Abstract
Various noninvasive liver functional reserve models have been proposed, but their prognostic ability in patients with hepatocellular carcinoma (HCC) is unclear. We aimed to investigate the performance of twelve noninvasive liver reserve models in HCC patients undergoing surgical resection. A total of 645 patients undergoing resection were prospectively identified and retrospectively analyzed. Tumor recurrence, overall survival, and independent prognostic factors were evaluated by the Cox proportional hazards model. Of the twelve models, the King’s score showed the highest homogeneity and lowest corrected Akaike information criterion (AICc) value, suggesting a better predictive ability for tumor recurrence. In multivariate Cox analysis, we confirmed that King’s score, tumor size and serum alpha-fetoprotein level were independent predictors associated with recurrence. In survival prediction, albumin-bilirubin (ALBI) revealed the highest homogeneity and lowest value among twelve invasive models, indicating a better prognostic performance. In the Cox model, ALBI grade, tumor burden, alpha-fetoprotein, vascular invasion, diabetes mellitus and performance status were independent predictors linked with overall survival. In summary, the currently used liver function models have differential predictive ability for HCC patients undergoing surgical resection. The King’s score is a feasible tool to predict tumor recurrence, whereas ALBI grade is a more robust model for prognostic prediction.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, accounting for more the 700,000 deaths each year1. Chronic hepatitis B and C virus (HBV, HCV) infection and alcoholism are the major risk factors for HCC2. HCC develops in the background of chronic liver disease or cirrhosis in 70–90% patients, and various degrees of liver dysfunction are usually present at the time of diagnosis. Surgical resection is the curative treatment option for patients with early stage HCC with well preserved liver function, and provides 5-year survival rate up to 70%3,4. However, tumor recurrence after surgery is common and is associated with a decreased overall survival5,6.
The prognosis and management of HCC is typically influenced by tumor burden, liver functional reserve and performance status3. The Child-Turcotte-Pugh (CTP) classification has been widely used for decades in assessing the severity of liver dysfunction. Many HCC staging systems, including the Barcelona Clínic Liver Cancer (BCLC) staging system, utilize CTP classification as an indicator of liver disease severity. However, the CTP classification is not an evidence-based practice. The model for end-stage liver disease (MELD) has been a prevailing system to prioritize cirrhotic patients awaiting liver transplantation7, and is used in assessing liver functional reserve and outcome for HCC patients8–10. Another important marker, indocyanine green retention rate at 15 minutes (ICG-15) test, has also been widely used to evaluate liver reserve in surgical HCC patients11.
Alternatively, the albumin-bilirubin (ALBI) and the platelet-albumin-bilirubin (PALBI) grade were recently proposed to assess liver functional reserve in HCC12,13. In addition, serum sodium concentration was found to inversely correlate with the severity of cirrhosis, and has been used to assess the degree of portal hypertension14–16. Other tools to evaluate liver functional reserve include aspartate aminotransferase-to-platelet ratio (APRI), fibrosis index based on 4 factors (FIB-4), King’s score, cirrhosis discriminate index (CDS), Lok index and the Göteborg University Cirrhosis Index (GUCI)17–22. These models incorporate different clinical parameters such as age and serum biochemistries. Up to date, twelve noninvasive liver reserve models are used to assess the degree of liver dysfunction, but the prognostic role of these models in HCC patients remains unclear. This study aimed to investigate the correlation of these noninvasive models and their prognostic impact on tumor recurrence and overall survival in HCC patients undergoing surgical resection.
Patients and Methods
Patients and follow-up
During a 12-year period between 2003 to 2015, patients with newly diagnosed HCC and admitted to Taipei Veterans General Hospital were prospectively identified and retrospectively analyzed. A total 645 patients undergoing surgical resection were enrolled in this study. The baseline demographics, etiology of liver disease, tumor status and serum biochemistries were collected at the time of diagnosis. Tumor recurrence, subsequent anti-cancer therapy, and overall survival were recorded. The inclusion criteria of surgery were (1) tumor involving no more than three Couinaud segments, (2) CTP class A or B and data for ICG-15, (3) no main portal vein trunk involvement or distant metastases, and (4) absence of other major diseases that may complicate resection23.
After surgery, the patients were followed up with imaging studies and serum a-fetoprotein (AFP) level every 3 to 6 months until death or dropout from the follow-up program. This study complies with the standard of the Declaration of Helsinki and current ethical guidelines, and has been approved by the Institutional Review Broad of Taipei Veterans General Hospital. Waiver of consent was obtained, and patient records/information was anonymized and de-identified prior to analysis.
Diagnosis and definition
The pre-operative diagnosis of HCC was histologically confirmed or based on the findings of typical four-phase multidetector contrast-enhanced dynamic computed tomography (CT) scan or magnetic resonance imaging (MRI)3. The performance status was assessed by using the Eastern Cooperative Oncology Group Performance scaling ranging from 0 (asymptomatic) to 4 (confined to bed). Intrahepatic recurrence was defined as residual disease within or adjacent to the previously treated tumor site, whereas extrahepatic recurrence was defined as emergence of the tumor elsewhere in or outside the liver24.
Treatment
Surgical resection was performed by our experienced surgical team. The operative procedures have been previously described in detail25–27. The resected liver tissue was sent for gross and microscopic examinations, and the recorded tumor size was based on the largest dimension of the resected specimen. The treatment of recurrence HCC included re-resection, local ablative treatment, transarterial chemoembolization, targeted therapy, chemotherapy, radiotherapy and best supportive care.
