Abstract
AIM
To examine the relationship between serum autotaxin (ATX) concentrations and clinicopathological findings in non-alcoholic fatty liver disease (NAFLD) patients.
METHODS
One hundred eighty-six NAFLD patients who had undergone liver biopsy between 2008 and 2017 were retrospectively enrolled. Serum samples were collected at the time of biopsy and ATX was measured by enzyme immunoassays. Sera obtained from 160 healthy, non-obese individuals were used as controls. Histological findings were graded according to an NAFLD scoring system and correlations with serum ATX were calculated by Spearman’s test. Diagnostic accuracy was evaluated using the area under the receiver operating characteristic curve (AUC). Cut-off values were identified by the Youden index, and the nearest clinically applicable value to the cutoff was considered the optimal threshold for clinical convenience.
RESULTS
Serum ATX levels were significantly higher in NAFLD patients than in controls (0.86 mg/L vs 0.76 mg/L, P < 0.001) and correlated significantly with ballooning score and fibrosis stage (r = 0.36, P < 0.001 and r = 0.45, P < 0.001, respectively). Such tendencies were stronger in female patients. There were no remarkable relationships between ATX and serum alanine aminotransferase, lipid profiles, or steatosis scores. The AUC values of ATX for predicting the presence of fibrosis (≥ F1), significant fibrosis (≥ F2), severe fibrosis (≥ F3), and cirrhosis (F4), were all more than 0.70 in respective analyses.
CONCLUSION
Serum ATX levels may at least partially reflect histological severity in NAFLD.
Keywords: Autotaxin, Non-alcoholic fatty liver disease, Fibrosis, Ballooning
Core tip: Patients with non-alcoholic fatty liver disease (NAFLD) exhibited significantly higher serum levels of autotaxin (ATX) than did healthy subjects. Serum ATX levels correlated significantly with ballooning score and fibrosis stage in NAFLD patients and may therefore reflect histological severity in NAFLD.
INTRODUCTION
The prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing worldwide[1,2]. NAFLD exhibits a wide spectrum, ranging from non-alcoholic fatty liver to non-alcoholic steatohepatitis (NASH) and ensuing cirrhosis and hepatocellular carcinoma[1-3]. Since the concept of NASH was developed using pathological characteristics, i.e., the presence of hepatocyte ballooning and lobular inflammation in addition to macrovesicular steatosis, liver biopsy is currently considered the gold standard for evaluating NAFLD/NASH activity. However, general limitations of liver biopsy are the costs and invasiveness, but also sampling error and inter- and intra-observer variability[4]. So, simple, accurate, non-invasive, quantitative alternatives are needed. Several studies have attempted to estimate histological severity in NAFLD using various serum biomarkers[5-8], but the accuracy of these techniques remains unsatisfactory.
Autotaxin (ATX) was originally discovered in conditioned medium from human melanoma cell cultures[9]. The protein is encoded by ectonucleotide pyrophosphatase/phosphodiesterase family member 2 gene (ENPP2) and catalyzes the hydrolysis of lysophosphatidylcholine (LPC) to lysophosphatidic acid (LPA), which functions as a phospholipase[10,11]. Signaling via a family of six G-protein-coupled receptors (LPA1-6) regulates the diverse cellular processes of ATX, including proliferation, migration, neurogenesis, angiogenesis, fibrogenesis, glucose homeostasis, insulin action, and cancer progression[12-18]. Disrupted LPC metabolism has been reported in murine NASH models[19,20].
ATX is synthesized by a variety of normal cells and tissues, secreted into the circulation as a glycoprotein, and later degraded by liver sinusoidal endothelial cells[21]. Serum ATX levels are reportedly increased during the progression of pregnancy[22] and in patients with idiopathic pulmonary fibrosis or some kinds of cancers[23-25]. Recently, elevated serum ATX has also been implicated in fibrosis progression in chronic hepatitis C[26,27], for which the retarded degradation of circulating ATX due to liver sinusoidal endothelial cell dysfunction from liver fibrosis was considered a main mechanism[28]. Perisinusoidal fibrosis is more frequently detected in alcoholic and non-alcoholic steatohepatitis than in viral hepatitis, with sinusoidal endothelial dysfunction also being reported in NAFLD[29].
Based on the above reports, we have hypothesized that serum ATX is increased in advanced stage NASH patients, but evidence is scarce on the relationship between circulating ATX concentration and histological severity in NAFLD. Accordingly, we measured serum ATX levels in 186 NAFLD patients who had undergone liver biopsy and examined for associations with clinicopathological findings.
MATERIALS AND METHODS
Patients and clinical examinations
This retrospective, cross-sectional study was approved by the Committee for Medical Ethics of Shinshu University School of Medicine (ID number: 3244) and performed in accordance with the Helsinki declaration of 1975, 1983 revision. Informed consent was obtained from all patients. We enrolled 186 biopsy-proven Japanese NAFLD patients who were admitted to Shinshu University Hospital (Matsumoto, Japan) between November 2008 and May 2017. NAFLD was suspected based on the following criteria: (1) the presence of hepatorenal contrast and increased hepatic echogenicity on abdominal ultrasonography; (2) An average daily consumption of < 20 g/d of ethanol; and (3) the absence of other causes of liver dysfunction, such as viral hepatitis, drug-induced liver injury, autoimmune liver disease, primary sclerosing cholangitis, Wilson’s disease, hereditary hemochromatosis, and citrin deficiency[30,31]. The diagnosis of NAFLD/NASH was confirmed with the histological findings of biopsied specimens. Body weight and height were measured before liver biopsy in a fasting state. All laboratory data were obtained in a fasting state on the day of liver biopsy. Homeostasis model assessment for insulin resistance (HOMA-IR), fibrosis-4 index (FIB-4), and aspartate aminotransferase (AST) to platelet ratio index (APRI) were calculated according to the following formulae: HOMA-IR = [fasting blood glucose (mg/dL) × fasting insulin (μU/mL)]/405[32,33], FIB-4 = [age (years) × AST (IU/L)]/[platelet count (109/L) × alanine aminotransferase (ALT) (IU/L)1/2][34], and APRI = [AST/upper limit of normal; 28 (IU/L)] × [100/platelet count (109/L)][35]. One hundred sixty subjects (80 male and 80 female) whose liver function tests and body mass index (BMI) were within normal levels and having no past medical history of NAFLD were selected as healthy controls, with equal age distribution among the male and female individuals (twenties: 20 subjects, thirties: 20 subjects, forties: 20 subjects, fifties: 20 subjects). These healthy controls were same as our previous report[26]. Sera were obtained after overnight fasting on the day of the liver biopsy and stored at -80 °C until testing.
