Abstract
Development of minimally invasive biomarker assays for early detection and effective clinical management of pancreatic cancer is urgently needed to reduce high morbidity and mortality associated with this malignancy. We hypothesized that if aberrantly expressing microRNAs (miRNAs) in pancreatic adenocarcinoma tissues are detected in blood plasma then plasma profiling of these miRNAs might serve as a minimally invasive early detection biomarker assay for this malignancy. By utilizing a modified protocol to isolate and quantify plasma miRNAs from heparin treated blood we show that miRNA profiling in plasma can differentiate pancreatic adenocarcinoma patients from healthy controls. We have profiled four miRNAs, miR-21, miR-210, miR-155 and miR-196a, all implicated in the development of pancreatic cancer with either proven or predicted target genes involved in critical cancer associated cellular pathways. Of these, miR-155 has recently been identified as a candidate biomarker of early pancreatic neoplasia while elevated expression of miR196a has been shown to parallel progression of disease. The results revealed a sensitivity of 64% and a specificity of 89% with the analyses of plasma levels for this panel of four miRNAs. The area under the receiver operating characteristic (ROC) curve were estimated at 0.82 and 0.78 without and with leave one out cross validation scheme respectively. These observations, although a “proof of principle” finding at this time, demonstrate the feasibility of developing plasma miRNA profiling as a sensitive and specific blood based biomarker assay for pancreatic cancer that has the potential of translation to the clinic with additional improvements in the future.
Introduction
Sensitive and specific cancer biomarkers are essential to early detection and diagnosis as well as for undertaking novel therapeutic trials and prevention strategies. Development of blood based biomarker assays for malignancies such as pancreatic cancer is critical since most patients with this neoplasm remain asymptomatic until they present with locally advanced or distally metastatic and surgically inoperable disease at the time of diagnosis. With pancreatic cancer being the fourth most common cause of cancer related deaths in the United States (1) and an average 5 year survival rate of <5%, early detection of this malignancy at a surgically resectable stage offers the best curative option for the patients (2).
The majority of pancreatic cancers arise from the epithelial lining of the exocrine pancreatic ducts as pancreatic ductal adenocarcinoma (PDAC) through a multi-step progression process involving non-invasive precursor lesions (3). The precursor lesions consist of microscopic pancreatic intraepithelial neoplasia (PanIN) within the duct (4) and macroscopic intra-ductal papillary mucinous neoplasms (IPMN) as cystic lesions connected with the main pancreatic duct or one of its branches (5). In both PanIN and IPMN, the epithelium lining demonstrates varying degrees of histologic atypia ranging form adenoma to carcinoma in situ (4). Interestingly, some of the seminal genetic alterations detected in invasive pancreatic carcinomas, such as mutations in KRAS2, DPC4/SMAD4 and TP53 are observed in both PanIN (6–8) and IPMN (3, 9) lending support to their roles as bona fide precursor lesions. Despite identification of such common genetic mutation signatures shared between non-invasive and invasive pancreatic lesions, their utility in discriminating between benign and malignant pancreatic disease remain unresolved primarily because minimally invasive diagnostic methods for screening of candidate biomarkers are still not available. It has been reported that even imaging techniques like computed tomography (CT) and magnetic resonance imaging (MRI) sometimes fail to differentiate between benign and malignant lesions with up to 6% of the cases suspected as malignant with these methods later found to be benign at surgery with subsequent post surgical complications developing among a significant number of these patients (10). Endoscopic ultrasound guided fine needle aspiration (EUS-FNA) has, of late, emerged as the preferred procedure for preoperative diagnosis and staging of pancreatic cancer (11–15). However, the invasive nature of the technique makes it unlikely that EUS-FNA could be routinely used for early detection or screening of pancreatic carcinomas. Development of plasma or serum based biomarker assays, therefore, remains the desired method of choice for undertaking pancreatic cancer screening efforts in the future.