Grading of 12 models
The calculation of 12 noninvasive liver functional reserve models was based on clinical variables and serum biochemistries at the time of diagnosis. The grading of these liver functional reserve models was according to published studies8–10,12,13,17–21,28,29. Grade 1 indicates adequate liver functions, and grade 3 is associated with poor liver reserve (Table 1).
Table 1.
Formula and grading of 12 noninvasive liver functional reserve models.
| Noninvasive blood testing for liver serve makers | Formula | ||
|---|---|---|---|
| ALBI, Grade 1/2/3 (<−2.6/−2.6–≤−1.39 />−1.39) | (log(Bilirubin[μmol/L]) × 0.66) + (Albumin[g/L] × −0.085) | ||
| APRI, Grade 1/2/3 (<0.5/0.5–1.5/>1.5) | [(AST/upper limit of normal)/Platelet Count (109/l)] × 100 | ||
| CTP, A/B/C, grade 1/2/3/ (5–6/7–9/10–15) | Encephalopathy: none = 1, grade 1 or 2 = 2, grade 3 or 4 = 3 Ascites: none = 1, mild to moderate = 2, severe = 3 Bilirubin(mg/dl): <2 = 1, 2–3 = 2, >3 = 3 Albumin(g/dl): >3.5 = 1, 2.8–3.5 = 2, <2.8 = 3 PT sec (INR): <4 (1.7) = 1, 4–6 (1.7–2.3) = 2, >6 (>2.3) = 3 | ||
| CDS, Grade 1/2/3 (<4/4–7/>7) | Platelet count ( × 109/L): >340 = 0; 280–339 = 1; 220–279 = 2; 160–219 = 3; 100–159 = 4; 40–99 = 5; <40 = 6 | ALT/AST ratio: >1.7 = 0; 1.2–1.7 = 1; 0.6–1.19 = 2; <0.6 = 3 | INR: <1.1 = 0; 1.1–1.4 = 1; >1.4 = 2 CDS is the sum of the above (possible value 0–11) |
| FIB-4 index, Grade 1/2/3 (<1.45/1.45–3.25/>3.25) | (Age[years] × AST[U/L])/(platelet [109] × ALT[U/L]1/2) | ||
| GUCI, Grade 1/2/3 (<0.5/0.5–1.56/>1.56) | [AST/TOPNORMAL AST] × INR × 100/(Platelets × 109) | ||
| Lok index, Grade 1/2/3 (<0.5/0.5–0.8/>0.8) | Lok Index = e(LogOddsLok)/(1 + e(LogOddsLok)) Log Odds Lok = (1.26 × AST/ALT) + (5.27 × INR) − (0.0089 × Platelets x109) − 5.56 | ||
| MELD, Grade 1/2/3 (<8/8–12/>12) | 10 × ((0.957 × ln(Creatinine)) + (0.378 × ln(Bilirubin)) + (1.12 × ln(INR))) + 6.43 | ||
| PABLI, Grade1/2/3 (≤−2.53, −2.53 and ≤−2.09, >−2.09) | (2.02 × log10 bilirubin) − [0.37 × (log10 bilirubin(umol/L))2] − 0.04 × albumin (g/L) − 3.48 × log10 platelets(109/L) + 1.01 × (log10 platelets(109/l))2 | ||
| King’s score (<7.6/7.6–16.7/16.7) | Age × AST × INR/[platelets (109/l)] | ||
| Serum sodium (≦135/>135 mmole/L) | |||
| ICG-15 test (%) (10/10–20/>20) | |||
Statistical Analysis
All statistical analyses were conducted using the SPSS for Windows version 21 release (SPSS Inc., Chicago, IL, USA). The X2 test or Fisher’s exact test was used to analyze categorical variables and the Mann-Whitney ranked sum test for continuous variables. The recurrence-free survival and overall survival were estimated by the Kaplan-Meier method and compared by a log-rank test. Independent prognostic factors that were possibly linked to recurrence-free survival and overall survival were analyzed. Factors that were significant in the univariate analysis were entered into the adjusted multivariate Cox proportional hazards model to determine adjusted hazard ratio (HR) and 95% confidence interval (CI)30.
The discriminatory ability of different models associated with tumor recurrence and overall survival was examined by using the Cox proportional hazards model, and the consequences of the Cox model were expressed with the corrected Akaike information criterion (AICc), which reveals how the model affects the dependent variable and represents an overall assessment of the model31,32. The lower the AIC, the more explanatory and informative the model is33. We also examined the correlation of ICG-15 and other 11 noninvasive liver functional reserve models. For all tests, a p < 0.05 was considered statistically significant.
Results
Baseline characteristics
A prospective data set of 645 patients who received surgical resection as curative treatment were enrolled during the study period. Baseline demographics and clinical information of these patients are shown in Table 2. The median age was 61 years with the majority being male (80%). Three hundred and twenty-seven (51%) patients had HBV infection, and 131(20%) had a history of diabetes mellitus. Two hundred and thirty (36%) patients had tumor size ≤3 cm and 633 (98%) of had ≤3 nodules at initial presentation. In these patients, 254 (39%) and 232 (36%) received lobectomy and bi-segmentectomy respectively, while 133 (21%) and 26 (4%) patients received segmentectomy and sub-segmentectomy respectively. All patients were histologically confirmed HCC and were free of surgical margin.
Table 2.
