Abstract
Purinergic receptors are implicated in the pathogenesis of gastrointestinal disorders and are being explored as potential therapeutic targets. Gut inflammation releases ATP that acts on neuron, glial, epithelial and immune cells. Purinergic signalling in glia and neurons is implicated in enteric neuropathies. Inflammation activates glia to increase ATP release and alter purinergic signalling. ATP release causes neuronal death and gut motor dysfunction in colitis via a P2X7-dependent neural-glial pathway and a glial purinergic-connexin-43 pathway. The latter pathway also mediates morphine-induced constipation and gut inflammation that may differ from opioid-induced constipation (OIC). P2X7R antagonists are protective in inflammatory bowel disease (IBD) models, where as AZD9056 is questionable in CD, but is potentially beneficial for chronic abdominal pain. Drug targets under investigation for IBD, IBS and motility disorders include P2X7R, P2X3R, P2Y2R, A2A/A2BAR, enzymes and transporters.
INTRODUCTION
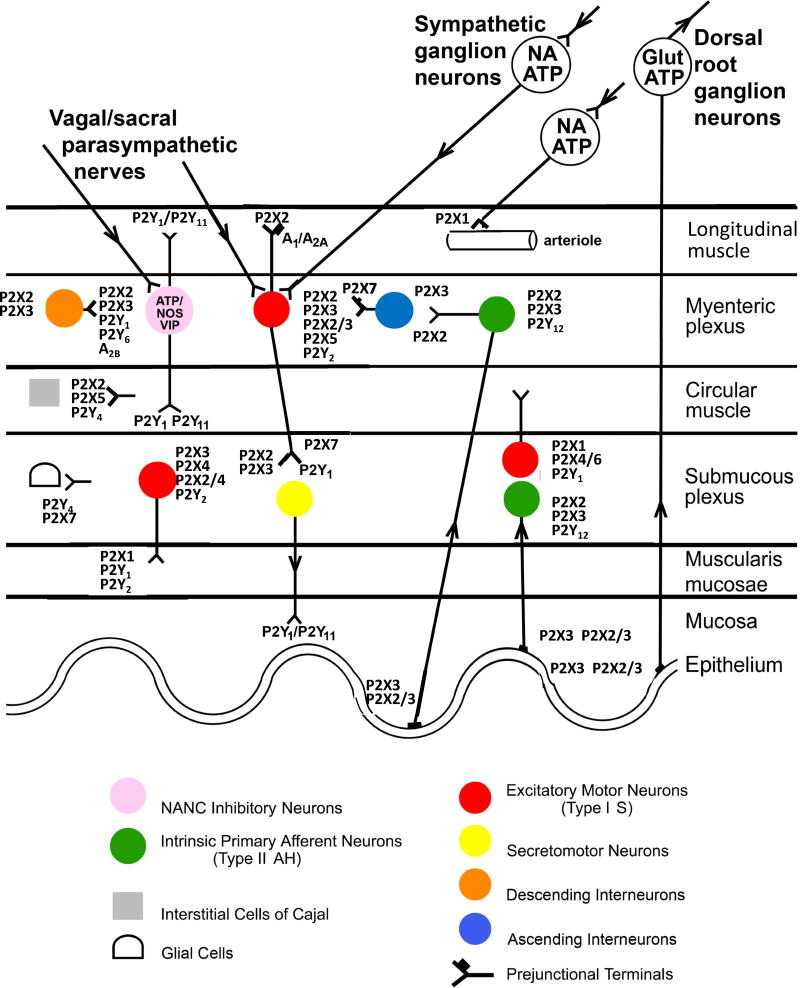
Purinergic receptors, which are widely distributed in the gastrointestinal tract, mediate signaling in the enteric nervous system and at the neuromuscular junction (Burnstock, 2014, 2016; Ochoa-Cortes et al., 2016; Grubišic and Gulbransen, 2017; Chaudhury et al., 2016). Figure 1 is a schematic showing the characterisation of purinoceptors in the gut. There are three classes of purinoceptors: 7 subtypes of P2X ligand-gated ion channels (P2XRs), 8 subtypes of nucleotide-activated G protein-coupled P2Y receptors (P2YRs) and 4 subtypes of G protein-coupled adenosine receptors (ARs, also designated P1 receptors). Our knowledge is based primarily on animal data, although recent studies described here focus more on purinergic signalling in the human gut. Purinergic receptors are key components of, and function at all levels of enteric neural reflexes and in both intrinsic and extrinsic neural pathways. Furthermore, purinergic signalling is an important regulator in activation and trafficking of immune/inflammatory cells (Cekic and Linden, 2016; Faas et al, 2017; Longhi et al, 2017). Activation of P2Rs generally boosts the immune response, while ARs tend to suppress it. Therefore, it is not surprising that there is a great deal of interest in further understanding the role of purines in pathogenic mechanisms and their therapeutic potential in gastrointestinal diseases, as reviewed (Antonioli et al., 2013; Ochoa-Cortes et al., 2014).
Figure 1.
Schematic showing the localisation of receptors to purines and pyrimidines on neurons and non-neuronal effector cells in the gut, although some of the interacting pathways are not yet known. Extrinsic vagal and sacral parasympathetic nerves connect with NANC inhibitory neurons in the myenteric plexus expressing P2X2, P2X3, P2Y1, P2Y6 and A2B receptors, as well as with cholinergic motor neurons; these neurons are also activated by descending interneurons. Extrinsic sympathetic nerves modulate motility via excitatory motor neurons and constrict blood vessels in the gut via P2X1R. Extrinsic sensory nerves arising from cell bodies in dorsal root ganglia and with subepithelial terminals mediate nociception. Intrinsic sensory neurons in both myenteric and submucosal plexuses express P2X2 and P2X3Rs, while a subpopulation also express P2Y12R; they connect with motor pathways involved in peristalsis. Excitatory motor neurons express P2X2, P2X3, P2X2/3, P2X5 and P2Y2Rs and connect with both interneurons and secretomotor neurons. Interneurons express P2X2 and P2X3Rs. Enteric glial cells express P2Y1, P2Y4 and P2X7Rs, while interstitial cells of Cajal express P2X2, P2X5 and P2Y4Rs. P2X7 and P1 receptors appear to act as prejunctional modulators of both motor and interneurons. (Reproduced from Burnstock, 2008, with permission). P2XRs are ligand-gated cation (Na+, K+ and Ca2+) channels. Perferential G protein-coupling of the GPCRs is: Gi: A1, A3, P2Y12, P2Y13, P2Y14; Gs: A2A, A2B: Gq: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11.