MicroRNAs (miRNAs) are 18-24 nucleotide long evolutionarily conserved RNA molecules (16) that regulate the stability and translational efficiency of target mRNAs by complementary base pairing with specific 3’UTR sequences. Such physiologic regulation of transcriptome function by miRNAs plays a significant role in the maintenance of cellular homeostasis and development. Extensive involvement of miRNAs in cell growth regulation and cancer has become evident in the last few years (17). Aberrant expression of miRNAs have been widely reported in human cancers with both over and under expression detected in neoplastic cells compared with their normal counterparts (18). A number of studies have identified multiple aberrantly expressing miRNAs associated with human pancreatic cancers (19–22). Plausible insights into the pathophysiological mechanisms of pancreatic ductal adenocarcinoma development involving miRNAs and their putative target genes implicated in this malignancy have been suggested along with the observation that expression levels of miRNA-196a and miRNA-217 can discriminate pancreatic cancer lesions from normal pancreas and chronic pancreatitis tissues (21). Preliminary validation of the same findings in pancreatic FNA biopsy samples indicated that miRNA analyses of FNA could provide a novel strategy for improving diagnosis of pancreatic disease (23). Furthermore, global miRNA expression profiling has also been reported to define distinct expression patterns differentiating normal pancreas, chronic pancreatitis and pancreatic ductal adenocarcinoma (20). More recently miRNA-155 and miRNA-21 were found significantly up-regulated in the majority of non-invasive IPMNs and corresponding pancreatic juice samples, which correlated with histological features of progression in these neoplasms (24). Varying miRNA expression signatures in normal, benign and malignant pancreatic tissues with their levels in clinical samples like pancreatic juice and FNA correlating with disease progression offer a new potentially sensitive method for improved detection and diagnosis of the disease.
Recent demonstration of tumor associated circulating miRNAs in the plasma of human prostate cancer xenograft mouse models and human prostate cancer patients, capable of distinguishing cancer bearing individuals from healthy controls, has raised the exciting possibility that assaying miRNA in plasma or serum may serve as a novel approach for blood based detection of human cancer (25). In the present study, we evaluated if plasma profiles of a set of miRNAs associated with pancreatic carcinoma could discriminate pancreatic cancer patients from normal healthy individuals. Our results provide compelling evidence that the levels of known pancreatic cancer associated miRNAs are elevated in the plasma of pancreatic carcinoma patients and that combined analyses of these circulating miRNAs can differentiate cancer patients from healthy individuals with an acceptable degree of sensitivity and specificity.
Materials and Methods
The study population consisting of patients with pathologically confirmed primary pancreatic ductal adenocarcinoma and controls were recruited at The University of Texas M. D. Anderson Cancer Center. Controls were healthy spouses, friends, or non–blood relatives of patients with various non-gastrointestinal and non–smoking related cancers. Controls were frequency-matched to cases by age at enrollment (±5 years), sex, and race. All study subjects were U.S. residents and gave written informed consent for the interviews and the collection of blood sample in accordance with the protocol approved by the Institutional Review Board of M. D. Anderson Cancer Center. Total of 49 cancer samples and 36 control samples collected between 2002 and 2008 were analyzed in this study. The patient population characteristics with respect to age, race, sex, stage of disease and survival durations are described in Table-1.
Table 1.
Characteristics of Patient Population.
| Characteristic | No. of Case (N=49) | No. of death (N=14) |
|---|---|---|
| Age (years) | ||
| <=50 | 4 | 1 |
| 51–60 | 23 | 8 |
| 61–70 | 17 | 3 |
| >70 | 5 | 2 |
| Sex | ||
| Male | 25 | 6 |
| Female | 24 | 8 |
| Race | ||
| White | 43 | 11 |
| Black | 2 | 0 |
| Hispanic | 3 | 2 |
| other | 1 | 1 |
| Stage | ||
| localized | 15 | 3 |
| Locally advanced | 13 | 5 |
| Metastatic | 21 | 6 |
Collection of Heparin Treated Blood Plasma and RNA isolation
Blood was collected from patients and controls in Sodium Heparin tubes (BD Vacutainer, Franklin Lakes, NJ) and processed within 2 h of collection by centrifugation at 1,300 × g at 4°C for 10 min. Plasma was transferred to a fresh tube and stored at −80°C.