Baseline characteristics of hepatocellular carcinoma undergoing surgical resection.
| Variables | Patients (n = 645) |
|---|---|
| Age (years, median [IQR]) | 61[52–70] |
| Male, n (%) | 518 (80) |
| Etiologies of liver disease | |
| HBV, n (%) | 327 (51) |
| HCV, n (%) | 116(18) |
| HBV + HCV, n (%) | 27 (4) |
| Alcohol, n (%) | 19 (3) |
| Diabetes mellitus, n (%) | 131 (20) |
| Performance status (0/1/2–4), n (%) | 507/104/34 (79/15/6) |
| Ascites, n (%) | 45 (7) |
| ICG (%, median [IQR]) | 10 [6–14] |
| Apha-fetoprotein (ng/mL) median [IQR] | 25.7 [7.27–301] |
| Tumor nodules (≦3/>3), n (%) | 633/12 (98/2) |
| Maximal tumor diameter (≤3/>3 cm), n(%) | 230/415(36/64) |
| Vascular invasion, n (%) | 68 (10%) |
| ALT (IU/L), median [IQR] | 45 [29–75.5] |
| AST (IU/L), median [IQR] | 44 [29–73.50] |
| Laboratory values (mean ± SD) | |
| Alkaline phosphatase (IU/L) | 99.80 ± 78.49 |
| Albumin (g/L) | 39.93 ± 5.03 |
| Total bilirubin (μmol/L) | 14.41 ± 10.38 |
| Creatinine (mg/dl) | 1.05 ± 0.74 |
| Platelets (1,000/μL) | 180.96 ± 82.00 |
| INR of prothrombin time (sec) | 1.03 ± 0.11 |
| Serum sodium (mmol/L) | 139.59 ± 2.75 |
| Non-invasive liver functional reserve models | |
| ALBI grade (1/2/3), n (%) | 402/234/9 (62/37/1) |
| APRI grade (1/2/3), n (%) | 225/284/136(35/44/21) |
| CDS grade (1/2/3), n (%) | 225/388/32 (35/60/5) |
| CTP classification (A/B-C), n (%) | 605/40 (94/6) |
| FIB-4 grade (1/2/3), n (%) | 135/282/228 (21/44/35) |
| GUCI grade (1/2/3), n (%) | 220/288/137 (34/45/21) |
| ICG (1/2/3), n(%) | 380/206/59 (59/31/9) |
| King’s score(1/2/3), n (%) | 112/207/326 (17/32/51) |
| Lok index grade (1/2/3), n (%) | 447/154/44 (69/24/7) |
| MELD score (<8/8–12/>12), n (%) | 419/190/36 (65/29/6) |
| Serum Na (1/2), n (%) | 616/49 (95/5) |
| PALBI grade (1/2/3), n (%) | 372/213/60 (58/33/9) |
| Extent of hepatic resection | |
| Sub-segmentectomy, n (%) | 26 (4) |
| Segmentectomy, n (%) | 133 (21) |
| Bi-segmentectomy, n (%) | 232 (36) |
| Lobectomy, n (%) | 254 (39) |
ALBI, Albumin-bilirubin; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; APRI, Aspartate transaminase-to-Platelet ratio; CDS, Cirrhosis discriminant index;
CTP, Child-Turcotte-Pugh score; FIB-4, Fibrosis-4 score; ICG, Indocyanine green;
HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, Model for End-stage liver disease;
GUCI, Göteborg University Cirrhosis Index; PALBI, platelet-albumin-bilirubin;
SD, standard deviation; IQR, interquartile range.
Tumor recurrence
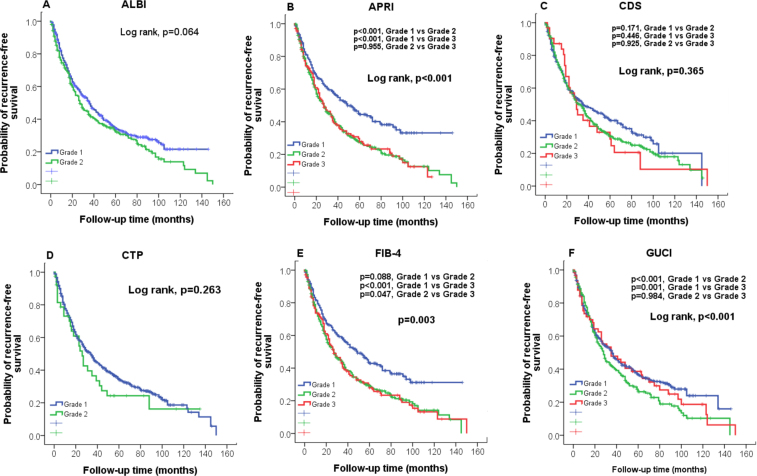
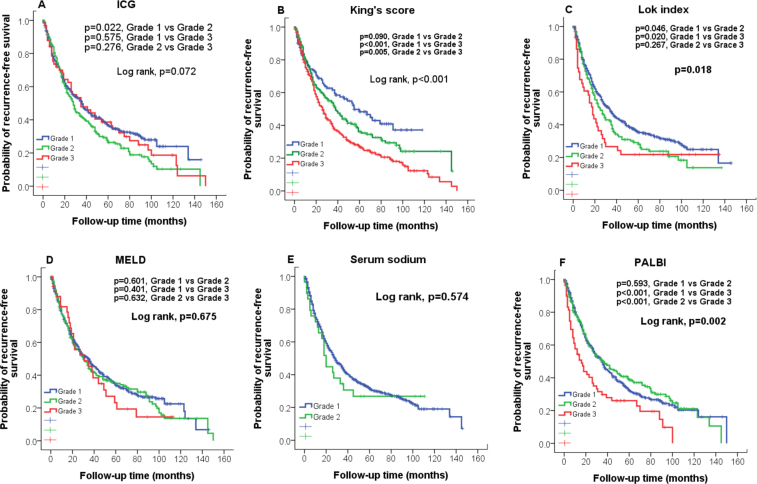
The median recurrence-free survival was 23 months, and 413 (64%) patients had tumor recurrence during the follow-up. The estimated 1-, 3-, and 5-year recurrence-free survival rates were 73%, 43% and 33%, respectively. The predictive role of 12 noninvasive liver functional reserve models on recurrence-free survival was evaluated according to their grading (Figs 1 and 2). A significant difference in recurrence-free survival were found only in APRI, FIB-4, GUCI, King’s score, Lok index and PALBI (all p < 0.05). Pairwise comparison showed that there was no significant difference between APRI grade 2 vs 3 (p = 0.995), GUCI grade 2 vs 3 (p = 0.984), Lok index grade 2 vs 3 (p = 0.267) and PALBI grade 1 and grade 2 (p = 0.593). Comparison of prognostic performance in terms of tumor recurrence prediction among 12 models reveals that the King’s score had the highest homogeneity and lowest AICc value (Table 3).