MEDICINAL CHEMISTRY OF PURINERGIC RECEPTORS
The X-ray crystallographic structures of ARs and P2YRs are aiding the design of novel ligands by rational structure-based approaches (Ciancetta and Jacobson, 2017). However, most commonly used AR ligand tools were discovered by empirical approaches (Figure 2, Jacobson and Müller, 2016; Ochoa-Cortes et al., 2016). Cl-ENBA (number 3 in Figure 2) is a more selective A1AR agonist for in vivo use than 1 and 2 (Carlin et al., 2017). CGS21680 (not shown) is an A2AAR agonist in rat, but has substantial human (h) A3AR affinity (Alnouri et al., 2015). Potent A2AAR agonist ATL-313 5 attenuates colitis in mice and reduces pro-inflammatory cytokines (Longhi et al, 2017). UK-432097 6 is a potent, selective A2AAR agonist with limited oral bioavailability. Regadenoson 4 is approved as a vasodilator for stress echocardiography but is only moderately potent and not selective at the A2AAR. Selective human A2AAR agonist PBS-0777 7 is water-soluble and does not diffuse across biological membranes. MRS5698 11 is a more selective A3AR agonist than 9 and 10 (Carlin et al., 2017). Selective antagonists are available for all four ARs (12 – 21), but their affinity is often species dependent. For example, typical heterocyclic A3AR agonists display only weak affinity at rodent A3ARs, but MRS1523 21 remains somewhat A3AR-selective across species (Alnouri et al., 2015).
Figure 2.
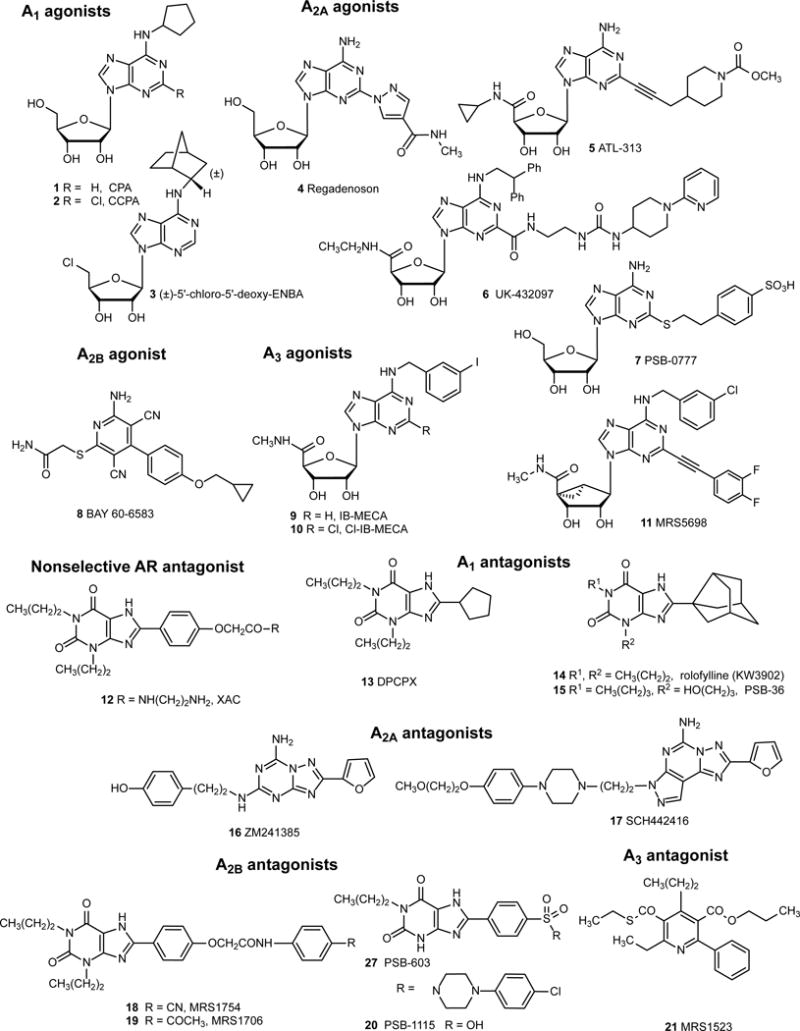
Structures of commonly used ligand probes, both agonists and antagonists, of the four AR subtypes. Ligand potencies and species dependence are reported (Jacobson and Müller, 2016; Müller and Jacobson, 2011). Only one ligand shown here, in addition to adenosine and theophylline, has been approved for human use: 4 (diagnostic).
Agonist and antagonist ligands are available for most P2YRs (Jacobson and Müller, 2016), but only antithrombotic P2Y12R antagonists (Figure 3, 22 – 25) are approved internationally for human use. Thienopyridines 9 and 10 act as prodrugs that are converted into irreversibly binding selective P2Y12R antagonists.
Figure 3.

Structures of commonly used ligand probes of the P2Rs. For P2YRs, only P2Y12R antagonists are shown, because those are used clinically. The only compounds shown here that are approved for human use are 22-25 (antithrombotics). 22 and 23 are prodrugs that require activation in vivo to form active metabolites.
Although there are seven subtypes of P2XRs, each functional channel consists of trimers, Each heterotrimeric combination may differ in ligand pharmacology from the homotrimer, which introduces complexity in the medicinal chemistry. Examples of such heterotrimers are P2X2/3, P2X2/4 and P2X4/6, (Figure 1). X-ray structures of the zebrafish P2X4R, human P2X3R and panda P2X7R are reported and are being utilized for the homology modeling of binding of various ligands at related P2X1R, P2X3R and P2X7R subtypes (Allsopp et al., 2017; Mansoor et al., 2016; Fryatt et al., 2016).
P2XR antagonists are available for P2X2R and P2X2/3R (26, 27), P2X3R (28), P2X4R (26, 27) and P2X7R (30 – 41) (Jacobson and Müller, 2016). Many of the nonpolar antagonists are, in fact, allosteric. Several nonpolar P2X7R antagonists have been in clinical trials for inflammatory disease, cancer, and depression (Park and Kim, 2017; Rech et al, 2016). Numerous pharmaceutical companies (with compound numbers) have been involved in discovery of P2X7R antagonists (e.g. Pfizer 30; Glaxo 31; Astra-Zeneca 32, 33; Lundbeck 34; Axxam 35; Abbvie 36; Janssen 37, 38, 40; Actelion 39). AZD9056 32 was the first P2X7R antagonist to be tested clinically, significantly improving in rheumatoid arthritis scores (Keystone et al., 2012). AZ10606120 33 binding at the P2X7R was probed structurally (Allsopp et al., 2017). Compounds 38 and 40 are PET ligands for imaging of P2X7R in the brain.