Total RNA containing small RNA was isolated from 1.5 ml of heparin plasma using Trizol LS reagent (Invitrogen Life Technologies, CA) according to the manufacturer’s protocol with the following modifications. The plasma was mixed with Trizol LS reagent (1:3 ratio), and after phase separation by centrifugation, the upper aqueous phase was carefully transferred to a fresh tube. The aqueous phase was then extracted twice with phenol/chloroform and added with 1.5 vol of ethanol before being applied directly to mirVana miRNA column (Ambion, Inc, Austin, TX) according to the manufacturer’s instructions. The bound RNA was cleaned with the buffers provided by the manufacturer to remove impurities and eluted in a final volume of 100 μl. To remove heparin associated contaminants 300 μl of 7.5 M LiCl was added to the RNA solution, incubated overnight at −20°C and then centrifuged at 12,000xg for 30 min at 4°C. The pellet was washed two times by centrifugation with 70% ethanol. The RNA pellet was dried for 10 min at room temperature and dissolved in 30 μl of DEPC-treated water for miRNA assay. The concentration of all RNA samples were quantified using Nano Drop 1000 (Nanodrop, Wilmington, USA).
Plasma RNA Pretreatment
20ng of RNA was pretreated with 1 unit of DNase (Invitrogen, CA) and 2 units of Heparinase I (Sigma Chemical Company) for 1 h at 25°C in 1 μl of 10 × DNase I Reaction Buffer (200 mM Tris-HCl pH 8.4, 20 mM MgCl2, 500 mM KCl) and 0.38 μl of RNAse inhibitor to remove any contaminating DNA and Heparin. After the enzyme digestions, 0.5 μl of 25 mM EDTA solution was added to the total reaction volume of 10ul and incubated at 65°C for 10min.
MicroRNA Real Time PCR
Taqman MicroRNA Assays were used to do expression profiling of the plasma miRNAs of interest. All reagents, primers and probes were obtained from Applied Biosystems (Applied Biosystems, Foster City, CA). 10 ng of DNAse and Heparinase treated plasma RNA for each sample was used for the individual assays in 15 μl reactions containing RT mixture and Taqman primer mix. The mix was incubated at 16°C for 30 min, 42°C for 60 min, and 85°C for 5 min. miRNA expression levels were quantified using the ABI prim 7900 HT Sequence detection system (Applied Biosystems). For the purpose, 15 μl reverse transcription (RT) reaction was diluted with 30μl of water and 11.25 μl of the diluted RT product was mixed with 12.5 μl of 2 x Taqman PCR mixture, 1.25 μl Taqman primer and probe mixture in a final volume of 25ul. Real time PCR was performed in triplicate, including no-template controls. Relative expression of the mature miRNAs was calculated utilizing the comparative CT (2−ΔΔCT) method (26) with miRNA-16 as the endogenous control to normalize the data (27). The cycle threshold (CT) is defined as the number of cycles required for the FAM signal to cross the threshold in real time PCR. ΔCT was calculated by subtracting the CT values of miR-16 from the CT values of the miRNA of interest. ΔΔCT was then calculated by subtracting mean ΔCT of the control samples from ΔCT of tested samples. Fold change of miRNA was calculated by the equation 2−ΔΔCT.
Statistical analysis
Student’s t-test was used to evaluate expression differences of miRNAs between cases and controls. Fisher’s exact test and Pearson’s Chi-Square Test were used to determine if there was significant association between the relative plasma levels of the four miRNAs. All tests of statistical significance were two-sided. P-values of less than 0.05 were considered statistically significant. Receiver operating characteristic curves (ROC) were constructed and the area under the curve (AUC) was calculated to evaluate the specificity and sensitivity of predicting cases and controls by each individual miRNA and by the combination of the four miRNAs. Since this study population was small, the cases and controls were not split into training and test sets. We, however, used a leave-one-out scheme to cross-validate the ROC analysis. In the leave-one-out analysis a subset of all but one observation is used to build a model, and then the model is used to predict the left-out recorded observation. When this process is repeated for each observation, a prediction is obtained for every record in the data set using a model that was blind to the predicted observation. These predictions generate a table of statistics that is used for cross-validating the ROC analysis.
All statistical analyses were performed using the Stata 8.0 software (Stata Corporation, College Station, TX).