Measurement of ATX
Serum ATX concentrations were determined with a specific two-site enzyme immunoassay using the automated immunoassay analyzer AIA-2000 system (Tosoh Co., Tokyo, Japan), as described previously[36]. To prepare the 2-site immunoassay, R10.23 was digested with pepsin and the purified F(ab)2 form using phenyl-5PW (Tosoh Co.) hydrophobic column chromatography in order to avoid the nonspecific binding of human antibodies against various animal IgG in human specimens, like human anti-mouse antibodies. Magnetic beads were coated with R10.23 F(ab)2 and placed in the reaction cup, and 35 ng of alkaline phosphatase-labeled R10.21 in assay buffer (5% BSA, 5% sucrose, 10 mmol/L Tris-HCl, 10 mmol/L MgCl2, pH 7.4) was added to the reaction cup. ATX assay reagent was prepared by immediate freeze-dry procedure of the reaction cup. The ATX assay reagent thus prepared can be used with AIA-system.
Histological findings
Liver specimens of at least 1.5 cm in length were obtained from segment 5 or 8 using 14-gauge needles, as described previously, and immediately fixed in 10% neutral formalin. Sections of 4 μm in thickness were cut and stained by means of the hematoxylin and eosin and Azan-Mallory methods. The histological activity of NAFLD was assessed by an independent expert pathologist (KS) in a blinded manner according to the NAFLD scoring system proposed by Kleiner et al[37]. Steatosis was graded as 0 to 3 based on the rate of steatotic hepatocytes (< 5%, 5%-33%, > 33-66%, and > 66%, respectively). Lobular inflammation was graded as 0 to 3 based on the overall assessment of all inflammatory foci (no foci, < 2 foci/200× field, 2-4 foci/200× field, and > 4 foci/200× field, respectively). Ballooning grade was scored as 0-2 by the frequency of ballooned hepatocytes (none, few, and many, respectively). NAFLD activity score (NAS) was calculated as the sum of steatosis, lobular inflammation, and ballooning scores, and NASH was defined as the presence of macrovesicular steatosis (> 5% of hepatocytes affected) and hepatocyte ballooning with or without lobular inflammation and fibrosis. Fibrosis stage was scored as follows: F0, none; F1, perisinusoidal or periportal; F2, perisinusoidal and portal/periportal; F3, bridging fibrosis; and F4, cirrhosis.
Statistical analysis
Clinical data are expressed as the number (percentage) or median (interquartile range). Statistical analyses were performed using StatFlex Ver. 6.0 (Artech Co., Ltd., Osaka, Japan) and SPSS 24.0 (IBM, Chicago, IL, United States) software. The Mann-Whitney U test was used for comparisons between two groups. Bonferroni’s correction test was performed for multiple comparisons. Correlation analysis was conducted by Spearman’s test. Diagnostic accuracy was evaluated using the area under the receiver operating characteristic (ROC) curve (AUC). Cut-off values were identified by the Youden index, with the nearest clinically applicable value to the cutoff being considered as the optimal threshold for clinical convenience. All statistical tests were two-sided and evaluated at the 0.05 level of significance.
RESULTS
Serum ATX levels were higher in NAFLD patients
The clinicopathological features of the 186 NAFLD patients enrolled in this study are summarized in Table 1. Eighty (43%) were male, and median age was 56 years. The number of patients according to fibrosis stage F0, F1, F2, F3, and F4 was 35, 89, 19, 34, and 9, respectively. Comparisons between genders revealed significant differences in fibrosis-related parameters, such as age, albumin, hyaluronic acid (HA), and FIB-4, but fibrosis stage distribution was comparable.
Table 1.
Clinicopathological features of 186 patients with non-alcoholic fatty liver disease
|
All (n = 186) |
Male (n = 80) |
Female (n = 106) |
||
| Median (IQR)/n | Median (IQR)/n | Median (IQR)/n | P value1 | |
| Age (yr) | 56 (46-65) | 50 (38-59) | 61 (54-66) | < 0.001 |
| BMI (kg/m2) | 26.2 (23.8-29.6) | 26.1 (24.3-29.4) | 26.5 (23.6-29.7) | NS |
| Laboratory data | ||||
