. According to the band structure in the main text, the Ured and Uox for PTEB equal to − 1.01 and 1.40 V, respectively. Both single site (Volmer–Heyrovsky) and dual sites (Volmer–Tafel) reaction pathways were investigated for HER. The elementary steps for single-site HER process are:
| 6 |
| 7 |
where * denotes catalysts surface. For dual sites HER, the first and second elementary steps are of electron transfer steps that are like Eq. 6, and the third step is a Tafel step:
| 8 |
Data availability
The data that support the findings of this study are available from the corresponding author on request.
Electronic supplementary material
Acknowledgements
This work was financially supported by the ERC Grant 2DMATER, ESF Young Researcher Group ‘GRAPHD,’ and the EC under the Graphene Flagship (number CNECTICT-604391). The German Excellence Initiative via the Cluster of Excellence EXC1056 “Center for Advancing Electronics Dresden” (cfaed) is gratefully acknowledged. We gratefully acknowledge Dr. Renhao Dong for helpful discussion and Kejun Liu for assistance in the characterization of SEM. D.O., A.M., and M.T. acknowledge funding support from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme ERC–Consolidator Grant (ERC CoG 2016 EspLORE grant agreement No 724610).
Author contributions
T.Z. and X.F. conceived and designed the experiments and wrote the paper. T.Z. carried out most of the experiments. Y.H. designed and performed PEC. V.D. and D.R.T.Z. measured and analysed XPS and UPS. Z.L. and E.Z. assisted with the TEM characterization. G.C. performed DFT calculations of band structure and H2 evolution reactions. M.L. measured SEM. D.O., A.M., and M.T. carried out DFT calculations of Raman spectrum and related data analysis. S.X. prepared the DET monomer. Z.Z. and R.J. rendered helpful discussions. All authors discussed the results and commented on the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Tao Zhang, Yang Hou.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-03444-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sivula K, van de Krol R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016;1:15010. doi: 10.1038/natrevmats.2015.10. [DOI] [Google Scholar]
- 2.Paracchino A, Laporte V, Sivula K, Gratzel M, Thimsen E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011;10:456–461. doi: 10.1038/nmat3017. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, et al. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science. 2015;347:970–974. doi: 10.1126/science.aaa3145. [DOI] [PubMed] [Google Scholar]
- 4.Landman A, et al. Photoelectrochemical water splitting in separate oxygen and hydrogen cells. Nat. Mater. 2017;16:646–651. doi: 10.1038/nmat4876. [DOI] [PubMed] [Google Scholar]
- 5.Zou ZG, Ye JH, Sayama K, Arakawa H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature. 2001;414:625–627. doi: 10.1038/414625a. [DOI] [PubMed] [Google Scholar]
- 6.Hou Y, Zuo F, Dagg A, Feng PY. Visible light-driven alpha-Fe2O3 nanorod/graphene/BiV1−xMoxO4 core/shell heterojunction array for efficient photoelectrochemical water splitting. Nano Lett. 2012;12:6464–6473. doi: 10.1021/nl303961c. [DOI] [PubMed] [Google Scholar]
- 7.Simon T, et al. Redox shuttle mechanism enhances photocatalytic H2 generation on Ni-decorated CdS nanorods. Nat. Mater. 2014;13:1013–1018. doi: 10.1038/nmat4049. [DOI] [PubMed] [Google Scholar]
- 8.McKone JR, Pieterick AP, Gray HB, Lewis NS. Hydrogen evolution from Pt/Ru-coated p-type WSe2 photocathodes. J. Am. Chem. Soc. 2013;135:223–231. doi: 10.1021/ja308581g. [DOI] [PubMed] [Google Scholar]