Figure 1.
Comparison of recurrence-free survival distribution according to (A) ALBI, (B) APRI, (C)CDS, (D) CTP, and (E) FIB-4, (F) GUCI grading. Significant survival differences are found in APRI, FIB4 and GUCI (p < 0.05).
Figure 2.
Comparison of recurrence-free survival distributions according to (A) ICG, (B) King’s score, (C) Lok index, (D) MELD, and (E) Serum sodium, (F) PALBI grading. Significant survival differences are found in King’s score, Lok index and PALBI (p < 0.05).
Table 3.
Predictive accuracy of tumor recurrence and overall survival in 12 noninvasive liver functional reserve models.
| Homogeneity (Wald χ2) | Corrected Akaike information criteria (AIC) | |
|---|---|---|
| Tumor recurrence | ||
| ALBI | 6.166 | 4968.555 |
| APRI | 17.027 | 4957.694 |
| CDS | 2.378 | 4972.344 |
| CTP | 0.535 | 4974.187 |
| FIB-4 | 13.176 | 4961.545 |
| GUCI | 15.276 | 4959.445 |
| ICG-15 | 6.025 | 4968.696 |
| King’s score | 21.248 | 4953.474 |
| Lok index | 6.186 | 4968.536 |
| MELD | 1.264 | 4973.457 |
| Serum Na | 0.298 | 4974.423 |
| PALBI | 4.831 | 4968.891 |
| Overall survival | ||
| ALBI | 14.822 | 3999.685 |
| APRI | 3.724 | 4010.784 |
| CDS | 0.244 | 4014.263 |
| CTP | 1.496 | 4013.011 |
| FIB-4 | 5.754 | 4008.753 |
| GUCI | 3.378 | 4011.130 |
| ICG-15 | 3.944 | 4010.563 |
| King’s score | 7.663 | 4006.845 |
| Lok index | 5.736 | 4008.815 |
| MELD | 0.908 | 4013.600 |
| Serum Na | 3.334 | 4009.173 |
| PALBI | 8.491 | 4006.017 |
In univariate analysis, positive HBsAg, alcoholism, high AFP, tumor number > 3 nodules, tumor size > 3 cm, presence of vascular invasion and King’s score grade 3 were associated with increased risk of recurrence (all p < 0.05; Table 4). In the Cox model, 4 independent predictors of tumor recurrence were identified: alcoholism (HR: 1.443; 95% CI: 1.062–1.960, p = 0.019), high AFP level (HR: 1.499; 95% CI: 1.185–1.897, p = 0.001), tumor size > 3 cm (HR: 1.562, 95% CI: 1.219–2.001, p < 0.001) and King’s score grade 3 (HR: 1.770, 95% CI: 1.318–2.378, p < 0.001).
Table 4.
Univariate and multivariate analysis of factors associated with tumor recurrence and overall survival.
| Tumor recurrence | Number | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | CI | p | HR | CI | p | ||
| Age (<65/≥65 years) | 399/246 | 1.030 | 0.845–1.255 | 0.769 | |||
| Sex (male/female) | 518/127 | 0.912 | 0.712–1.68 | 0.465 | |||
| HBsAg (negative/positive) | 235/410 | 1.251 | 1.020–1.535 | 0.032 | |||
| Anti-HCV (negative/positive) | 490/155 | 1.028 | 0.822–1.286 | 0.811 | |||
| Alcoholism (no/yes) | 552/93 | 1.379 | 1.062–1.789 | 0.016 | 1.443 | 1.062–1.960 | 0.019 |
| DM (no/yes) | 514/131 | 0.915 | 0.714–1.172 | 0.481 | |||
| Ascites (absent/present) | 600/45 | 1.185 | 0.796–1.764 | 0.402 | |||
| Alpha-fetoprotein (<20/≥20 ng/mL) | 301/344 | 1.399 | 1.152–1.699 | 0.001 | 1.499 | 1.185–1.897 | 0.001 |
| Tumor nodules ( ≦ 3/ > 3nodules) | 633/12 | 1.869 | 0.927–3.767 | 0.080 | |||
| Tumor size ( ≦ 3 cm/ > 3 cm) | 230/415 | 1.374 | 1.121–1.684 | 0.002 | 1.543 | 1.203–1.978 | 0.001 |