| Albumin (g/dL) | 4.5 (4.3-4.7) | 4.6 (4.4-4.8) | 4.4 (4.2-4.7) | < 0.001 |
| T-bil (mg/dL) | 0.87 (0.69-1.17) | 0.94 (0.74-1.26) | 0.81 (0.67-1.07) | < 0.05 |
| AST (IU/L) | 41 (30-65) | 39 (30-62) | 42 (30-69) | NS |
| ALT (IU/L) | 63 (38-97) | 68 (43-103) | 53 (33-89) | NS |
| γ-GT (IU/L) | 54 (35-92) | 64 (43-99) | 50 (32-81) | < 0.05 |
| TG (mg/dL) | 122 (92-159) | 122 (91-159) | 121 (95-159) | NS |
| LDL-C (mg/dL) | 130 (107-151) | 132 (105-154) | 130 (109-149) | NS |
| HDL-C (mg/dL) | 51 (44-60) | 48 (44-56) | 55 (47-63) | |
| Plt (× 104/μL) | 23.1 (18.5-26.8) | 23.0 (19.6-26.7) | 23.3 (17.6-26.9) | NS |
| HbA1c (%) | 5.9 (5.7-6.6) | 5.9 (5.6-6.5) | 5.9 (5.7-6.6) | NS |
| FBG (mg/dL) | 108 (98-121) | 108 (98-121) | 108 (97-121) | NS |
| IRI (mU/L) | 11.2 (7.2-16.7) | 10.5 (6.8-16.3) | 11.5 (7.4-17.2) | NS |
| HOMA-IR | 3.0 (1.9-4.6) | 2.9 (1.8-4.5) | 3.2 (2.0-4.7) | NS |
| Fe (μg/dL) | 111 (90-137) | 120 (92-146) | 104 (88-129) | < 0.05 |
| Ferritin (ng/mL) | 146 (79-274) | 172 (126-293) | 113 (58-236) | < 0.001 |
| AFP (ng/mL) | 3.2 (2.2-4.8) | 2.8 (2.1-4.0) | 3.4 (2.6-5.2) | < 0.01 |
| Fibrosis markers | ||||
| HA (ng/mL) | 51 (28-91) | 41 (25-62) | 63 (34-118) | < 0.001 |
| 4C7S (ng/mL) | 4.6 (3.8-5.7) | 4.5 (3.8-5.5) | 4.7 (3.8-6.6) | NS |
| FIB-4 | 1.35 (0.94-2.18) | 1.12 (0.77-1.88) | 1.53 (1.13-2.51) | < 0.001 |
| APRI | 0.69 (0.46-1.13) | 0.66 (0.44-1.03) | 0.71 (0.46-1.25) | NS |
| Histological findings | ||||
| Steatosis (1/2/3) | 57/90/39 | 24/41/15 | 33/49/24 | NS |
| Lobular inflammation (0/1/2/3) | 9/101/69/7 | 6/48/23/3 | 3/53/46/4 | < 0.05 |
| Ballooning (0/1/2) | 43/98/45 | 22/44/14 | 21/54/31 | NS |
| Fibrosis (0/1/2/3/4) | 35/89/19/34/9 | 16/43/8/13/0 | 19/46/11/21/9 | NS |
Comparison between male and female subjects. IQR: Interquartile range; BMI: Body mass index; T-bil: Total bilirubin; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; γ-GT: Gamma-glutamyltransferase; TG: Triglyceride; LDL-C: Low density lipoprotein cholesterol; HDL-C: High density lipoprotein cholesterol; Plt: Platelet; FBG: Fasting blood glucose; IRI: Immunoreactive insulin; HOMA-IR: Homeostasis model assessment of insulin resistance; AFP: Alpha-fetoprotein; HA: Hyaluronic acid; 4C7S: Type 4 collagen•7S; FIB-4: Fibrosis-4 index; APRI: AST to platelet ratio; NS: Not significant.
Median serum ATX levels were significantly higher in NAFLD patients than in healthy controls (0.86 vs 0.76 mg/L, P < 0.001) (Figure 1A). In agreement with a previous report demonstrating a gender difference in serum ATX levels[26], serum ATX levels were higher in female patients and controls than in their male counterparts (Figure 1B). The degree of a serum ATX concentration increase was significant in female NAFLD patients (Figure 1B).
Figure 1.
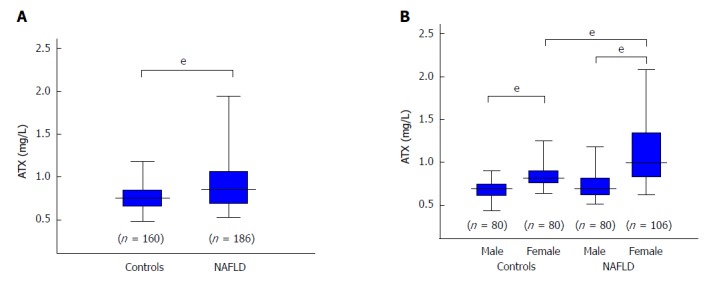
Comparison of autotaxin levels between controls and all patients with non-alcoholic fatty liver disease (A) and according to gender (B). The box plot shows the interquartile range, 95% confidence interval, and median. The difference between each group was tested with the Mann Whitney U test. eP < 0.001. ATX: Autotaxin; NAFLD: Non-alcoholic fatty liver disease.
Relationship between serum ATX levels and clinicopathological features in NAFLD patients
We observed significant but weak correlations between ATX and glucose metabolism, BMI, and iron status, but none with lipid profiles. ATX was significantly and positively correlated to the factors of age, AST, HA, type 4 collagen 7S (4C7S), FIB-4, and APRI and was significantly and negatively correlated to platelet count (Table 2), which supported an association with fibrosis stage in NAFLD[38]. Indeed, ATX was significantly and positively correlated with ballooning grade (r = 0.36, P < 0.001) and fibrosis stage (r = 0.45, P < 0.001) overall, with no significant relationships for steatosis grades (Table 2, Figure 2). These correlations were stronger for women than for men, as were the correlation coefficients for ballooning score and fibrosis stage (Table 2, Figure 3).
Table 2.