Enzymatic transformation of extracellular purines can also affect the levels of activation of purinergic receptors. Intestinal inflammation may cause downregulation of the ectonucleotidases CD39 (E-NTPDase1) and CD73 (5′-nucleotidase), leading to excess nucleotide-dependent proinflammatory effects and deficiency of adenosine-dependent anti-inflammatory effects in the immune and nervous systems (Longhi et al, 2017). CD39 hydrolyzes ATP and ADP to AMP, and CD73 hydrolyzes AMP to adenosine. Thus, soluble ectonucleotidases and selective A2AAR agonists have been suggested as therapies for correcting an imbalance of purinergic signaling in inflammatory bowel disease (IBD) and gastrointestinal autoimmunity (Longhi et al, 2017).
Modulators of nucleoside transport, such as the vasodilator dipyridamole, and inhibitors of adenosine deaminase (ADA), such as anticancer drug pentostatin, indirectly raise the level of AR activation.
P2X7R IN INFLAMMATORY BOWEL DISEASE (IBD)
IBD includes two main clinical forms, ulcerative colitis (UC) and Crohn’s disease (CD). Under inflammatory conditions, ATP is released. P2X7R is preferentially expressed by immune cells, particularly macrophages, which, when activated, release inflammatory cytokines, mediate inflammasome formation and enhance phagocytosis. Reviews discussing the role of P2X7R in IBD are available (Kurashima et al., 2015; Diezmos et al., 2016).
P2X7R is involved in colonic motor dysfunction associated with bowel inflammation. P2X7R modulate the activity of excitatory cholinergic nerves through a facilitation of inhibitory nitrergic pathways (Antonioli et al., 2014).
Damage to the intestinal mucosa epithelial barrier by chemical or physical stressors induces inflammation, which results in ATP release. The ATP activates mast cells, which further promote inflammatory processes (Kurashima and Kiyono, 2016). Oestrogen receptor-β activation may play a therapeutic role in IBD by down-regulatiing P2X3R and P2X7R (Ma et al., 2016). P2X7R is over-expressed in IBD patients’ gut mucosa. P2X7R knockout mice were protected against gut inflammation (Figliuolo et al., 2017). P2X7R activates the NLRP3 inflammasome in T cells, macrophages, dendritic cells, and neutrophils (Gombault et al, 2012). However, no studies have assessed the utility of P2X7R antagonists in humans with IBD except for one study in Crohn’s Disease (CD) patients (Eser et al., 2015).
ULCERATIVE COLITIS (UC)
Extracellular ATP mediates inflammatory responses in colitis via P2×7R signalling (Wan et al., 2016b). Colitis differentially affects P2X7R-expressing enteric neurons based on their chemical codes (da Silva et al., 2015). P2X7R activation also triggers mucosal regulatory T cell death (Figliuolo et al., 2017). P2X7R antagonist A438079 35 down-regulates the production of pro-inflammatory cytokines in colonic tissues, and attenuates murine colitis, indicating P2X7R-dependent triggering of immune responses during colitis (Wan et al., 2016b).
Extracellular ATP mediates mast cell-dependent intestinal inflammation via P2X7R. It was shown that leukocyte mono-immunoglobulin-like receptor 3–deficient colonic mast cells were pivotal in the exacerbation of experimental colitis (Matsukawa et al., 2016). P2X7R is predominantly located on myenteric neurons, but in colitis its neuromuscular layer expression increased, with a marked reduction of electrically induced contractions by the P2X7R agonist 2′ (3′)-O- (4-benzoylbenzoyl) adenosine 5′-triphosphate 41 (Antonioli et al., 2014).
P2X3R mediate visceral hypersensitivity during 2,4,6-trinitrobenzene sulphonic-acid-induced colitis, identifying it as a potential new target for abdominal pain syndrome treatment (Deiteren et al., 2015).
Intestinal epithelial adenosine A2BAR signalling, including activation by selective agonist Bay60-6583 9, in vivo protects against acute intestinal inflammation by regulating (Ser157) phosphorylation of vasodilator stimulated phosphoprotein (VASP) at epithelial cell junctions to enhance mucosal barrier responses (Aherne et al., 2015). This pathway may be exploited as a therapeutic strategy in IBD. However, A2BAR antagonism or gene knockout showed benefit in a mouse model of colitis (Kolachala et al., 2008). Systemic administration of P2X7R antagonist A740003 protects against sepsis-induced disruption of the intestinal barrier (Wu et al., 2017).
MicroRNA-16 in human UC regulates the increase in inflammatory responses by suppressing the expression of the A2AAR to control the activation of the NF-κB signalling pathway (Tian et al., 2016; Figure 4). MicroRNA-206 has a pro-inflammatory role in UC by downregulating A3AR expression and activating NF-κB signalling (Wu et al., 2017).
Figure 4.