| Performance status (0/1–4) | 507/138 | 1.287 | 1.016–1.629 | 0.036 | |||
| Vascular invasion (no/yes)) | 577/68 | 1.780 | 1.309–2.421 | <0.001 | |||
| King’s score | |||||||
| Grade 1 | 112 | 1 | 1 | ||||
| Grade 2 | 207 | 1.205 | 0.876–1.658 | 0.252 | 1.327 | 0.958–1.838 | 0.088 |
| Grade 3 | 326 | 1.741 | 1.297–2.337 | <0.001 | 1.770 | 1.318–2.378 | <0.001 |
| Overall survival | |||||||
| Age (<65/≥65 years) | 399/246 | 1.283 | 1.036–1.589 | 0.023 | |||
| Sex (male/female) | 518/127 | 0.941 | 0.716–1.236 | 0.661 | |||
| HBsAg (negative/positive) | 235/410 | 0.854 | 0.687–1.061 | 0.153 | |||
| Anti-HCV (negative/positive) | 490/155 | 1.130 | 0.889–1.436 | 0.319 | |||
| Alcoholism (no/yes) | 552/93 | 1,346 | 1.010–1.794 | 0.043 | |||
| DM (no/yes) | 514/131 | 1.429 | 1.108–1.841 | 0.006 | 1.489 | 1.152–1.925 | 0.002 |
| Ascites (absent/present) | 600/45 | 1.621 | 1.098–2.391 | 0.015 | |||
| Alpha-fetoprotein (<20/≥20 ng/mL) | 301/344 | 1.695 | 1.365–2.106 | <0.001 | 1.513 | 1.211–1.891 | <0.001 |
| Tumor nodules ( ≦ 3/ > 3 nodules) | 633/12 | 2.816 | 1.496–5.298 | 0.001 | 2.599 | 1.372–4.924 | 0.003 |
| Tumor size ( ≦ 3 cm/ > 3 cm) | 230/415 | 2.035 | 1.602–2.584 | <0.001 | 1.747 | 1.365–2.236 | <0.001 |
| Performance status (0/1–4) | 507/138 | 1.589 | 1.228–2.058 | <0.001 | 1.311 | 1.006–1.710 | 0.045 |
| Vascular invasion (no/yes) | 577/68 | 2.964 | 2.217–3.963 | <0.001 | 2.334 | 1.723–3.162 | <0.001 |
| ALBI grade | |||||||
| Grade 1 | 402 | 1 | 1 | ||||
| Grade 2–3 | 243 | 1.526 | 1.231–1.891 | <0.001 | 1.439 | 1.158–1.790 | 0.001 |
Treatment after tumor recurrence
During the follow-up period, 377 (58%) patients had intrahepatic recurrence and 36 (9%) had extrahepatic recurrence. Treatment of recurrent HCC included re-resection (n = 35), local ablative therapy (n = 131), transarterial chemoembolization (n = 187), sorafenib (n = 6), chemotherapy (n = 16), radiotherapy (n = 10) and best supportive care (n = 27).
Overall survival
The median overall survival was 55 months and 343 (53%) of patient died during follow-up. The cause of death was tumor recurrence in 213 (62.2%) patients. Another 73 (20.2%) patients died of liver failure or complications of portal hypertension with (66 patients) or without (7 patients) tumor recurrence, and the remaining 57 (16.6%) died of non-liver related causes.
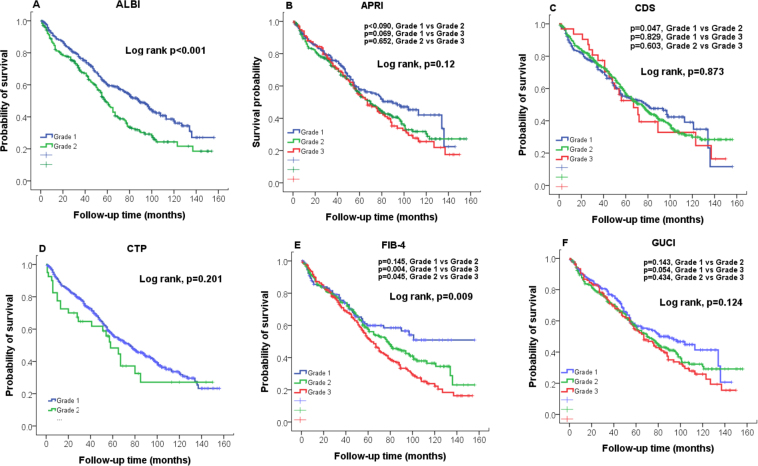
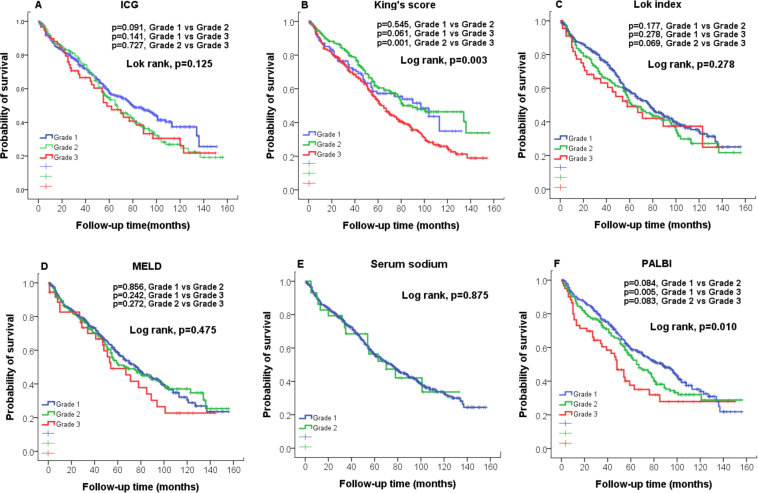
The estimated 1-, 3-, and 5-year survival rates were 88%, 74% and 56%, respectively. The survival distribution according to the grading of 12 noninvasive liver reserve models are shown in Figs 3 and 4. Significant differences in overall survival were found only in ALBI, FIB-4, King’s core and PALBI (all p < 0.05). Pairwise comparison showed that there were no significant differences in FIB-4 grade 1 vs 3 (p = 0.145), King’s score grade 1 vs 3 (p = 0.545), PALBI grade 1 vs grade 3 (p = 0.084) and grade 2 vs grade 3 (p = 0.083). The prognostic role of these 12 models for survival analysis showed that the ALBI grade had the highest homogeneity and lowest AICc value (Table 3).