Results
We have developed a modified RNA isolation protocol for real time RT-PCR assay of plasma derived miRNAs from blood collected in heparin tubes. This protocol yielded 100ng–500ng of total RNA from about 1.5 ml plasma samples. The isolated RNA samples could quantify relative miRNA levels in a reproducible manner as evident from the results of at least two repeated experiments of every sample run in either duplicate or triplicate in each instance. The results of these independent experiments did not show significant differences (t-test, P=0.41). Furthermore, Pearson’s correlation coefficient revealed significant positive correlation between the relative miRNA levels quantified in independent experiments (r=0.705, P=0.0002). The modifications introduced in the published methods to eliminate heparin and other contaminants including DNA were critical for successful real time RT-PCR reactions for plasma miRNAs. To obtain reproducible real time results some samples had to be processed through multiple cycles of purification steps, which affected the final yield of RNA isolated in each case. Since miR-16 has been reported to be one of the most stably expressed miRNAs across 40 normal human tissue types (28) that is detectable at modest levels in normal human plasma (25) and given that expression of miR-16 has also been used as the calibrator for assaying relative miRNA expression in human tissues, we decided to evaluate its utility as the endogenous control in this study. It was observed that the cycle threshold (CT) values for miR-16 did not vary significantly (P>.05) in the different reaction batches for control and cancer plasma samples thus validating miR-16 as a reliable endogenous control. Due to this observation, we routinely ran the real time RT-PCR reactions for miR-16 in triplicate first to check the quality of each plasma RNA sample and reproducibility of their assay performance before analyzing them for additional miRNAs of interest. In this study, besides assaying for miR-16 as the endogenous normalization control we selected a panel of four miRNAs implicated in pancreatic cancer, miR-21, miR-210, miR-155 and miR-196a, to interrogate their plasma levels in 49 pancreatic cancer patients and 36 normal healthy individuals. However, due to varying yield of the isolated RNA in each case, a total of 28 cancer and 19 control samples could be finally analyzed for all the four miRNAs.
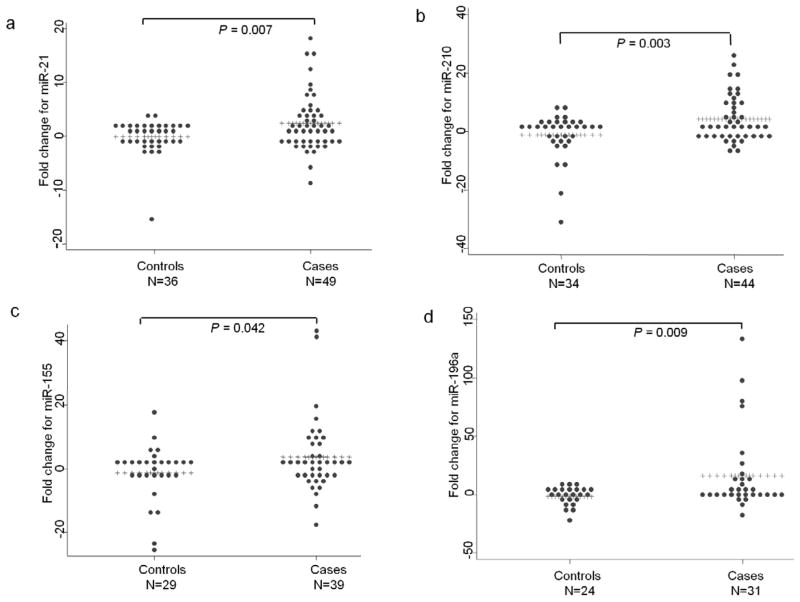
The relative levels of miR-21, miR-210, miR-155 and miR-196a normalized to the level of the miR-16 endogenous control were elevated overall in the plasma of pancreatic adenocarcinoma patients (Fig. 1a-d). The mean fold changes (2−ΔΔCt) in relative levels and P values reflected segregation between normal healthy controls and PDAC samples. Differences in the mean fold change for each miRNA was, however, also a function of the relative abundance of the respective miRNAs in plasma. While the distribution of miR-21, miR-210 and miR-155 levels were spread over a broader range in the healthy controls, those of miR-196a were significantly narrower with its abundance being distinctly less in all the control samples. For miR-21, thirty one of the forty nine cancer samples revealed about 2–20 fold elevation in plasma levels while among the controls, nineteen of the thirty six displayed only 2–4 fold increase. In case of miR-210, twenty eight of the forty four cancer cases had plasma levels elevated by about 2–28 fold but the increase was limited to 2–8 fold in twenty one of the thirty four control samples. 2–40 fold increase of miR-155 was detected in twenty three of the thirty nine cancer cases as opposed to 2–18 fold increase in sixteen of the twenty nine controls. With the relative abundance of miR-196a being low in most of the control plasma, the cancer samples revealed elevation in plasma levels ranging from about 5–140 fold in fifteen of the thirty one cases compared with only about 5–10 fold elevation seen in ten of the twenty four control samples. With the rest of the samples showing either no change or negative fold change values, the overall mean fold increase for each of the four miRNAs in the plasma of cancer samples compared with the controls were significant with the P values of .007 for miR21, .003 for miR-210, .042 for miR-155 and .009 for miR-196a (Table-2). Examination of individual mean fold increases in the cancer samples indicated that a relatively limited number of outliers contributed greatly to the overall highly significant differences observed between the cancer and the control samples. However, no significant differences in the plasma levels of the four miRNAs, both individually and in combination, (P-value >0.20) were observed for the cancer samples at different stages of the disease. These observations suggested that the overall mean fold increase in plasma miRNA levels were detected even in patients with localized disease and was, therefore, not a marker of patients with only late stage metastatic cancer.