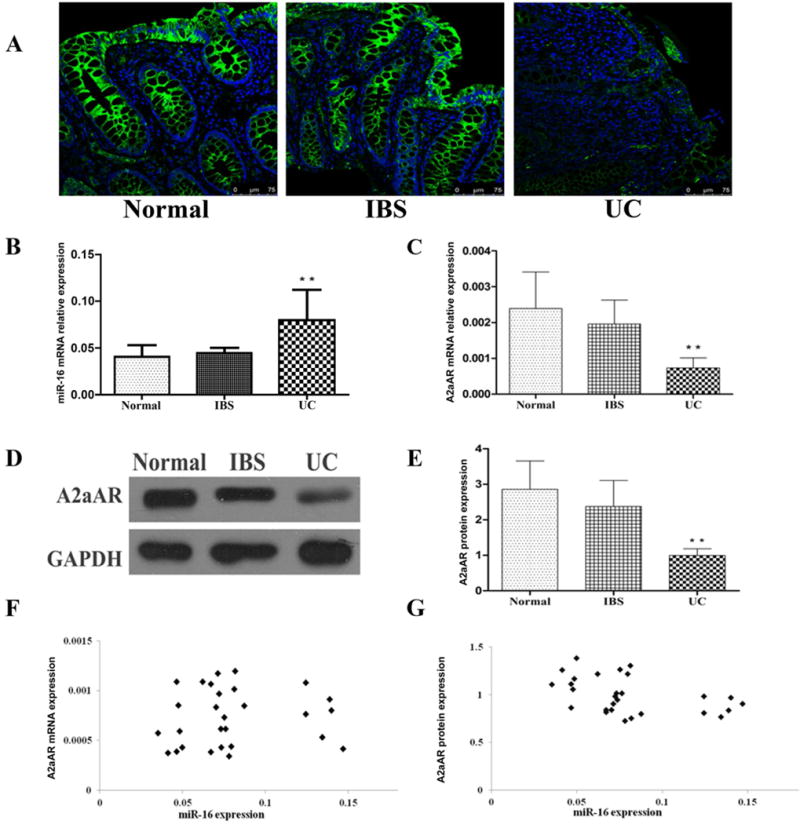
(A) Immunofluorescence staining of sigmoid biopsy section using specific antibody to label A2AAR protein and DAPI for counterstaining the nuclei (blue). The strong green fluorescence signal indicating A2AAR was observed mainly in the sigmoid colonic epithelial cells. The expression of A2AAR was reduced in ulcerative colitis (UC) inflamed tissues. (B) MicroRNA-16 expression, and (C) A2AAR mRNA expression in individual sigmoid colon tissues (normal controls n = 20, irritable bowel syndrome (IBS) n = 22 and active UC, n = 28), were measured by qRT-PCR (Data are shown as mean ± SD, **P < 0.01). (D) A representative western blot image of A2AAR protein expression in sigmoid colon tissues. (E) A2AAR protein expression in sigmoid colon tissues was based on the ratio of band density of A2AAR to GAPDH protein. The western blot band density was measured using Quantity One software (normal controls n = 20, IBS n = 22 and active UC, n = 28. Data represent mean ± SD, **P < 0.01). (F) Correlation between miR-16 and A2AAR mRNA expression in active UC inflamed sigmoid tissues (n = 28, Pearson correlation r = 0.095, P > 0.05). (G) Correlation between miR-16 and A2AAR protein expression, miR-16 expression showed a strong negative correlation with A2AAR protein level in active UC inflamed sigmoid tissues (n = 28, Pearson correlation r = − 0.438, P < 0.05). (Reproduced from Tian et al., 2016, with permission (Creative Commons Attribution 4.0 International License).
In a rat TNBS-induced colitis model, A3AR agonist 10, at a relatively high dose (3 mg/kg), reduced weight loss, diarrhea, occult blood, and mucosal inflammation (Guzman et al., 2006). However, the results in other studies with A3AR agonists were variable (Ochoa-Cortes et al., 2016). The role of the A3AR in inflammatory diseases was recently reviewed (Jacobson et al., 2017).
CROHN’S DISEASE (CD)
The inflammasome is a multiprotein complex belonging to the innate immune system and activation of P2X7R results in secretion of inflammatory cytokines. A significant difference in expression of the inflammasome in CD patients was observed, with increased expression of interleukin-1β and activation of the P2X7R (Zelante et al., 2015). There is overexpression of P2X7R in the inflamed mucosa in CD, suggesting that P2X7R may be a novel target for CD (Neves et al., 2014).
A randomized placebo-controlled, double blind, Phase IIa Study in 34 patients (24 AZD9056 and 10 placebo) was done to assess the safety and efficacy of an oral P2X7R antagonist, AZD9056 32 in adult patients with moderately to severely active CD (Eser et al., 2015). This is the first study done to evaluate a purinergic drug in IBD. AZD9056 was well tolerated and there were no serious adverse events reported. AZD9056 caused a significant improvement in CDAI (the primary end point) versus placebo. A decrease in disease activity was observed for pain and general well being, two subcomponents of CDAI. The improvement in abdominal pain was significantly greater for AZD9056 than placebo. The remission and response rates (secondary end point) and the reduction in inflammatory biomarkers (including serum C-reactive protein and fecal calprotectin) were not different versus placebo. Therefore, assuming AZD9056 is an effective P2X7R antagonist in humans, this study does not provide any evidence for a therapeutic effect for AZD9056 as an anti-inflammatory drug in CD, despite the evidence in animal models. Overall, the study demonstrates that AZD9056 has the potential to improve symptoms an in particular abdominal pain, in patients with moderate to severe CD. Clearly, further studies are needed to determine whether AZD9056 (or other P2X7R antagonists) is an effective therapy for chronic abdominal pain and whether it has anti-inflammatory potential. The former possibility is supported by accumulating evidence for a role of P2X7R in nociception [Jorge et al, 2012; Ochoa-Cortes-et al, 2014]. Furthermore, a vesicular ATP release inhibitor is an effective treatment of neuropathic and inflammatory pain in mice (Kato et al, 2017), presumably by acting at P2X7R or other P2XR.
Pentostatin has shown benefit in colitis patients in which IL10 is absent (Brown et al., 2008). The plasma levels of the two ADA isozymes are considered a biomarker of inflammation in CD (Maor et al, 2011).
PURINERGIC SIGNALING PATHWAYS IN ENTERIC GLIA AND NEURONS AS A TARGET FOR IBD AND MOTILITY DISORDERS
Emerging evidence suggests that reactive enteric glia or alterations in glia induced by inflammation, infection or surgical intestinal manipulation/trauma may contribute to disturbed motility in intestinal diseases including postoperative ileus, GI symptoms in Parkinson’s Disease (i.e. constipation), idiopathic severe slow transit constipation, diverticular disease, IBD, infectious colitis, and necrotizing enterocolitis. Common features in these diseases that suggest a role for glia in pathogenesis are alterations in the expression of glial proteins (GFAP, GDNF, s100B), a sign of glial activation or reductions in numbers of glia or size of the glial network. In an IBS model, glia are involved in a stress-induced colonic hypercontractility. In a gut surgical manipulation model of postoperative ileus, enteric glia are involved in abnormal motility (slow transit) (reviewed by Ochoa-Cortes et al., 2016).
Enteric glial cells contribute to intestinal disease via neuronal P2X7R. ATP release during inflammation causes neuronal death and gut motor dysfunction by activating neuronal P2X7R to trigger ATP release and activation of surrounding enteric glia (Gulbrensan et al, 2012). Glia also modulate enteric neuron death in colitis through purinergic – connexin-43 pathways driven by NO production from iNOS in glia. Data suggest that the glial responses to ATP contribute to neurotoxic effects and motility disorders. Enteric glial activation is an important mechanism in the development of enteric neuropathy and glial purinergic signalling pathways represent a potential new target for the development of more effective treatments for functional bowel disorders (Brown et al, 2016).