Correlation between autotaxin and clinicopathological findings
|
All (n = 186) |
Male (n = 80) |
Female (n = 106) |
||||
| r | P value | r | P value | r | P value | |
| Age (yr) | 0.48 | < 0.001 | 0.45 | < 0.001 | 0.28 | < 0.01 |
| BMI (kg/m2) | 0.18 | < 0.05 | 0.06 | NS | 0.31 | < 0.01 |
| Platelet (× 104/μL) | -0.32 | < 0.001 | -0.28 | < 0.05 | -0.43 | < 0.001 |
| Albumin (g/dL) | -0.32 | < 0.001 | -0.10 | NS | -0.31 | < 0.01 |
| AST (IU/L) | 0.31 | < 0.001 | 0.34 | < 0.01 | 0.40 | < 0.001 |
| ALT (IU/L) | 0.06 | NS | 0.14 | NS | 0.24 | < 0.05 |
| TG (mg/dL) | -0.09 | NS | -0.14 | NS | -0.08 | NS |
| LDL-C (mg/dL) | -0.04 | NS | -0.01 | NS | -0.06 | NS |
| HDL-C (mg/dL) | 0.13 | NS | -0.04 | NS | -0.04 | < 0.001 |
| FBG (mg/dL) | 0.22 | < 0.01 | 0.36 | 0.001 | 0.21 | < 0.05 |
| IRI (mU/L) | 0.20 | < 0.01 | 0.15 | NS | 0.31 | 0.002 |
| HOMA-IR | 0.22 | < 0.01 | 0.22 | < 0.05 | 0.31 | 0.001 |
| Fe (μg/dL) | 0.09 | NS | 0.12 | NS | 0.35 | < 0.001 |
| Ferritin (ng/mL) | 0.04 | NS | 0.22 | NS | 0.31 | 0.002 |
| HA (ng/mL) | 0.49 | < 0.001 | 0.47 | < 0.001 | 0.46 | < 0.001 |
| 4C7S (ng/mL) | 0.40 | < 0.001 | 0.30 | < 0.01 | 0.50 | < 0.001 |
| FIB-4 | 0.58 | < 0.001 | 0.51 | < 0.001 | 0.60 | < 0.001 |
| APRI | 0.43 | < 0.001 | 0.45 | < 0.001 | 0.55 | < 0.001 |
| Histological findings | ||||||
| Steatosis score | 0.02 | NS | 0.12 | NS | -0.03 | NS |
| Lobular inflammation score | 0.22 | < 0.01 | 0.06 | NS | 0.25 | < 0.01 |
| Ballooning score | 0.36 | < 0.001 | 0.34 | < 0.01 | 0.38 | < 0.001 |
| NAS | 0.27 | < 0.001 | 0.27 | < 0.05 | 0.26 | < 0.01 |
| Fibrosis stage | 0.45 | < 0.001 | 0.44 | < 0.001 | 0.53 | < 0.001 |
Correlations were calculated using Spearman’s test. ATX: Autotaxin; BMI: Body mass index; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; TG: Triglyceride; LDL-C: Low density lipoprotein cholesterol; HDL-C: High density lipoprotein cholesterol; FBG: Fasting blood glucose; IRI: Immunoreactive insulin; HOMA-IR: Homeostasis model assessment of insulin resistance; HA: Hyaluronic acid; 4C7S: Type 4 collagen•7S; FIB-4: Fibrosis-4 index; APRI: AST to platelet ratio; NAS: NAFLD activity score; NS: Not significant.
Figure 2.
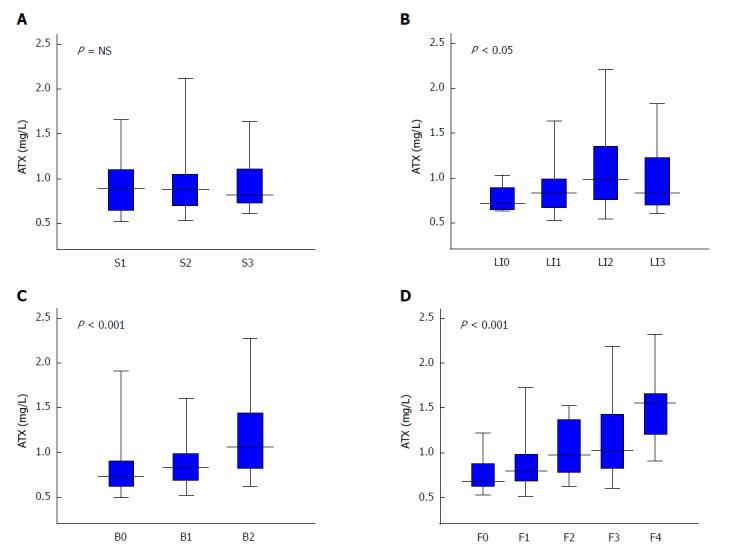
Relationship between autotaxin and histological grade in non-alcoholic fatty liver disease patients for steatosis (A), lobular inflammation (B), ballooning (C), and fibrosis (D). Table 1 presents the number of subjects for each histological stage. The Kruskal-Wallis test was used for multi-group simultaneous comparisons. P values are displayed in the upper left of each graph. ATX: Autotaxin; NAFLD: Non-alcoholic fatty liver disease; NS: Not significant.
Figure 3.
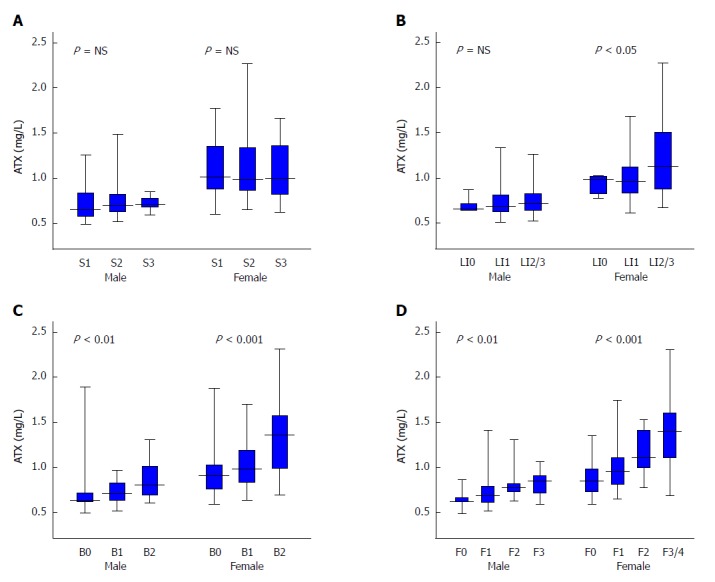
Relationship between autotaxin and histological grade in non-alcoholic fatty liver disease patients by gender for steatosis (A), lobular inflammation (B), ballooning (C), and fibrosis (D). Table 1 presents the number of subjects for each histological stage. The Kruskal-Wallis test was used for multi-group simultaneous comparisons. P values are displayed in the upper left of each graph. ATX: Autotaxin; NAFLD: Non-alcoholic fatty liver disease; NS: Not significant.
Performance of ATX for diagnosing fibrosis status
To assess the significance of ATX as a predictor of fibrosis stage, ROC analysis was performed. Cut off values, sensitivities, specificities, positive predictive values, negative predictive values, and accuracies for predicting the presence of fibrosis (≥ F1), significant fibrosis (≥ F2), severe fibrosis (≥ F3), and cirrhosis (F4) in overall, male, and female NAFLD patients are shown in Table 3, and these ROC curves are shown in Figure 4. The AUC values of ATX for predicting the presence of fibrosis (≥ F1), significant fibrosis (≥ F2), severe fibrosis (≥ F3), and cirrhosis (F4), were all more than 0.70 in respective analyses.