- 9.Yu XY, Prevot MS, Guijarro N, Sivula K. Self-assembled 2D WSe2 thin films for photoelectrochemical hydrogen production. Nat. Commun. 2015;6:7596. doi: 10.1038/ncomms8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Septina W, et al. Photosplitting of water from wide-gap Cu(In,Ga)S2 thin films modified with a CdS layer and Pt nanoparticles for a high-onset-potential photocathode. J. Phys. Chem. C. 2015;119:8576–8583. doi: 10.1021/acs.jpcc.5b02068. [DOI] [Google Scholar]
- 11.Wang XC, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009;8:76–80. doi: 10.1038/nmat2317. [DOI] [PubMed] [Google Scholar]
- 12.Zhang GG, Lan ZA, Wang XC. Conjugated polymers: catalysts for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016;55:15712–15727. doi: 10.1002/anie.201607375. [DOI] [PubMed] [Google Scholar]
- 13.Hasan T, et al. Nanotube-polymer composites for ultrafast photonics. Adv. Mater. 2009;21:3874–3899. doi: 10.1002/adma.200901122. [DOI] [Google Scholar]
- 14.Zhang MW, Wang XC. Two dimensional conjugated polymers with enhanced optical absorption and charge separation for photocatalytic hydrogen evolution. Energ. Environ. Sci. 2014;7:1902–1906. doi: 10.1039/c3ee44189j. [DOI] [Google Scholar]
- 15.Wang XC, et al. Polymer semiconductors for artificial photosynthesis: hydrogen evolution by mesoporous graphitic carbon nitride with visible light. J. Am. Chem. Soc. 2009;131:1680–1681. doi: 10.1021/ja809307s. [DOI] [PubMed] [Google Scholar]
- 16.Zheng DD, Cao XN, Wang XC. Precise formation of a hollow carbon nitride structure with a janus surface to promote water splitting by photoredox catalysis. Angew. Chem. Int. Ed. 2016;55:11512–11516. doi: 10.1002/anie.201606102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo YF, et al. A rapid microwave-assisted thermolysis route to highly crystalline carbon nitrides for efficient hydrogen generation. Angew. Chem. Int. Ed. 2016;55:14693–14697. doi: 10.1002/anie.201608453. [DOI] [PubMed] [Google Scholar]
- 18.Han Q, Wang B, Gao J, Qu LT. Graphitic carbon nitride/nitrogen-rich carbon nanofibers: highly efficient photocatalytic hydrogen evolution without cocatalysts. Angew. Chem. Int. Ed. 2016;55:10849–10853. doi: 10.1002/anie.201605591. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YJ, Thomas A, Antonietti M, Wang XC. Activation of carbon nitride solids by protonation: morphology changes, enhanced ionic conductivity, and photoconduction experiments. J. Am. Chem. Soc. 2009;131:50–51. doi: 10.1021/ja808329f. [DOI] [PubMed] [Google Scholar]
- 20.Schwab MG, et al. Photocatalytic hydrogen evolution through fully conjugated poly(azomethine) networks. Chem. Commun. 2010;46:8932–8934. doi: 10.1039/c0cc04057f. [DOI] [PubMed] [Google Scholar]
- 21.Stegbauer L, Schwinghammer K, Lotsch BV. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 2014;5:2789–2793. doi: 10.1039/C4SC00016A. [DOI] [Google Scholar]
- 22.Vyas VS, et al. A tunable azine covalent organic framework platform for visible light-induced hydrogen generation. Nat. Commun. 2015;6:8508. doi: 10.1038/ncomms9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YJ, et al. Biopolymer-activated graphitic carbon nitride towards a sustainable photocathode material. Sci. Rep. 2013;3:2163. doi: 10.1038/srep02163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sprick RS, et al. Tunable organic photocatalysts for visible-light-driven hydrogen evolution. J. Am. Chem. Soc. 2015;137:3265–3270. doi: 10.1021/ja511552k. [DOI] [PubMed] [Google Scholar]