Figure 1.
The relative fold change of four miRNAs in the plasma of pancreatic adenocarcinoma patients and normal healthy controls. The horizontal line of pluses (+) represent the mean fold change for each miRNA. (a) miR-21, (b) miR-210, (c) miR-155, (d) miR-196a.
Table 2.
Mean Fold Change of Plasma miRNA Levels in PDAC and Control Samples
| miRNA | PDAC Mean Fold change ± SE | Control Mean Fold change ± SE | p-value |
|---|---|---|---|
| miR-21 | 2.42 ± 0.76 | −0.13 ± 0.54 | 0.007 |
| miR-210 | 4.22 ± 1.19 | −1.56 ± 1.33 | 0.003 |
| miR-155 | 3.74 ± 1.81 | −1.31 ± 1.63 | 0.042 |
| miR-196a | 16.05 ± 6.11 | −1.56 ± 1.63 | 0.009 |
Interestingly, with a five fold or more increase as the cutoff for individual samples, the data revealed a significant association between the elevated plasma levels of miR-155 and miR-210 in cancer patients (p-value=0.004). For miR-155, eleven of the thirty nine (28%) and for miR-210, fourteen of the forty four (32%) cancer samples showed increase in plasma levels spanning this range. Of these, eight cancer samples revealed identical increases in relative plasma levels for miR-155 (73%) and miR-210 (57%).
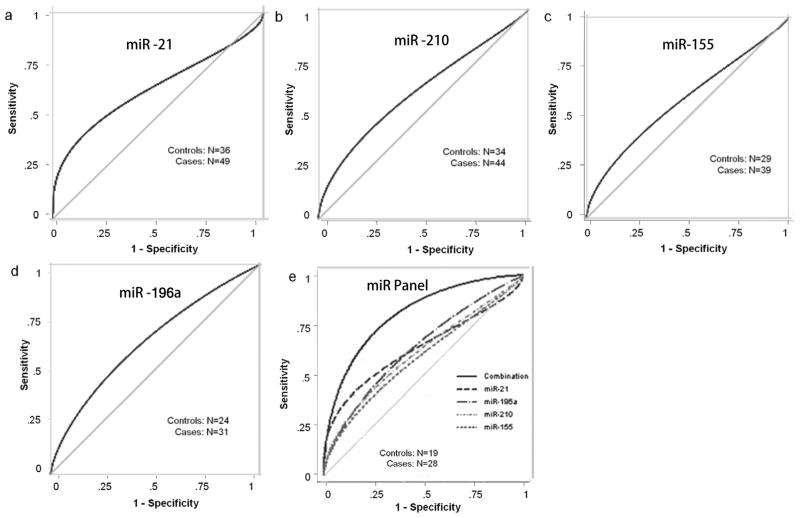
In order to determine if the relative fold changes in the four plasma miRNAs could significantly differentiate between pancreatic cancer patients and healthy controls, receiver operating characteristic (ROC) curves were constructed (Fig. 2a-d). The area under the ROC curve (AUC) for miR-21 was 0.63 (95% confidence interval [CI]: 0.51–0.75); for miR-210 was 0.62 (95% CI: 0.49–0.74); for miR-155 was 0.60 (95% CI: 0.46–0.74); and for miR-196a was 0.66 (95% CI: 0.51–0.80). As mentioned above, due to varying yields of the isolated RNAs in each case, a total of 28 cancer and 19 control samples could be finally analyzed for all the four miRNAs. We separately compared the AUC for each miRNA and the combination of the four miRNAs in this sample set also. Results revealed that there was a highly significant difference in the AUC values obtained for the four individual miRNAs and the panel of four in combination (p = 0.0008) in this set of 28 cancer and 19 control samples. The area under the ROC curve (AUC) for miR-21 was 0.62 (95% confidence interval [CI]: 0.45–0.77); for miR-210 was 0.65 (95% CI: 0.49–0.80); for miR-155 was 0.67 (95% CI: 0.51–0.82); and for miR-196a was 0.69 (95% CI: 0.53–0.84). Remarkably, the AUC for the combination of these four miRNAs was 0.82 (95% CI: 0.70–0.94). Thus the AUC increased from 0.62–0.69 range for each individual miRNA to 0.82 for the four miRNAs combined (Fig. 2e). The ROC curves also helped determine the sensitivities and specificities for the plasma miRNAs at various cut-off values. Using the optimal cut-point, the sensitivity and specificity were 46% and 89% for miR-21; 42% and 73% for miR-210; 53% and 78% for Mir155; and 43% and 84% for Mir196a respectively. On the other hand, the sensitivity and specificity for the four miRNAs combined were 64% and 89%. Finally, the composite panel of the four miRNAs in plasma revealed a sensitivity of 46% given a specificity of 100% and a specificity of 37% given a sensitivity of 100% in this study.