Much of our knowledge of purinergic signalling in the enteric nervous system (ENS) relies on data from animals (see Figure 1). A recent study in isolated human enteric glial cells in culture obtained from non-diseased colon surgical specimens obtained from colectomy patients for polyp removal have revealed that inflammation (i.e. bacterial lipopolysaccharide) can induce a reactive glial phenotype associated with upregulation of cytokines, chemokines and alterations in purinergic gene expression, including P2XRs, P2YRs and enzymes involved in purine metabolism (Linan-Rico et al, 2016). In reactive glia induced by chronic stimulation with lipopolysaccharide, there is an increase in ATP release, and there is disruption of Ca2+ waves, responses to ATP and mechanically evoked glial Ca2+ responses. All together, data indicate that inflammation has a profound influence on purinergic signalling pathways in glia and Ca2+ waves, which are required for normal motility and intestinal transit. Such effects are expected to cause disturbances in motility in the human intestinal tract.
Ca2+ imaging was also used to study the purinergic receptor neuropharmacology in the human submucous plexus of surgical specimens. The study revealed that stimulatory P2X1R, P2X2R and P2X3R, and inhibitory P2YR and A3AR are involved in neurotransmission. Several types of neurons were identified based on their P2X receptor subtype expression (Linan-Rico et al, 2015). P2X1R is highly expressed in human submucous neurons, and this could represent a potential species difference from rodents. Purines regulate neurotransmission at several synapses in the human enteric nervous system. One or more neural receptors identified are novel targets in GI diseases and enteric neuropathies.
MOTILITY DISORDERS
A review, focussed on the physiological mechanisms responsible for nerve-mediated purinergic relaxation, provides a functional basis for clinical and pharmacological studies on defective GI motility (Jiménez et al., 2014).
The phytoalkaloid berberine is traditionally used to treat GI motility disorders, but it is not used as a treatment strategy in the USA. In a recent study, berberine was shown to decrease the amplitude and frequency of pacemaker potentials via ATP sensitive K+ channels (Kim et al., 2016). The purinergic fast inhibitory junction potential may be impaired in Hirschsprung’s disease (Jiménez et al., 2015).
A side effect of morphine pain treatment is persistent constipation and inflammation. Prolonged treatment with morphine causes a ‘leaky gut’ that predisposes to colonic inflammation. Connexin-purinergic signalling in enteric glia likely mediates the opioid induced constipation (OIC) and inflammation caused by long-term treatment with morphine (Bhave et al., 2017). The mouse model of opioid-induced constipation is associated with colonic inflammation and other features that could be markedly different from OIC in humans. For instance, it is evident that systemic inflammation occurs with chronic opioid use in man (Xu et. al., 2017) but whether the gut is the origin remains unclear. It is also not yet known whether glial cells are activated by OIC in man. Targeting the connexin-purinergic pathway is a potential therapeutic target.
P2Y1R and Ca2+ activated small-conductance K+ (SK) channels are key components of inhibitory neurotransmission in the colon. An endogenous vasoconstrictor, adenosinyl uridinyl tetraphosphate (Up4A), activates P2Y1R and SK channels in human and mouse colon. Up4A is a novel mediator of purinergic signalling in human gut and in enteric inhibitory motor neurotransmission (Durnin et al, 2014), and is another mediator for consideration in gastrointestinal motility disorders.
IRRITABLE BOWEL SYNDROME (IBS)
Diarrhoea-predominant IBS is characterised by abdominal pain and increased expression of P2Y1R and P2Y2R in the rectosigmoid mucosa of IBS patients (Luo et al., 2016). P2Y2R expression may be correlated with abdominal pain. Visceral hypersensitivity is often seen in IBS. It has been suggested that P2X7R of rat dorsal root ganglia may play a role in the transmission of the nociceptive signal (Liu et al., 2015). Emerging evidence suggests that electroacupuncture may downregulate the expression of the P2X3R and ease the sensitivity to IBS visceral pain (Weng et al., 2015). It may be worth investigating AZD9056 32 in IBS and in related functional gastrointestinal disorders, since the P2X7R antagonist was shown to have some efficacy against chronic abdominal pain in CD (Eser et al., 2015).
GUT PAIN
ATP release from mucosal epithelial cells, to activate P2X3R expressed on nociceptive primary afferent sensory nerve endings in the submucosa, was proposed to relay messages via the spinal cord to the conscious pain centres in the brain (Burnstock, 2001). P2X3R antagonists and anti-P2X3R antibodies are being explored as therapeutic agents against colic and colitis pain (Deiteren et al., 2015; Shcherbatko et al., 2016).
GASTROESOPHAGEAL REFLUX DISEASE
Roles for adenosine, ATP and UTP in the pathogenesis of gastroesophageal reflux disease symptoms have been considered (Altomare et al., 2013). Purinergic receptors have been demonstrated in various species, including humans, and there is recent evidence to implicate a role for P2Y2 and A2A receptors in esophageal hypersensitivity.
Clinical studies have shown that that adenosine contributes to esophageal mechanical hypersensitivity and non-cardiac chest pain originating in the esophagus (Achem et al, 2007). Stimulation of A2AAR induces mechanical sensitization of esophageal vagal nodose C fibers by a mechanism involving TRPA1. This may contribute to the effects of adenosine on hypersensitivity (Brozmanova et al, 2016).
Immune-mediated injury and IL-8 secretion has been proposed to play a role in the pathogenesis of gastroesophageal reflux disease (GERD). A recent study showed that ATP-induced activation of P2Y2R and IL-8 release maybe implicated in the pathogenesis of refractory GERD (Wu et al, 2017).
GUT CANCER
High expression of the ecto-nucleotidase CD39 occurs in malignant epithelial cells of human rectal adenocarcinoma (Zhang et al., 2015). P2X7R activation mediated inflammatory responses and suppressed colitis associated cancer development. Inactivation of the purinergic P2X7R dampens inflammation in a colitis model, but increases tumor incidence in a mouse colitis-associated cancer model (Hofman et al, 2015). Therefore, the use of P2X7R antagonists to treat IBD should be approached with some caution.
The protein kinase inhibitor H89 acts as an ATP mimetic synergizing with a nitric oxide donor to trigger apoptosis in aggressive colonic cancer cells (Cortier et al., 2015).