Table 3.
Diagnostic performance of autotaxin for predicting liver fibrosis stage in patients with non-alcoholic fatty liver disease
| Cut off | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
| All patients | |||||||
| ≥ F1 | 0.73 | 0.71 | 77 | 57 | 89 | 36 | 73 |
| ≥ F2 | 1.19 | 0.75 | 45 | 94 | 80 | 77 | 78 |
| ≥ F3 | 1.19 | 0.75 | 51 | 91 | 63 | 86 | 82 |
| F4 | 1.20 | 0.87 | 78 | 85 | 21 | 99 | 84 |
| Male | |||||||
| ≥ F1 | 0.70 | 0.73 | 58 | 94 | 97 | 36 | 65 |
| ≥ F2 | 0.71 | 0.75 | 81 | 68 | 47 | 91 | 71 |
| F3 | 0.82 | 0.74 | 62 | 82 | 40 | 92 | 79 |
| Female | |||||||
| ≥ F1 | 1.03 | 0.76 | 53 | 95 | 98 | 31 | 60 |
| ≥ F2 | 1.19 | 0.80 | 66 | 91 | 82 | 81 | 81 |
| ≥ F3 | 1.19 | 0.78 | 73 | 86 | 67 | 89 | 82 |
| F4 | 1.20 | 0.78 | 78 | 74 | 22 | 97 | 75 |
ATX: Autotaxin; AUC: Area under the receiver operating characteristic curve; PPV: Positive predictive value; NPV: Negative predictive value.
Figure 4.
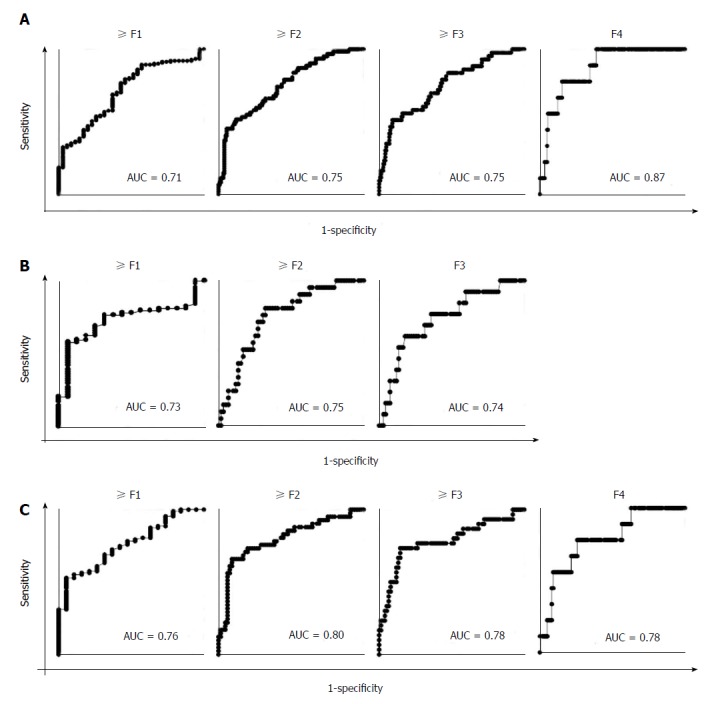
Receiver operating characteristic analysis of autotaxin for the estimation of the presence of fibrosis (≥ F1), significant fibrosis (≥ F2), severe fibrosis (≥ F3), and cirrhosis (F4) in all (A), male (B), and female (C) patients. The areas under the receiver operating characteristic curve are displayed in the lower right of each graph. AUC: Receiver operating characteristic curve; F: Fibrosis.
For comparison, ROC analysis of serum ATX and conventional fibrosis indicators (HA, 4C7S, APRI, and FIB-4) for determination of severe fibrosis (≥ F3) were performed (Table 4). Although sensitivity of ATX is lower than those of HA, 4C7S, APRI, and FIB-4, specificity of ATX was highest (91%) compared to others.
Table 4.
Diagnostic performance of autotaxin and conventional fibrosis indicators for predicting severe fibrosis (≥ F3) in patients with non-alcoholic fatty liver disease
| AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
| All patients | ||||||
| ATX | 0.75 | 51 | 91 | 63 | 86 | 82 |
| HA | 0.82 | 93 | 63 | 44 | 96 | 70 |
| 4C7S | 0.87 | 75 | 88 | 64 | 92 | 85 |
| APRI | 0.82 | 60 | 89 | 62 | 88 | 82 |
| FIB-4 | 0.85 | 79 | 74 | 48 | 92 | 75 |
| Male | ||||||
| ATX | 0.74 | 62 | 82 | 40 | 92 | 79 |
| HA | 0.76 | 85 | 72 | 41 | 95 | 75 |
| 4C7S | 0.81 | 69 | 89 | 56 | 94 | 86 |
| APRI | 0.74 | 77 | 64 | 29 | 93 | 66 |
| FIB-4 | 0.81 | 92 | 75 | 41 | 98 | 78 |
| Female | ||||||
| ATX | 0.78 | 73 | 86 | 67 | 89 | 82 |
| HA | 0.86 | 78 | 86 | 68 | 91 | 83 |
| 4C7S | 0.89 | 78 | 90 | 75 | 92 | 87 |
| APRI | 0.86 | 63 | 95 | 83 | 87 | 86 |
| FIB-4 | 0.85 | 80 | 75 | 56 | 90 | 76 |
AUC: Area under the receiver operating characteristic curve; PPV: Positive predictive value; NPV: Negative predictive value; ATX: Autotaxin; HA: Hyaluronic acid; 4C7S: Type 4 collagen•7S; APRI: AST to platelet ratio; FIB-4: Fibrosis-4 index.