Figure 2.
Receiver operating characteristic (ROC) curves of different sample sets analyzed for the plasma levels of the four individual miRNAs (a-d) and an identical sample set analyzed for the four miRNA individually and in combination as a composite panel (e).
Since the sample size in the study was small, we applied leave-one-out scheme to further validate our ROC results. The estimate of the AUC, as obtained by leave-one-out cross-validation, was 0.78 (95% CI 0.64–0.91) for the four miRNAs combined, which still showed a good discriminating power. Using the optimal cut-point, the sensitivity and specificity for the four miRNAs combined were 64% and 89% after cross-validation, which is same as that obtained before cross-validation. Similarly, the composite panel of the four miRNAs in plasma revealed a sensitivity of 46% given a specificity of 100% and a specificity of 32% given a sensitivity of 100% after cross-validation. The results, therefore, document that the combined analysis of this four miRNAs in a panel had a reasonable power to differentiate pancreatic cancer patients from healthy controls.
Discussion
Major ongoing research efforts in the field of cancer detection and diagnosis concentrate on the development of biomarker assays that could be performed on blood samples or other body fluids involving non-invasive or minimally invasive techniques. Assays must be capable of differentiating patients with precursor and advanced malignant lesions at different stages of malignancy from normal healthy individuals. Blood plasma and serum are obviously preferred choices for developing such detection, diagnostic and prognostic markers. Several studies have attempted to utilize proteomic profiling of plasma and serum samples to identify peptide biomarkers reflective of physiologic or pathologic state of malignancy in human cancer patients as well as in genetically engineered mouse models of cancer (29). However, with the challenge of tumor associated proteins possibly constituting only a minor fraction within the vastly abundant dynamic range of plasma proteins, proteomic based strategies of cancer biomarker identification have had limited success to date (30). Recent reports that miRNAs are present in blood plasma and serum in a remarkably stable form together with the findings that circulating miRNAs can distinguish patients with prostate (25) cancer from healthy individuals have suggested that miRNAs may serve as reliable blood based biomarkers for cancer detection. Our present findings that plasma miRNA analyses can help differentiate pancreatic adenocarcinoma patients from healthy controls now provide compelling evidence in support of miRNA profiling in blood plasma being a viable novel approach for developing a minimally invasive biomarker assay for pancreatic cancer. Developing a specific and sensitive blood based miRNA biomarker assay is particularly relevant to pancreatic cancer since current limitations in diagnostic methods and lack of early stage disease symptoms are considered the major cause of high mortality rate among these patients.