Figure 3.
Comparison of overall survival distribution according to (A) ALBI, (B) APRI, (C)CDS, (D) CTP, and (E) FIB-4, (F) GUCI grading. Significant survival differences are found in ALBI and FIB-4 (p < 0.05).
Figure 4.
Comparison of overall survival distributions according to (A) ICG, (B) King’s score, (C) Lok index, (D) MELD, and (E) Serum sodium, (F) PALBI grading. Significant survival differences are found in King’s score and PALBI (p < 0.05).
In univariate analysis of overall survival among surgical patients, older age (≥65 years), alcoholism, presence of diabetes mellitus, presence of ascites, high AFP level, larger tumor (>3 cm) and multi-nodularity (>3 nodules), poor performance status, presence of vascular invasion and ALBI grade 2–3 were associated with decreased survival (all p < 0.05, Table 4). In the Cox model, seven independent adverse prognostic predictors were found: diabetes mellitus (HR: 1.489, 95% CI: 1.152–1.925, p = 0.002), AFP ≥ 20 ng/ml (HR: 1.513; 95% CI: 1.211–1.891, p < 0.001), >3 tumor nodules (HR: 2.599, 95% CI: 1.372–4.924, p = 0.003), main tumor size > 3 cm (HR: 1.747, 95% CI: 1.365–2.236, p < 0.001), poor performance status (HR: 1.311, 95% CI: 1.006–1.710, p = 0.045), vascular invasion (HR: 2.334; 95% CI: 1.723–3.162, p < 0.001), and ALBI grade 2–3 (HR: 1.439, 95% CI: 1.158–1.790, p < 0.001).
Correlation analysis
Except for serum sodium, the scores of other 10 noninvasive liver functional reserve models all significantly increased with higher ICG-15 value (Table 5).
Table 5.
Correlation between ICG-15 and different liver functional reserve models.
| ICG-15 retention rate (%) | |||||
|---|---|---|---|---|---|
| <10 | 10–15 | 15–20 | > 20 | p | |
| ALBI (Mean ± SD) | −3.81 ± 0.45 | −3.69 ± 0.46 | −3.53 ± 0.041 | −3.31 ± 0.57 | <0.001 |
| APRI (Mean ± SD) | 0.84 ± 1.23 | 1.26 ± 1.90 | 1.45 ± 1.47 | 1.66 ± 1.22 | <0.001 |
| CDS (Mean ± SD) | 4.78 ± 1.46 | 5.02 ± 1.36 | 5.55 ± 1.65 | 5.98 ± 1.70 | <0.001 |
| FIB-4 (Mean ± SD) | 2.62 ± 2.36 | 3.85 ± 3.95 | 4.67 ± 2.90 | 5.33 ± 2.80 | <0.001 |
| GUCI (Mean ± SD) | 0.98 ± 1.50 | 1.44 ± 2.08 | 1.76 ± 1.84 | 2.05 ± 1.71 | <0.001 |
| Lok index (Mean ± SD) | 0.39 ± 0.21 | 0.42 ± 0.19 | 0.50 ± 0.23 | 0.56 ± 0.24 | <0.001 |
| MELD (Mean ± SD) | 7.83 ± 2.01 | 8.03 ± 1.73 | 8.49 ± 2.47 | 8.89 ± 2.89 | <0.001 |
| PALBI (Mean ± SD) | −2.60 ± 0.35 | −2.55 ± 0.32 | −2.52 ± 0.26 | −2.34 ± 0.40 | <0.001 |
| King’s score (Mean ± SD) | 22.41 ± 31.00 | 36.74 ± 52.79 | 47.91 ± 52.07 | 49.98 ± 39.67 | <0.001 |
| CTP (Mean ± SD) | 5.25 ± 0.53 | 5.29 ± 0.60 | 5.46 ± 0.68 | 5.80 ± 1.35 | <0.001 |
| Serum sodium (mmole/L; Mean ± SD) | 139.71 ± 2.64 | 139.65 ± 2.61 | 139.40 ± 3.18 | 138.90 ± 2.74 | 0.181 |
ALBI, Albumin-bilirubin; APRI, Aspartate transaminase-to-Platelet ratio; CDS, Cirrhosis discriminant index; CTP, Child-Turcotte-Pugh score; FIB-4, Fibrosis-4 score; MELD, Model for End-stage liver disease; GUCI, Göteborg University Cirrhosis Index; PALBI, platelet-albumin-bilirubin; SD, standard deviation.
Discussion
Liver functional reserve is a crucial prognostic predictor for HCC. In this study, we utilize a prospective HCC cohort to evaluate the prognostic role of these noninvasive models on tumor recurrence and overall survival in HCC patients undergoing surgical resection. We show that among these noninvasive models, the King’s score is a more feasible marker to predict tumor recurrence and ALBI is the most accurate model in the discrimination of survival for HCC patients.