DISCUSSION
Rachakonda et al[39] recently reported increased serum ATX levels in NAFLD patients. In severely obese and non-diabetic women, serum ATX was higher in those with NAFLD compared with those without NAFLD and positively correlated with insulin resistance. However, they did not assess liver pathology in their cohort of female subjects only. In this study, we compared serum ATX levels with clinicopathological background factors in biopsy-proven NAFLD patients and found that serum ATX levels were significantly related to hepatic fibrosis stage and ballooning score, implicating at least a partial reflection of histological severity in NAFLD.
The correlation between serum ATX levels and the severity of hepatic fibrosis has been explained by a mechanism of impaired circulating ATX degradation in damaged or impaired sinusoidal endothelial cells[28]. However, a recent study documented that ATX expression in hepatocytes activated hepatic stellate cells and amplified the fibrotic process, suggesting direct fibrosis-promoting properties of ATX[40]. Since ATX is a novel biomarker for hepatic fibrosis in chronic hepatitis C patients[26,27], we presumed similar results in NAFLD patients, but the correlation between ATX and fibrosis stage was comparatively weaker.
Thus, other mechanisms determining circulating ATX concentrations may exist as ATX is present in various tissues, such as white adipose tissue and the nervous system[41-43]. The importance of visceral fat has also been discussed[44], but in this study, we have not been able to examine waist circumference or waist-to-hip ratio, so this point is the limitation of this study.
In this study, we also conducted AUC analysis of ATX for determination of severe fibrosis (≥ F3) compared to conventional fibrosis indicators (HA, 4C7S, APRI, and FIB-4). AUC values and sensitivity of ATX was inferior to those other indicators[41], but specificity of ATX was highest among those other indicators. So ATX might be useful as a biomarker to exclude severe hepatic fibrosis.
Serum ATX levels were significantly associated with hepatocyte ballooning in our cohort, and a correlation was detected between fibrosis stage and ballooning grade (r = 0.56, P < 0.001). Ballooning degeneration is caused by an impaired intracellular cytoskeleton and resultant protein transport and appears after exposure to oxidative and endoplasmic reticulum stresses and during lipoapoptotic processes[45]. ATX expression was up-regulated by oxidative stress in microglia[46] and by LPC (18:1), an inducer of lipoapoptosis[47], in isolated hepatocytes[42]. Additionally, intravenous injection of LPC (18:1) into mice increased hepatic Enpp2 mRNA expression and hepatocyte apoptosis[40]. These findings may explain how circulating ATX concentrations are positively correlated with the prevalence of hepatocytes with ballooning degeneration.
In this study, we examined the relationship between NAFLD activity score as the severity of NAFLD/NASH and ATX, the correlation coefficient was significant but not high (r = 0.27, P < 0.001, Table 2). It seems difficult to predict the histological severity of NAFLD with ATX alone.
In conclusion, serum ATX levels were significantly higher in NAFLD patients over controls and correlated with ballooning score and fibrosis stage, especially in female patients. Further prospective research in larger cohorts is necessary for understanding the metabolism of circulating ATX in NAFLD.
ARTICLE HIGHLIGHTS
Research background
The prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing worldwide. NAFLD exhibits a wide spectrum, ranging from non-alcoholic fatty liver to non-alcoholic steatohepatitis (NASH) and ensuing cirrhosis and hepatocellular carcinoma. Although the evaluation of NAFLD/NASH depends on the histological findings, there is a limitation and an alternative method is required.
Research motivation
Several studies have attempted to estimate histological severity in NAFLD using various serum biomarkers, but the accuracy of these techniques remains unsatisfactory.
Research objectives
Recently, elevated serum autotaxin (ATX) has been implicated in fibrosis progression in chronic liver disease, especially hepatitis C. So, we examine the relationship between serum ATX concentrations and clinicopathological findings in NAFLD patients.
Research methods
One hundred eighty-six NAFLD patients who had undergone liver biopsy between 2008 and 2017 were retrospectively enrolled. Serum samples were collected at the time of biopsy and ATX was measured by enzyme immunoassays. Sera obtained from 160 healthy, non-obese individuals were used as controls. Histological findings were graded according to an NAFLD scoring system and correlations with serum ATX were calculated by Spearman’s test. Diagnostic accuracy was evaluated using the area under the receiver operating characteristic curve (AUC). Cut-off values were identified by the Youden index, and the nearest clinically applicable value to the cutoff was considered the optimal threshold for clinical convenience.
Research results
Serum ATX levels were significantly higher in NAFLD patients than in controls (0.86 vs 0.76 mg/L, P < 0.001) and correlated significantly with ballooning score and fibrosis stage (r = 0.36, P < 0.001 and r = 0.45, P < 0.001, respectively). Such tendencies were stronger in female patients. There were no remarkable relationships between ATX and serum alanine aminotransferase, lipid profiles, or steatosis scores. The AUC values of ATX for predicting the presence of fibrosis (≥ F1), significant fibrosis (≥ F2), severe fibrosis (≥ F3), and cirrhosis (F4), were all more than 0.70 in respective analyses.
Research conclusions
Serum ATX levels may at least partially reflect histological severity in NAFLD.
Research perspectives
In order to evaluate the severity of NAFLD, it is considered that a method that can simultaneously evaluate activity and fibrosis is necessary.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Committee for Medical Ethics of Shinshu University School of Medicine Institutional Review Board.
Informed consent statement: Written informed consent was obtained from all patients.
Conflict-of-interest statement: The authors declare that no conflict of interest exists.
Data sharing statement: No additional data are available.
Peer-review started: January 17, 2018
First decision: January 25, 2018
Article in press: March 3, 2018
P- Reviewer: Abenavoli L, Esmat S, Tarantino G S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
Contributor Information
Naoyuki Fujimori, Department of Internal Medicine, Division of Gastroenterology, Shinshu University School of Medicine, Matsumoto 390-8621, Japan.
Takeji Umemura, Department of Internal Medicine, Division of Gastroenterology, Shinshu University School of Medicine, Matsumoto 390-8621, Japan.
Takefumi Kimura, Department of Internal Medicine, Division of Gastroenterology, Shinshu University School of Medicine, Matsumoto 390-8621, Japan. t_kimura@shinshu-u.ac.jp.