Several studies have shown that unique miRNA expression profiles are present in a number of human cancer tissues such as those of breast, lung, esophagus, prostate and pancreas and differential expression of miRNAs correlate with important histopathologic features like tumor stage, proliferation capacity and vascular invasion (31).
In pancreatic cancer, varying expression profiles of a number of miRNAs distinguishing malignant lesions from normal pancreatic tissue and chronic pancreatitis has been published (20,21,32). For the present study, we selected a panel of four miRNAs, miR-21, miR-210, miR-155 and miR-196a, reported to be over expressed in pancreatic ductal adenocarcinomas, which have been characterized for their functional regulatory interactions with genes associated with critical cancer associated phenotypes. This biased approach for the analysis of only a few selected miRNAs was undertaken solely as a proof of principle pilot study to evaluate their utility in developing a blood plasma based biomarker assay for pancreatic cancer. Among the four miRNAs analyzed, up-regulation of miR-21 in cancer cells has been associated with apoptosis inhibition and acquisition of invasive properties (33–34), likely mediated by its down-regulating effects on the expression of two target tumor suppressor genes PTEN (35) and PDCD4 (36). Increased expression of miR-210, on the other hand, has recently been shown to be regulated in a hypoxia inducible factor-1 alpha dependent manner with possible downstream effects on DNA repair genes affecting genomic instability (37). A functional role for miR-155 in pancreatic cancer has been implied based on the observations that it represses the function of the pro-apoptotic protein TP53INP1, which enhances tumorigenicity of pancreatic cancer cells in vivo (38). Interestingly, miR-155 has been also identified as a biomarker of early pancreatic neoplasia consequent to the finding that it is over expressed in about 80% of precursor IPMN lesions (24). Finally, over expression of miR-196a paralleling disease progression was reported to be a predictor of survival for pancreatic cancer patients (20–21).
Our results herein document that the combined analyses of these four miRNAs in plasma can discriminate pancreatic adenocarcinoma patients from normal healthy individuals with fairly good sensitivity and specificity. While the findings demonstrate the feasibility of this approach in designing blood based miRNA biomarker panels, it is important to point out that the small sample size consisting of two extremes of adenocarcinomas and healthy controls, included in the study, allows it to have only limited clinical implications towards developing into pancreatic cancer screening and early detection modality at this time. The results, nonetheless, justify continued development of this strategy towards a more refined modality for efficacy in disease assessment according to the developmental pathways proposed by the Translational Research Working Group of the National Cancer Institute (39). In order for the plasma miRNA panel to be developed as biomarkers for early detection of cancer, it is imperative that adequate sample size of training and test sets spanning different grades and stages of the disease be evaluated through the progressive phases of clinical assay development according to the recommended phased biomarker development concept for early detection of cancer (40). We are in the process of initiating such studies in collaboration with the members of Early Detection Research Network (EDRN) of the National Cancer Institute.
Acknowledgments
Grant Support: The National Cancer Institute Grant UO1CA111302 The National Cancer Institute Cancer Center Support Grant CA16672
References
- 1.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–88. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goggins M. Identifying molecular markers for the early detection of pancreatic neoplasia. Semin Oncol. 2007;34:303–10. doi: 10.1053/j.seminoncol.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am. 2007;36:831–49. doi: 10.1016/j.gtc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 5.Adsay NV, Basturk O, Cheng JD, Andea AA. Ductal neoplasia of the pancreas: Nosologic, clinicopathologic and biologic aspects. Semin Radiat Oncol. 2005;15:254–64. doi: 10.1016/j.semradonc.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Luttges J, Schlehe B, Menke MA, et al. The K-ras mutation pattern in pancreatic ductal adenocarcinoma usually is identical to that in associated normal, hyperplastic, and metaplastic ductal epithelium. Cancer. 1999;85:1703–10. [PubMed] [Google Scholar]
- 7.Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002–6. [PubMed] [Google Scholar]
- 8.DiGiuseppe JA, Hruban RH, Goodman SN, et al. Overexpression of p53 protein in adenocarcinoma of the pancreas. Am J Clin Pathol. 1994;101:684–8. doi: 10.1093/ajcp/101.6.684. [DOI] [PubMed] [Google Scholar]
- 9.Maitra A, Fukushima N, Takaori K, Hruban RH. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 2005;12:81–91. doi: 10.1097/01.pap.0000155055.14238.25. [DOI] [PubMed] [Google Scholar]