In the prediction of tumor recurrence, our results disclose that the King’s score, APRI and FIB-4 are the three most accurate models associated with recurrence according to AICc analysis. Among these models, the King’s score has the greatest homogeneity of recurrence pattern among HCC patients, indicating it is a more useful tool for recurrence prediction. In multivariate Cox model, King’s score grade 3 had 77% increased risk of recurrence as compared with those with grade 1. In addition to King’s score, tumor size, high AFP and alcoholism are also independent predictors associated with tumor recurrence. These findings suggest that liver functional reserve and tumor status are closely linked with a more aggressive tumor behavior.
In survival analysis, consistent with previous report12,13,34, we found that the ALBI and PALBI grade are the best models for discriminating patient survival. We further show that ALBI grade has the highest homogeneity for survival prediction, suggesting that ALBI is a more robust tool for outcome prediction. In multivariate Cox model, ALBI grade 2–3 was associated with 43% increased risk of mortality compared with ALBI grade 1.
Our analyses indicate that tumor size and number are closely related to survival of HCC patients. In addition, in accordance with previous studies35,36, performance status and vascular invasion are crucial prognostic predictors. Moreover, consistent with published series37–40, high serum AFP level and diabetes mellitus may strongly impact the outcome of HCC patients. Taken together, the severity of liver reserve, tumor burden and performance status are the hallmarks for survival prediction.
The CTP classification has been used to evaluate the severity of liver function and prognosis of HCC. However, in our study, CTP was not significantly linked with tumor recurrence and overall prognosis. This is probably because the majority (94%) of the patients were CTP class A and hence its prognostic ability is impaired. The MELD score and serum sodium level are used to evaluate the prognosis of end-stage cirrhotic patients in the process of organ allocation in liver transplantation8,14. However, these two models could not accurately discriminate tumor recurrence and survival because the patients in this study are mostly mildly cirrhotic or non-cirrhotic. Serum ICG-15 has been a useful adjunct to quantify hepatic reserve in HCC, but its performance is not superior to other markers in this study. Other noninvasive models (CDS, GUCI and Lok index) have not been used to evaluate the prognosis of HCC patients. Importantly, of all models, the ALBI grade, based simply on serum albumin and bilirubin level, is more objective and a readily available marker that can be used for survival discrimination in surgical HCC patients.
Among the 12 liver functional reserve models, APRI, FIB-4 and King’s score are principally designated as liver fibrosis models. Previous studies showed that these models could be used to predict the prognosis of HCC41–43. Notably, these models are associated with liver fibrosis which might be associated with an increased risk of tumor recurrence via carcinogenesis pathway. Interestingly, of these models, the King’s score is the best in predicting tumor recurrence in HCC patients undergoing surgical resection. Other noninvasive liver reserve models (CDS, GUCI and Lok index) have not been used to assess liver reserve and prognosis in HCC. Alternatively, the PALBI grade, an updated version of ALBI classification, is a new promising prognostic tool for HCC and more studies are required to validate its clinical role.
The correlation between ICG-15 and other noninvasive liver functional reserve models was investigated in this study. Our results show that, except for serum sodium, there is a strong correlation between ICG-15 and other 10 models, indicating that most models are clinically relevant in evaluating the degree of liver injury.
Liver functional reserve plays an important role in determining the extent of surgical resection for HCC. The major surgical resection was performed in patients who had good liver functional reserve. However, for those with relatively poor liver reserve, limited surgical resection was done, and these patients might have a higher risk of recurrence after surgery. As a result, liver function may have indirect impact on tumor recurrence via the choice of extent of surgical resection.
There are some study limitations. This is a single-center study in an HBV endemic area, thus external validation is needed from other study groups. In addition, the results are based on HCC patients undergoing surgical resection, therefore the prognostic accuracy of ALBI and King’s score in patients receiving different treatment modalities needs further study to establish. Lastly, since our hospital is a tertiary medical center, referral bias cannot be completely avoided.
In conclusion, the currently used liver functional reserve models have differential predictive ability for HCC patients undergoing surgical resection.The King’s score may serve as a feasible model in predicting tumor recurrence, whereas ALBI grade is the best prognostic tool among the 12 noninvasive liver reserve models. Appropriate models should be considered to integrate into cancer staging in future clinical practice.
Acknowledgements
This study was supported by the grant from the Center of Excellence for Cancer Research at Taipei Veterans General Hospital (MOHW106-TDU-B-211-144-003), Taiwan, and grants from Taipei Veterans General Hospital (V107A-008, VN107-04, V107C-003), Taipei, Taiwan.