Naoki Tanaka, Department of Metabolic Regulation, Shinshu University Graduate School of Medicine, Matsumoto, Japan, and Research Center for Agricultural Food Industry, Shinshu University, Matsumoto, 390-8621, Japan.
Ayumi Sugiura, Department of Internal Medicine, Division of Gastroenterology, Shinshu University School of Medicine, Matsumoto 390-8621, Japan.
Tomoo Yamazaki, Department of Internal Medicine, Division of Gastroenterology, Shinshu University School of Medicine, Matsumoto 390-8621, Japan.
Satoru Joshita, Department of Internal Medicine, Division of Gastroenterology, Shinshu University School of Medicine, Matsumoto 390-8621, Japan.
Michiharu Komatsu, Department of Internal Medicine, Division of Gastroenterology, Shinshu University School of Medicine, Matsumoto 390-8621, Japan.
Yoko Usami, Department of Laboratory Medicine, Shinshu University Hospital, Matsumoto 390-8621, Japan.
Kenji Sano, Department of Laboratory Medicine, Shinshu University Hospital, Matsumoto 390-8621, Japan.
Koji Igarashi, Bioscience Division, TOSOH Corporation, Kanagawa 252-1123, Japan.
Akihiro Matsumoto, Department of Internal Medicine, Division of Gastroenterology, Shinshu University School of Medicine, Matsumoto 390-8621, Japan.
Eiji Tanaka, Department of Internal Medicine, Division of Gastroenterology, Shinshu University School of Medicine, Matsumoto 390-8621, Japan.
References
- 1.Tanaka N, Aoyama T, Kimura S, Gonzalez FJ. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Ther. 2017;179:142–157. doi: 10.1016/j.pharmthera.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37 Suppl 1:81–84. doi: 10.1111/liv.13299. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobyliak N, Abenavoli L. The role of liver biopsy to assess non-alcoholic fatty liver disease. Rev Recent Clin Trials. 2014;9:159–169. doi: 10.2174/1574887109666141216102231. [DOI] [PubMed] [Google Scholar]
- 5.Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475–485. doi: 10.3748/wjg.v20.i2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giraudi PJ, Gambaro SE, Ornelas Arroyo S, Chackelevicius CM, Giuricin M, Silvestri M, Macor D, Crocé LS, Bonazza D, Soardo G, et al. A simple in silico strategy identifies candidate biomarkers for the diagnosis of liver fibrosis in morbidly obese subjects. Liver Int. 2018;38:155–163. doi: 10.1111/liv.13505. [DOI] [PubMed] [Google Scholar]
- 7.Tsutsui M, Tanaka N, Kawakubo M, Sheena Y, Horiuchi A, Komatsu M, Nagaya T, Joshita S, Umemura T, Ichijo T, et al. Serum fragmented cytokeratin 18 levels reflect the histologic activity score of nonalcoholic fatty liver disease more accurately than serum alanine aminotransferase levels. J Clin Gastroenterol. 2010;44:440–447. doi: 10.1097/MCG.0b013e3181bdefe2. [DOI] [PubMed] [Google Scholar]
- 8.Kitabatake H, Tanaka N, Fujimori N, Komatsu M, Okubo A, Kakegawa K, Kimura T, Sugiura A, Yamazaki T, Shibata S, et al. Association between endotoxemia and histological features of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:712–722. doi: 10.3748/wjg.v23.i4.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, Liotta LA. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524–2529. [PubMed] [Google Scholar]
- 10.Nakanaga K, Hama K, Aoki J. Autotaxin--an LPA producing enzyme with diverse functions. J Biochem. 2010;148:13–24. doi: 10.1093/jb/mvq052. [DOI] [PubMed] [Google Scholar]
- 11.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moolenaar WH. Lysophospholipids in the limelight: autotaxin takes center stage. J Cell Biol. 2002;158:197–199. doi: 10.1083/jcb.200206094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradère JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pradère JP, Tarnus E, Grès S, Valet P, Saulnier-Blache JS. Secretion and lysophospholipase D activity of autotaxin by adipocytes are controlled by N-glycosylation and signal peptidase. Biochim Biophys Acta. 2007;1771:93–102. doi: 10.1016/j.bbalip.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 16.Castelino FV, Seiders J, Bain G, Brooks SF, King CD, Swaney JS, Lorrain DS, Chun J, Luster AD, Tager AM. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 2011;63:1405–1415. doi: 10.1002/art.30262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai N, Chun J, Duffield JS, Wada T, Luster AD, Tager AM. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 2013;27:1830–1846. doi: 10.1096/fj.12-219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aikawa S, Hashimoto T, Kano K, Aoki J. Lysophosphatidic acid as a lipid mediator with multiple biological actions. J Biochem. 2015;157:81–89. doi: 10.1093/jb/mvu077. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology. 2012;56:118–129. doi: 10.1002/hep.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka N, Takahashi S, Hu X, Lu Y, Fujimori N, Golla S, Fang ZZ, Aoyama T, Krausz KW, Gonzalez FJ. Growth arrest and DNA damage-inducible 45α protects against nonalcoholic steatohepatitis induced by methionine- and choline-deficient diet. Biochim Biophys Acta. 2017;1863:3170–3182. doi: 10.1016/j.bbadis.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen S, Andries M, Vekemans K, Vanbilloen H, Verbruggen A, Bollen M. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett. 2009;284:216–221. doi: 10.1016/j.canlet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Masuda A, Fujii T, Iwasawa Y, Nakamura K, Ohkawa R, Igarashi K, Okudaira S, Ikeda H, Kozuma S, Aoki J, et al. Serum autotaxin measurements in pregnant women: application for the differentiation of normal pregnancy and pregnancy-induced hypertension. Clin Chim Acta. 2011;412:1944–1950. doi: 10.1016/j.cca.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 23.Oikonomou N, Mouratis MA, Tzouvelekis A, Kaffe E, Valavanis C, Vilaras G, Karameris A, Prestwich GD, Bouros D, Aidinis V. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;47:566–574. doi: 10.1165/rcmb.2012-0004OC. [DOI] [PubMed] [Google Scholar]