- 25.Li LW, et al. Rational design of porous conjugated polymers and roles of residual palladium for photocatalytic hydrogen production. J. Am. Chem. Soc. 2016;138:7681–7686. doi: 10.1021/jacs.6b03472. [DOI] [PubMed] [Google Scholar]
- 26.Vyas VS, Lotsch BV. Materials chemistry: organic polymers form fuel from water. Nature. 2015;521:41–42. doi: 10.1038/521041a. [DOI] [PubMed] [Google Scholar]
- 27.Diederich F, Kivala M. All-carbon scaffolds by rational design. Adv. Mater. 2010;22:803–812. doi: 10.1002/adma.200902623. [DOI] [PubMed] [Google Scholar]
- 28.Klappenberger F, et al. On-surface synthesis of carbon-based scaffolds and nanomaterials using terminal alkynes. Acc. Chem. Res. 2015;48:2140–2150. doi: 10.1021/acs.accounts.5b00174. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh S, et al. Conducting polymer nanostructures for photocatalysis under visible light. Nat. Mater. 2015;14:505–511. doi: 10.1038/nmat4220. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasu K, Ghosh SK. Graphyne and graphdiyne: promising materials for nanoelectronics and energy storage applications. J. Phys. Chem. C. 2012;116:5951–5956. doi: 10.1021/jp212181h. [DOI] [Google Scholar]
- 31.Zhang T, et al. Wafer-scale synthesis of defined polymer brushes under ambient conditions. Polym. Chem. 2015;6:8176–8183. doi: 10.1039/C5PY01274K. [DOI] [Google Scholar]
- 32.Zhang T, Du YH, Muller F, Amin I, Jordan R. Surface-initiated Cu(0) mediated controlled radical polymerization (SI-CuCRP) using a copper plate. Polym. Chem. 2015;6:2726–2733. doi: 10.1039/C5PY00093A. [DOI] [Google Scholar]
- 33.Li YN, Wang JL, He LN. Copper(II) chloride-catalyzed Glaser oxidative coupling reaction in polyethylene glycol. Tetrahedron Lett. 2011;52:3485–3488. doi: 10.1016/j.tetlet.2011.04.118. [DOI] [Google Scholar]
- 34.Siemsen P, Livingston RC, Diederich F. Acetylenic coupling: a powerful tool in molecular construction. Angew. Chem. Int. Ed. 2000;39:2633–2657. doi: 10.1002/1521-3773(20000804)39:15<2632::AID-ANIE2632>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Cataldo F. Spectroscopical characterization of carbonaceous matter prepared through the Glaser coupling reaction route. Carbon N. Y. 1999;37:161–163. doi: 10.1016/S0008-6223(98)90106-3. [DOI] [Google Scholar]
- 36.Du R, et al. CMP aerogels: ultrahigh-surface-area carbon-based monolithic materials with superb sorption performance. Adv. Mater. 2014;26:8053–8058. doi: 10.1002/adma.201403058. [DOI] [PubMed] [Google Scholar]
- 37.Zhang SQ, et al. Raman spectra and corresponding strain effects in graphyne and graphdiyne. J. Phys. Chem. C. 2016;120:10605–10613. doi: 10.1021/acs.jpcc.5b12388. [DOI] [Google Scholar]
- 38.Zhang X, Zhou Q, Huang Y, Li ZC, Zhang ZJ. Contrastive analysis of the raman spectra of polychlorinated benzene: hexachlorobenzene and benzene. Sensors. 2011;11:11510–11515. doi: 10.3390/s111211510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaffer HE, Chance RR, Silbey RJ, Knoll K, Schrock RR. Conjugation length dependence of raman-scattering in a series of linear polyenes-implications for polyacetylene. J. Chem. Phys. 1991;94:4161–4170. doi: 10.1063/1.460649. [DOI] [Google Scholar]
- 40.Hu, A., Rybachuk, M., Lu, Q. B. & Duley, W. W. Direct synthesis of sp-bonded carbon chains on graphite surface by femtosecond laser irradiation. Appl. Phys. Lett. 91 131906 (2007).