Reviews focus on the pathophysiological roles of P2YR in inflammation and cancer (Wan et al., 2016b, Ferrari et al, 2017).
GUT INFECTION
Aged mice are less able to deal with inflammation from Candida albicans infection due to lower gut density of A2AAR, which is an inflammation stop signal (Rodrigues et al., 2016).
Purinergic receptors have been proposed to be key mediators of human immunodeficiency virus type 1 (HIV-1) infection and inflammation (Swartz et al, 2015). Chronic HIV-1 infection leads to a decrease in the integrity of the mucosal epithelial barrier, bacterial translocation and high bacterial lipopolysaccharide levels in HIV-infected individuals. A better understanding of these pathways will provide further insight into disease pathogenesis and may enable more targeted therapies for HIV-infected patients.
CONCLUSIONS
Antagonists of P2X receptors, and P2X7R and P2X3R, in particular, are potential therapeutic targets for treating IBD, IBS and motility disorders. Other targets include A2AAR agonists, A2BAR modulators, A3AR agonists and P2Y2R antagonists. Furthermore, modulation of the levels of endogenous purinoceptor agonists indirectly through enzymes and transporters can provide benefit in gastrointestinal disorders. MicroRNAs can also tune the levels of purinergic mediator proteins. Medicinal chemistry is facilitating the development of new purinergic agonists and antagonists with higher selectivity, potency, and a more favourable pharmacokinetic and safety profile for future clinical trials. Overall, pre-clinical studies provide compelling evidence for the potential therapeutic use of purinergic drugs for GI diseases and Disorders (Ochoa-Cortes et al, 2014). Purinergic drugs have a favourable risk benefit profile and carefully designed clinical trials to test feasibility, safety and efficacy in GI disorders should be encouraged.
Highlights.
-
➢
New drug-like purinergic agonists and antagonists with higher selectivity are being developed for future clinical trials.
-
➢
Antagonists of P2X and P2Y receptors are potential therapeutic targets for treating IBD, IBS and motility disorders.
-
➢
A2AAR agonists and enzymes and transporter modulators provide benefit in gastrointestinal disorders.
Acknowledgments
We acknowledge support from the NIH Intramural Research Program to K.A. Jacobson; NIH NIDDK grants DK093499 and DK113943 and Office of Research & Dean’s Bridge Grant, College of Medicine at The Ohio State University to F.L. Christofi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors report no conflict of interest.
References
- Achem SR. New frontiers for the treatment of noncardiac chest pain: the adenosine receptors. Am J Gastroenterol. 2007 May;102(5):939–41. doi: 10.1111/j.1572-0241.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- Aherne CM, Saeedi B, Collins CB, Masterson JC, McNamee EN, Perrenoud L, Rapp CR, Curtis VF, Bayless A, Fletcher A, Glover LE, Evans CM, Jedlicka P, Furuta GT, de Zoeten EF, Colgan SP, Eltzschig HK. Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis. Mucosal Immunol. 2015;8:1324–1338. doi: 10.1038/mi.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp RC, Dayl S, Schmid R, Evans RJ. Unique residues in the ATP gated human P2X7 receptor define a novel allosteric binding pocket for the selective antagonist AZ10606120. Scientific Reports. 2017;7:725. doi: 10.1038/s41598-017-00732-5. http://doi.org/10.1038/s41598-017-00732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnouri MW, Jepards S, Casari A, Schiedel AC, Hinz S, Muller CE. Selectivity is species-dependent: characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors. Purinergic Signal. 2015;11(3):389e407. doi: 10.1007/s11302-015-9460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomare A, Guarino MP, Emerenziani S, Cicala M, Drewes AM, Krarup AL, Brock C, Lottrup C, Frøkjaer JB, Souza RF, Nardone G, Compare D. Gastrointestinal sensitivity and gastroesophageal reflux disease. Ann N Y Acad Sci. 2013;1300:80–95. doi: 10.1111/nyas.12236. [DOI] [PubMed] [Google Scholar]
- Antonioli L, Colucci R, Pellegrini C, Giustarini G, Tuccori M, Blandizzi C, Fornai M. The role of purinergic pathways in the pathophysiology of gut diseases: pharmacological modulation and potential therapeutic applications. Pharmacol Ther. 2013;139:157–188. doi: 10.1016/j.pharmthera.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Antonioli L, Giron MC, Colucci R, Pellegrini C, Sacco D, Caputi V, Orso G, Tuccori M, Scarpignato C, Blandizzi C, Fornai M. Involvement of the P2X7 purinergic receptor in colonic motor dysfunction associated with bowel inflammation in rats. PLoS One. 2014;9:e116253. doi: 10.1371/journal.pone.0116253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Bhave S, Gade A, Kang M, Hauser KF, Dewey WL, Akbarali HI. Connexin-purinergic signaling in enteric glia mediates the prolonged effect of morphine on constipation. FASEB J. 2017;31(6):2649–2660. doi: 10.1096/fj.201601068R. Morphine is a narcotic used for the treatment of pain, but persistent constipation and antinociceptive tolerance limit its clinical efficacy. Prolonged morphine treatment results in a “leaky” gut, predisposing to colonic inflammation in a mouse model. Long-term morphine treatment in vivo enhanced P2X receptor activity in enteric glia. Bacterial LPS increased ATP and P2X-induced currents in glia, and increased expression of P2X4, P2X7, IL6 and IL1, as well as connexin-43 (a gap, as well as connexin junction protein), and ATP release. The effects of LPS induction were blocked with connexion-43 inhibitors. In addition, colonic inflammation, gut wall disruption and morphine- induced constipation were blocked by connexion-43 inhibition. The study suggests that the prolonged effect of morphine on constipation involves the connexin-purinergic signalling pathway in enteric glia in a mouse model. The mouse model of opioid-induced constipation is associated with colonic inflammation and other features that may be differ from OIC in humans. This remains to be determined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JB, Lee G, Grimm GR, et al. Therapeutic benefit of pentostatin in severe IL-102/2 colitis. Inflamm Bowel Dis. 2008;14:880–887. doi: 10.1002/ibd.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozmanova M, Mazurova L, Ru F, Tatar M, Hu Y, Yu S, et al. Mechanisms of the adenosine A2A receptor-induced sensitization of esophageal C fibers. Am J Physiol Gastrointest Liver Physiol. 2016;310(3):G215–23. doi: 10.1152/ajpgi.00350.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Commentary. Purinergic receptors as future targets for treatment of functional GI disorders. Gut. 2008;57:1193–1194. doi: 10.1136/gut.2008.151134. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signalling. 2014;10:3–50. doi: 10.1007/s11302-013-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling in the gut. In: Brierley S, Costa M, editors. The Enteric Nervous System 30 Years Later. Springer; Heidelberg/Berlin: 2016. pp. 91–112. [Google Scholar]