- 24.Nakai Y, Ikeda H, Nakamura K, Kume Y, Fujishiro M, Sasahira N, Hirano K, Isayama H, Tada M, Kawabe T, et al. Specific increase in serum autotaxin activity in patients with pancreatic cancer. Clin Biochem. 2011;44:576–581. doi: 10.1016/j.clinbiochem.2011.03.128. [DOI] [PubMed] [Google Scholar]
- 25.Xu A, Ahsanul Kabir Khan M, Chen F, Zhong Z, Chen HC, Song Y. Overexpression of autotaxin is associated with human renal cell carcinoma and bladder carcinoma and their progression. Med Oncol. 2016;33:131. doi: 10.1007/s12032-016-0836-7. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki T, Joshita S, Umemura T, Usami Y, Sugiura A, Fujimori N, Shibata S, Ichikawa Y, Komatsu M, Matsumoto A, et al. Association of Serum Autotaxin Levels with Liver Fibrosis in Patients with Chronic Hepatitis C. Sci Rep. 2017;7:46705. doi: 10.1038/srep46705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa H, Ikeda H, Nakamura K, Ohkawa R, Masuzaki R, Tateishi R, Yoshida H, Watanabe N, Tejima K, Kume Y, et al. Autotaxin as a novel serum marker of liver fibrosis. Clin Chim Acta. 2011;412:1201–1206. doi: 10.1016/j.cca.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda H, Yatomi Y. Autotaxin in liver fibrosis. Clin Chim Acta. 2012;413:1817–1821. doi: 10.1016/j.cca.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Pasarín M, La Mura V, Gracia-Sancho J, García-Calderó H, Rodríguez-Vilarrupla A, García-Pagán JC, Bosch J, Abraldes JG. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS One. 2012;7:e32785. doi: 10.1371/journal.pone.0032785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komatsu M, Yazaki M, Tanaka N, Sano K, Hashimoto E, Takei Y, Song YZ, Tanaka E, Kiyosawa K, Saheki T, et al. Citrin deficiency as a cause of chronic liver disorder mimicking non-alcoholic fatty liver disease. J Hepatol. 2008;49:810–820. doi: 10.1016/j.jhep.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Komatsu M, Kimura T, Yazaki M, Tanaka N, Yang Y, Nakajima T, Horiuchi A, Fang ZZ, Joshita S, Matsumoto A, et al. Steatogenesis in adult-onset type II citrullinemia is associated with down-regulation of PPARα. Biochim Biophys Acta. 2015;1852:473–481. doi: 10.1016/j.bbadis.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka N, Nagaya T, Komatsu M, Horiuchi A, Tsuruta G, Shirakawa H, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, et al. Insulin resistance and hepatitis C virus: a case-control study of non-obese, non-alcoholic and non-steatotic hepatitis virus carriers with persistently normal serum aminotransferase. Liver Int. 2008;28:1104–1111. doi: 10.1111/j.1478-3231.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 34.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 35.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura K, Igarashi K, Ide K, Ohkawa R, Okubo S, Yokota H, Masuda A, Oshima N, Takeuchi T, Nangaku M, et al. Validation of an autotaxin enzyme immunoassay in human serum samples and its application to hypoalbuminemia differentiation. Clin Chim Acta. 2008;388:51–58. doi: 10.1016/j.cca.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 38.Nagaya T, Tanaka N, Komatsu M, Ichijo T, Sano K, Horiuchi A, Joshita S, Umemura T, Matsumoto A, Yoshizawa K, et al. Development from simple steatosis to liver cirrhosis and hepatocellular carcinoma: a 27-year follow-up case. Clin J Gastroenterol. 2008;1:116–121. doi: 10.1007/s12328-008-0017-0. [DOI] [PubMed] [Google Scholar]
- 39.Rachakonda VP, Reeves VL, Aljammal J, Wills RC, Trybula JS, DeLany JP, Kienesberger PC, Kershaw EE. Serum autotaxin is independently associated with hepatic steatosis in women with severe obesity. Obesity (Silver Spring) 2015;23:965–972. doi: 10.1002/oby.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaffe E, Katsifa A, Xylourgidis N, Ninou I, Zannikou M, Harokopos V, Foka P, Dimitriadis A, Evangelou K, Moulas AN, et al. Hepatocyte autotaxin expression promotes liver fibrosis and cancer. Hepatology. 2017;65:1369–1383. doi: 10.1002/hep.28973. [DOI] [PubMed] [Google Scholar]
- 41.Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, Fujita K, Chayama K, Saibara T, Kawada N, Fujimoto K, Kohgo Y, Yoshikawa T, Okanoue T; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD) Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. doi: 10.1186/1471-230X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Souza K, Kane DA, Touaibia M, Kershaw EE, Pulinilkunnil T, Kienesberger PC. Autotaxin Is Regulated by Glucose and Insulin in Adipocytes. Endocrinology. 2017;158:791–803. doi: 10.1210/en.2017-00035. [DOI] [PubMed] [Google Scholar]
- 43.Yuelling LM, Fuss B. Autotaxin (ATX): a multi-functional and multi-modular protein possessing enzymatic lysoPLD activity and matricellular properties. Biochim Biophys Acta. 2008;1781:525–530. doi: 10.1016/j.bbalip.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finelli C, Sommella L, Gioia S, La Sala N, Tarantino G. Should visceral fat be reduced to increase longevity? Ageing Res Rev. 2013;12:996–1004. doi: 10.1016/j.arr.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Bedossa P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017;37 Suppl 1:85–89. doi: 10.1111/liv.13301. [DOI] [PubMed] [Google Scholar]
- 46.Awada R, Rondeau P, Grès S, Saulnier-Blache JS, Lefebvre d’Hellencourt C, Bourdon E. Autotaxin protects microglial cells against oxidative stress. Free Radic Biol Med. 2012;52:516–526. doi: 10.1016/j.freeradbiomed.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Kakisaka K, Cazanave SC, Fingas CD, Guicciardi ME, Bronk SF, Werneburg NW, Mott JL, Gores GJ. Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G77–G84. doi: 10.1152/ajpgi.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