- 41.Sergushin IP, et al. X-ray electron and X-ray spectral study of carbyne. J. Struct. Chem. 1977;18:553–555. doi: 10.1007/BF00745293. [DOI] [Google Scholar]
- 42.Szilluweit R, et al. Low-temperature preparation of tailored carbon nanostructures in water. Nano Lett. 2012;12:2573–2578. doi: 10.1021/nl300822f. [DOI] [PubMed] [Google Scholar]
- 43.Zhu YP, Ma TY, Jaroniec M, Qiao SZ. Self-templating synthesis of hollow Co3O4 microtube arrays for highly efficient water electrolysis. Angew. Chem. Int. Ed. 2016;55:1324–1328. doi: 10.1002/anie.201508540. [DOI] [PubMed] [Google Scholar]
- 44.Liang L, Liu J, Windisch CF, Exarhos GJ, Lin YH. Direct assembly of large arrays of oriented conducting polymer nanowires. Angew. Chem. Int. Ed. 2002;41:3665–3668. doi: 10.1002/1521-3773(20021004)41:19<3665::AID-ANIE3665>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, et al. Templateless assembly of molecularly aligned conductive polymer nanowires: a new approach for oriented nanostructures. Chem. Eur. J. 2003;9:604–611. doi: 10.1002/chem.200390064. [DOI] [PubMed] [Google Scholar]
- 46.Rajesh Ahuja T, Kumar D. Recent progress in the development of nano-structured conducting polymers/nanocomposites for sensor applications. Sens. Actuators, B. 2009;136:275–286. doi: 10.1016/j.snb.2008.09.014. [DOI] [Google Scholar]
- 47.Nie ZH, Kumacheva E. Patterning surfaces with functional polymers. Nat. Mater. 2008;7:277–290. doi: 10.1038/nmat2109. [DOI] [PubMed] [Google Scholar]
- 48.De S, et al. Silver nanowire networks as flexible, transparent, conducting films: extremely high DC to optical conductivity ratios. ACS Nano. 2009;3:1767–1774. doi: 10.1021/nn900348c. [DOI] [PubMed] [Google Scholar]
- 49.Zheng QB, et al. Transparent conductive films consisting of ultra large graphene sheets produced by Langmuir-Blodgett assembly. ACS Nano. 2011;5:6039–6051. doi: 10.1021/nn2018683. [DOI] [PubMed] [Google Scholar]
- 50.Liu JK, et al. Boron carbides as efficient, metal-free, visible-light-responsive photocatalysts. Angew. Chem. Int. Ed. 2013;52:3241–3245. doi: 10.1002/anie.201209363. [DOI] [PubMed] [Google Scholar]
- 51.Zhang JS, et al. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew. Chem. Int. Ed. 2010;49:441–444. doi: 10.1002/anie.200903886. [DOI] [PubMed] [Google Scholar]
- 52.Chana, D. K. L. & Yu, J. C. Facile synthesis of carbon- and oxygen-rich graphitic carbon nitride with enhanced visible-light photocatalytic activity. Catal. Today, 10.1016/j.cattod.2017.05.017 (2017).