Author Contributions
S.-Y. Ho, P.-H. Liu, C.-Y. Hsu and T.-I. Huo performed the research. Y.-H. Lee, C.-W. Su, Y.-H. Huang and C.-Y. Hsia collected and analyzed the data. S.-Y. Ho, P.-H. Liu and T.-I. Huo designed the research study and wrote the paper. M.-C. Hou and F.-Y. Lee contributed to the design of the study. All authors approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Park JW, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol56, 908–943 (2012). [DOI] [PubMed]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imamura H, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/S0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 7.Olthoff KM, et al. Summary report of a national conference: Evolving concepts in liver allocation in the MELD and PELD era. December 8, 2003, Washington, DC, USA. Liver Transpl. 2004;10:A6–22. doi: 10.1002/lt.20247. [DOI] [PubMed] [Google Scholar]
- 8.Botta F, et al. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut. 2003;52:134–139. doi: 10.1136/gut.52.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huo TI, et al. The sequential changes of the model for end-stage liver disease score correlate with the severity of liver cirrhosis in patients with hepatocellular carcinoma undergoing locoregional therapy. J Clin Gastroenterol. 2006;40:543–550. doi: 10.1097/00004836-200607000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Huo TI, et al. Model for end-stage liver disease score to serum sodium ratio index as a prognostic predictor and its correlation with portal pressure in patients with liver cirrhosis. Liver Int. 2007;27:498–506. doi: 10.1111/j.1478-3231.2007.01445.x. [DOI] [PubMed] [Google Scholar]
- 11.Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39:107–116. doi: 10.1111/j.1872-034X.2008.00441.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnson PJ, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, P. H. et al. ALBI and PALBI Grade Predict Survival for HCC across Treatment Modalities and BCLC Stages in the MELD Era. J Gastroenterol Hepatol (2016). [DOI] [PubMed]
- 14.Kim WR, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biggins SW, et al. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32–39. doi: 10.1002/hep.20517. [DOI] [PubMed] [Google Scholar]
- 16.Ruf AE, et al. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–343. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 17.Vallet-Pichard A, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 18.Wai CT, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 19.Cross TJ, et al. King’s Score: an accurate marker of cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol. 2009;21:730–738. doi: 10.1097/MEG.0b013e32830dfcb3. [DOI] [PubMed] [Google Scholar]
- 20.Lok AS, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42:282–292. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]
- 21.Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92:1302–1304. [PubMed] [Google Scholar]
- 22.Westin J, et al. A non-invasive fibrosis score predicts treatment outcome in chronic hepatitis C virus infection. Scand J Gastroenterol. 2008;43:73–80. doi: 10.1080/00365520701514461. [DOI] [PubMed] [Google Scholar]
- 23.Chang WT, et al. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection? Surgery. 2012;152:809–820. doi: 10.1016/j.surg.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 24.N’Kontchou G, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475–1483. doi: 10.1002/hep.23181. [DOI] [PubMed] [Google Scholar]
- 25.Hung HH, et al. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. 2010;4:691–699. doi: 10.1007/s12072-010-9213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei HJ, et al. Prognostic value and clinical relevance of the6th Edition 2002 American Joint Committee on Cancer staging system in patients with resectable hepatocellular carcinoma. J Am Coll Surg. 2006;203:426–435. doi: 10.1016/j.jamcollsurg.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Kao WY, et al. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg. 2011;35:858–867. doi: 10.1007/s00268-010-0928-z. [DOI] [PubMed] [Google Scholar]
- 28.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 29.Lau H, et al. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255–1259. doi: 10.1002/bjs.1800840917. [DOI] [PubMed] [Google Scholar]
- 30.Bradburn MJ, Clark TG, Love SB, Altman DG. Survival analysis part II: multivariate data analysis–an introduction to concepts and methods. Br J Cancer. 2003;89:431–436. doi: 10.1038/sj.bjc.6601119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinstein AR. Clinical biostatistics. XVI. The process of prognostic stratification. 2. Clin Pharmacol Ther. 1972;13:609–624. doi: 10.1002/cpt1972134609. [DOI] [PubMed] [Google Scholar]
- 32.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16:965–980. doi: 10.1002/(SICI)1097-0258(19970515)16:9<965::AID-SIM509>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 33.Forster MR. Key Concepts in Model Selection: Performance and Generalizability. J Math Psychol. 2000;44:205–231. doi: 10.1006/jmps.1999.1284. [DOI] [PubMed] [Google Scholar]
- 34.Toyoda H, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer. 2016;114:744–750. doi: 10.1038/bjc.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu PH, et al. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol. 2014;21:1825–1833. doi: 10.1245/s10434-014-3510-3. [DOI] [PubMed] [Google Scholar]
- 36.Hsu CY, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology. 2013;57:112–119. doi: 10.1002/hep.25950. [DOI] [PubMed] [Google Scholar]
- 37.Hsu CY, et al. Using serum alpha-fetoprotein for prognostic prediction in patients with hepatocellular carcinoma: what is the most optimal cutoff? PLoS One. 2015;10:e0118825. doi: 10.1371/journal.pone.0118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu PH, et al. Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. J Hepatol. 2016;64:601–608. doi: 10.1016/j.jhep.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Huo TI, et al. Diabetes mellitus is a risk factor for hepatic decompensation in patients with hepatocellular carcinoma undergoing resection: a longitudinal study. Am J Gastroenterol. 2003;98:2293–2298. doi: 10.1111/j.1572-0241.2003.07688.x. [DOI] [PubMed] [Google Scholar]
- 40.Huo TI, et al. Differential mechanism and prognostic impact of diabetes mellitus on patients with hepatocellular carcinoma undergoing surgical and nonsurgical treatment. Am J Gastroenterol. 2004;99:1479–1487. doi: 10.1111/j.1572-0241.2004.30024.x. [DOI] [PubMed] [Google Scholar]
- 41.Kao WY, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: the clinical implication of aspartate aminotransferase-platelet ratio index. Eur J Gastroenterol Hepatol. 2011;23:528–536. doi: 10.1097/MEG.0b013e328346d529. [DOI] [PubMed] [Google Scholar]
- 42.Okamura Y, et al. The FIB-4 index is a significant prognostic factor in patients with non-B non-C hepatocellular carcinoma after curative surgery. Langenbecks Arch Surg. 2016;401:195–203. doi: 10.1007/s00423-016-1389-0. [DOI] [PubMed] [Google Scholar]
- 43.Pinato DJ, et al. The Kings Score refines prognostic prediction in hepatocellular carcinoma: a novel application. Liver Int. 2015;35:2458–2465. doi: 10.1111/liv.12841. [DOI] [PubMed] [Google Scholar]