- 10.Van Gulik TM, Reeders JW, Bosma A, et al. Incidence and clinical findings of benign, inflammatory disease in patients resected for presumed pancreatic head cancer. Gastrointest Endosc. 1997;46:417–23. doi: 10.1016/s0016-5107(97)70034-8. [DOI] [PubMed] [Google Scholar]
- 11.Vilmann P, Saftoiu A. Endoscopic ultrasoundguided fine needle aspiration biopsy: equipment and technique. J Gastroenterol Hepatol. 2006;21:1646–55. doi: 10.1111/j.1440-1746.2006.04475.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Zheng B, Robbins DH, et al. Accurate discrimination of pancreatic ductal adenocarcinoma and chronic pancreatitis using multimarker expression data and samples obtained by minimally invasive fine needle aspiration. Int J Cancer. 2007;120:1511–7. doi: 10.1002/ijc.22487. [DOI] [PubMed] [Google Scholar]
- 13.Eloubeidi MA, Jhala D, Chhieng DC, et al. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285–92. doi: 10.1002/cncr.11643. [DOI] [PubMed] [Google Scholar]
- 14.Jhala N, Jhala D, Vickers SM, et al. Biomarkers in diagnosis of pancreatic carcinoma in fine-needle aspirates. Am J Clin Pathol. 2006;126:572–9. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 15.Jhala NC, Jhala D, Eltoum I, et al. Endoscopic ultrasound- guided fine-needle aspiration biopsy: a powerful tool to obtain samples from small lesions. Cancer. 2004;102:239–46. doi: 10.1002/cncr.20451. [DOI] [PubMed] [Google Scholar]
- 16.Chang TC, Mendell JT. microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet. 2007;8:215–39. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 17.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94:776–80. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croce CM, Calin GA. miRNAs, cancer and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 19.Volinia S, Calin GA, Liu CG, et al. A microRNA expressionsignature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–8. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 21.Szafranska AE, Davison TS, John J, et al. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26:4442–52. doi: 10.1038/sj.onc.1210228. [DOI] [PubMed] [Google Scholar]
- 22.Lee EJ, Gusev Y, Jiang J, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046–54. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szafranska AE, Doleshal M, Edmunds HS, et al. Analysis of microRNAs in pancreatic fine-needle aspirates can classify benign and malignant tissues. Clin Chem. 2008;54:1716–24. doi: 10.1373/clinchem.2008.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habbe N, Koorstra JB, Mendell JT, et al. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther. 2009;8:340–6. doi: 10.4161/cbt.8.4.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 27.Wong TS, Liu XB, Wong BY, et al. Mature miR-184 as Potential Oncogenic microRNA of Squamous Cell Carcinoma of Tongue. Clin Cancer Res. 2008;14:2588–92. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 28.Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8:166–185. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faca VM, Song KS, Wang H, et al. A mouse to human search for plasma proteome changes associated with pancreatic tumor development. PLoS Med. 2008;5:953–967. doi: 10.1371/journal.pmed.0050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanash SM, Pitteri SJ, Faca VM. Mining the plasma proteome for cancer biomarkers. Nature. 2008;452:571–579. doi: 10.1038/nature06916. [DOI] [PubMed] [Google Scholar]
- 31.Lynam-Lennon N, Maher SG, Reynolds JV. The roles of microRNA in cancer and apoptosis. Biol Rev Camb Philos Soc. 2009;84:55–71. doi: 10.1111/j.1469-185X.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 32.Roldo C, Missiaglia E, Hagan JP, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–84. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 33.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–33. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 34.Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–36. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Kong W, He L, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–33. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 36.Frankel LB, Christoffersen NR, Jacobsen A, et al. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. Bio Chem. 2008;283:1026–33. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 37.Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69:1221–9. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gironella M, Seux M, Xie MJ, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci USA. 2007;104:16170–5. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawk ET, Matrisian LM, Nelson WG, Dorfman GS, Stevens L, Kwok J, Viner J, hautala J, Grad O. The translational research working group developmental pathways: Introduction and overview. Clin Cancer Res. 2008;14:5664–71. doi: 10.1158/1078-0432.CCR-08-1268. [DOI] [PubMed] [Google Scholar]
- 40.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–61. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]