- 53.Wang F, et al. Red phosphorus: an elemental photocatalyst for hydrogen formation from water. Appl. Catal. B Environ. 2012;111:409–414. doi: 10.1016/j.apcatb.2011.10.028. [DOI] [Google Scholar]
- 54.Hu ZF, Yuan LY, Liu ZF, Shen ZR, Yu JC. An elemental phosphorus photocatalyst with a record high hydrogen evolution efficiency. Angew. Chem. Int. Ed. 2016;55:9579–9584. doi: 10.1002/anie.201603331. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, et al. Mesoporous silicon carbide nanofibers with in situ embedded carbon for co-catalyst free photocatalytic hydrogen production. Nano Res. 2016;9:886–898. doi: 10.1007/s12274-015-0971-z. [DOI] [Google Scholar]
- 56.Hou Y, Wen ZH, Cui SM, Feng XL, Chen JH. Strongly coupled ternary hybrid aerogels of N-deficient porous graphitic-C3N4 nanosheets/N-doped graphene/NiFe-layered double hydroxide for solar-driven photoelectrochemical water oxidation. Nano Lett. 2016;16:2268–2277. doi: 10.1021/acs.nanolett.5b04496. [DOI] [PubMed] [Google Scholar]
- 57.Bao JM. Photoelectrochemical water splitting a new use for bandgap engineering. Nat. Nanotechnol. 2015;10:19–20. doi: 10.1038/nnano.2014.322. [DOI] [PubMed] [Google Scholar]
- 58.Bronstein H, et al. Thieno[3,2-b]thiophene-diketopyrrolopyrrole containing polymers for inverted solar cells devices with high short circuit currents. Adv. Funct. Mater. 2013;23:5647–5654. doi: 10.1002/adfm.201300287. [DOI] [Google Scholar]
- 59.Ghosh S, Priyam A, Bhattacharya SC, Saha A. Mechanistic aspects of quantum dot based probing of Cu (II) ions: role of dendrimer in sensor efficiency. J. Fluoresc. 2009;19:723–731. doi: 10.1007/s10895-009-0468-9. [DOI] [PubMed] [Google Scholar]
- 60.Wang Q, et al. Particulate photocatalyst sheets based on carbon conductor layer for efficient z-scheme pure-water splitting at ambient pressure. J. Am. Chem. Soc. 2017;139:1675–1683. doi: 10.1021/jacs.6b12164. [DOI] [PubMed] [Google Scholar]
- 61.Wang Q, et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1% Nat. Mater. 2016;15:611–615. doi: 10.1038/nmat4589. [DOI] [PubMed] [Google Scholar]
- 62.Chai GL, et al. Active sites and mechanisms for oxygen reduction reaction on nitrogen-doped carbon alloy catalysts: Stone-Wales defect and curvature effect. J. Am. Chem. Soc. 2014;136:13629–13640. doi: 10.1021/ja502646c. [DOI] [PubMed] [Google Scholar]
- 63.Enriquez A, et al. Efficient thiol-yne click chemistry of redox-active ethynylferrocene. Organometallics. 2014;33:7307–7317. doi: 10.1021/om501110w. [DOI] [Google Scholar]
- 64.Li YR, Ashizawa M, Uchida S, Michinobu T. Colorimetric sensing of cations and anions by clicked polystyrenes bearing side chain donor-acceptor chromophores. Polym. Chem. 2012;3:1996–2005. doi: 10.1039/c2py20230a. [DOI] [Google Scholar]
- 65.Yam VWW, Hui CK, Yu SY, Zhu NY. Syntheses, luminescence behavior, and assembly reaction of tetraalkynylplatinate(II) complexes: crystal structures of [Pt(tBu3trpy)(C⋮CC5H4N)Pt(tBu3trpy)] (PF6)3 and [Pt2Ag4(C⋮CC⋮CC6H4CH3-4)8(THF)4] Inorg. Chem. 2004;43:812–821. doi: 10.1021/ic034877l. [DOI] [PubMed] [Google Scholar]
- 66.Frisch, M. J. et al. Gaussian 09 (revision D.01) (Gaussian, Inc., Wallingford CT, 2016).
- 67.Dovesi R, et al. CRYSTAL14: a program for the ab initio investigation of crystalline solids. Int. J. Quantum Chem. 2014;114:1287–1317. doi: 10.1002/qua.24658. [DOI] [Google Scholar]
- 68.Paolo G, et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter. 2009;21:395502. doi: 10.1088/0953-8984/21/39/395502. [DOI] [PubMed] [Google Scholar]
- 69.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 70.Nørskov JK, Rossmeisl J, Logadottir A, Lindqvist L. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B. 2004;108:17886–17892. doi: 10.1021/jp047349j. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on request.