- **.Brown IAM, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric Glia Mediate Neuron Death in Colitis Through Purinergic Pathways That Require Connexin-43 and Nitric oxide. Cell Mol Gastroenterol Hepatol. 2016;3:77–91. doi: 10.1016/j.jcmgh.2015.08.007. It was shown in this study that activated enteric glia mediate neuron death in colitis through purinergic pathways that require connexion-43 driven by NO production from iNOS in enteric glia. Data suggest that the glial response to ATP contributes to neurotoxic effects and motility disorders. The study suggests that activation of enteric glia is an important mechanism in the development of enteric neuropathy. Glial purinergic signalling pathways could potentially represent a new target for the development of more effective treatments for functional bowel disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin JL, Jain S, Gizewski E, Wan TC, Tosh DK, Xiao C, Auchampach JA, Jacobson KA, Gavrilova O, Reitman ML. Hypothermia in mouse is caused by adenosine A1 and A3 receptor agonists and AMP via three distinct mechanisms. Neuropharmacology. 2017;114:101–113. doi: 10.1016/j.neuropharm.2016.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16(3):177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- Chaudhury A, Dendi VS, Mirza W. Colligative property of ATP: implications for enteric purinergic neuromuscular neurotransmission. Front Physiol. 2016;7:500. doi: 10.3389/fphys.2016.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciancetta A, Jacobson KA. Breakthrough in crystallography of GPCRs and its impact on computer-aided drug design. In: Heifetz A, editor. Computational Methods for GPCR Drug Discovery. Springer; 2017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortier M, Boina-Ali R, Racoeur C, Paul C, Solary E, Jeannin JF, Bettaieb A. H89 enhances the sensitivity of cancer cells to glyceryl trinitrate through a purinergic receptor-dependent pathway. Oncotarget. 2015;6:6877–6886. doi: 10.18632/oncotarget.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva MV, Marosti AR, Mendes CE, Palombit K, Castelucci P. Differential effects of experimental ulcerative colitis on P2X7 receptor expression in enteric neurons. Histochem Cell Biol. 2015;143:171–184. doi: 10.1007/s00418-014-1270-6. [DOI] [PubMed] [Google Scholar]
- Deiteren A, van der Linden L, de Wit A, Ceuleers H, Buckinx R, Timmermans JP, Moreels TG, Pelckmans PA, De Man JG, De Winter BY. P2X3 receptors mediate visceral hypersensitivity during acute chemically-induced colitis and in the post-inflammatory phase via different mechanisms of sensitization. PLoS One. 2015;10:e0123810. doi: 10.1371/journal.pone.0123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diezmos EF, Bertrand PP, Liu L. Purinergic signaling in gut inflammation: the role of connexins and pannexins. Front Neurosci. 2016;10:311. doi: 10.3389/fnins.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Durnin L, Hwang SJ, Kurahashi M, Drumm BT, Ward SM, Sasse KC, Sanders KM, Mutafova-Yambolieva VN. Uridine adenosine tetraphosphate is a novel neurogenic P2Y1 receptor activator in the gut. Proc Natl Acad Sci USA. 2014;111(44):15821–15826. doi: 10.1073/pnas.1409078111. P2Y1 purinergic receptors and Ca2+ activated small-conductance K+ (SK) channels are key components of inhibitory neurotransmission in the colon. Uridine adenosine tetraphosphate (Up4A) activates P2Y1 receptors and SK channels in human and mouse colon. Up4A is a novel mediator of purinergic signalling in human gut, and in enteric inhibitory motor neurotransmission (Durnin et al 2014). Therefore, Up4A is another purine candidate for consideration in gastrointestinal motility disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Eser A, Colombel JF, Rutgeerts P, Vermeire S, Vogelsang H, Braddock M, Persson T, Reinisch W. Safety and efficacy of an oral inhibitor of the purinergic receptor P2X7 in adult patients with moderately to severely active Crohn’s disease: a randomized placebo-controlled, double-blind, phase IIa study. Inflamm Bowel Dis. 2015;21:2247–2253. doi: 10.1097/MIB.0000000000000514. A randomized placebo-controlled, double blind, Phase IIa Study in 34 patients (24 AZD9056 and 10 placebo) was done to assess the safety and efficacy of an oral P2X7R antagonist, AZD9056 in adult patients with moderately to severely active CD. AZD9056 was well tolerated and there were no serious adverse events reported. AZD9056 improved the general well being component of CDAI and the pain symptoms of patients with moderate-to-severe CD and the results suggest P2X7 antagonism for the treatment of chronic abdominal pain. However, findings in the clinical study question the anti-inflammatory potential of P2X7 antagonism, This is the first study showing efficacy of a P2X7 antagonist in IBD. [DOI] [PubMed] [Google Scholar]
- Faas MM, Sáez T, de Vos P. Extracellular ATP and adenosine: The Yin and Yang in immune responses? Molecular Aspects of Medicine. 2017;55:9–19. doi: 10.1016/j.mam.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Malavasi F, Antonioli L. A Purinergic Trail for Metastases. Trends Pharmacol Sci. 2017;38:277–290. doi: 10.1016/j.tips.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Figliuolo VR, Savio LEB, Safya H, Nanini H, Bernardazzi C, Abalo A, de Souza HSP, Kanellopoulos J, Bobé P, Coutinho CMLM, Coutinho-Silva R. P2X7 receptor promotes intestinal inflammation in chemically induced colitis and triggers death of mucosal regulatory T cells. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2017 doi: 10.1016/j.bbadis.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Fryatt AG, Dayl S, Cullis PM, Schmid R, Evans RJ. Mechanistic insights from resolving ligand-dependent kinetics of conformational changes at ATP-gated P2X1R ion channels. Sci Rep. 2016;12(6):32918. doi: 10.1038/srep32918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombault A, Baron L, Couillin I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol. 2012;3:414. doi: 10.3389/fimmu.2012.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubišic V, Gulbransen BD. Enteric glia: the most alimentary of all glia. J. Physiol. 2017;595:557–570. doi: 10.1113/JP271021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hofman P, Cherfils-Vicini J, Bazin M, Ilie M, Juhel T, Hébuterne X, Gilson E, Schmid-Alliana A, Boyer O, Adriouch S, Vouret-Craviari V. Genetic and pharmacological inactivation of the purinergic P2RX7 receptor dampens inflammation but increases tumor incidence in a mouse model of colitis-associated cancer. Cancer Res. 2015;75:835–845. doi: 10.1158/0008-5472.CAN-14-1778. P2X7R activation mediates inflammatory responses and acts to suppress colitis associated cancer development. Inactivation of the purinergic P2X7R dampens inflammation in a dextran sulphate model of IBD, but increases tumor incidence in the mouse model of colitis-associated cancer. Therefore, the use of P2X7R antagonists to treat IBD should be approached with caution, since it may increase the risk of colitis-associated cancer, a complication of IBD. [DOI] [PubMed] [Google Scholar]
- Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor–pannexin-1 mediates death of enteric neurons during colitis. Nature Medicine. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman J, Yu JG, Suntres Z, et al. ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm Bowel Dis. 2006;12:766–789. doi: 10.1097/00054725-200608000-00014. [DOI] [PubMed] [Google Scholar]
- **.Jacobson KA, Müller CE. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology. 2016;104:31–49. doi: 10.1016/j.neuropharm.2015.12.001. Describes receptor distribution, key ligands and intended therapeutic applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Merighi S, Varani K, Borea PA, Baraldi S, Tabrizi MA, Romagnoli R, Baraldi PG, Ciancetta A, Tosh DK, Gao ZG, Gessi S. A3 adenosine receptors as modulators of inflammation: from medicinal chemistry to therapy. Med Res Rev. 2017 doi: 10.1002/med.21456. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez M, Clavé P, Accarino A, Gallego D. Purinergic neuromuscular transmission in the gastrointestinal tract; functional basis for future clinical and pharmacological studies. Br J Pharmacol. 2014;171:4360–4375. doi: 10.1111/bph.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez M, De Diego M, Martínez-Cutillas M, Mañé Reed N, Gallego D, Ojanguren M, Ibanez MM, Serra J. Purinergic and nitrergic inhibitory neuromuscular transmission in ganglionic, transitional and aganglionic segments from Hirschsprung’s disease patients. Neurogastroenterology & Motility. 2015;27:71. [Google Scholar]
- Keystone Edward C, Wang Millie M, Layton Mark, Hollis Sally, McInnes Iain B. Annals of the Rheumatic Diseases. 2012;71(10):1630–1635. doi: 10.1136/annrheumdis-2011-143578. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim H, Jung MH, Kwon YK, Kim BJ. Berberine induces pacemaker potential inhibition via cGMP-dependent ATP-sensitive K+ channels by stimulating mu/delta opioid receptors in cultured interstitial cells of Cajal from mouse small intestine. Mol Med Rep. 2016;14:3985–3991. doi: 10.3892/mmr.2016.5698. [DOI] [PubMed] [Google Scholar]
- Kato Y, Hiasa M, Ichikawa R, Hasuzawa N, Kadowaki A, Iwatsuki K, et al. Identification of a vesicular ATP release inhibitor for the treatment of neuropathic and inflammatory pain. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1704847114. pii: 201704847. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolachala VL, Vijay-Kumar M, Dalmasso G, et al. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology. 2008;135:861–870. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashima Y, Kiyono H, Kunisawa J. Pathophysiological role of extracellular purinergic mediators in the control of intestinal inflammation. Mediators Inflamm. 2015;2015:427125. doi: 10.1155/2015/427125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashima Y, Kiyono H. Physiological and Pathological Inflammation at the Mucosal Frontline. In: Miyasaka M, Takatsu K, editors. Chronic Inflammation: Mechanisms and Regulation. Springer; Japan, Tokyo: 2016. pp. 567–590. [Google Scholar]
- Maor I, Rainis T, Lanir A, et al. Adenosine deaminase activity in patients with Crohn’s disease: distinction between active and nonactive disease. Eur J Gastroenterol Hepatol. 2011;23:598–602. doi: 10.1097/MEG.0b013e328346e205. [DOI] [PubMed] [Google Scholar]
- Mansoor SE, Lü W, Oosterheert W, Shekhar M, Tajkhorshid E, Gouaux E. X-ray structures define human P2X3 receptor gating cycle and antagonist action. Nature. 2016;538:66–71. doi: 10.1038/nature19367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Cortes F, Turco F, Linan-Rico A, Soghomonyan S, Whitaker E, Wehner S, Cuomo R, Christofi FL. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Infl Bowel Dis. 2016;22(2):433–449. doi: 10.1097/MIB.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Liñán-Rico A, Wunderlich JE, Enneking JT, Tso DR, Grants I, Williams KC, Otey A, Michel K, Schemann M, Needleman B, Harzman A, Christofi FL. Neuropharmacology of purinergic receptors in human submucous plexus: Involvement of P2X1, P2X2, P2X3 channels, P2Y and A3 metabotropic receptors in neurotransmission. Neuropharmacology. 2015;95:83–99. doi: 10.1016/j.neuropharm.2015.02.014. Ca2+ imaging was used to study the neuropharmacology of purinergic receptors in vitro in human submucous plexus. The neuropharmacology suggests that stimulatory P2X1R, P2X2R, P2X3R and inhibitory P2Y and A3R are involved in neurotransmission. Several types of neurons were identified based on their expression of P2XR subtypes. P2X1R expression in human submucous neurons may represent an important species difference. Overall, findings suggest that purines regulate neurotransmission at several synapses in the human enteric nervous system. One or more of these neural receptors are potential targets of investigation in GI diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Linan-Rico A, Turco F, Ochoa-Cortes F, Harzman A, Needleman BJ, Arsenescu R, Abduel-Rasoul M, Fadda P, Grants I, Whitaker E, Cuomo R, Christofi FL. Molecular Signaling and Dysfunction of the Human Reactive Enteric Glial Cell Phenotype: Implications for GI Infection, IBD, POI, Neurological, Motility and GI Disorders. Infl Bowel Dis. 2016;22(8):1812–34. doi: 10.1097/MIB.0000000000000854. Enteric glial cells are implicated in the pathogenesis of gut inflammatory diseases, postoperative ileus, intestinal infections, functional bowel disorders and motility disorders, and in particular slow transit constipation. In this study, human enteric glial cells in culture were used to identify a component of the ‘reactive human enteric glial phenotype.’ Bacterial LPS induction (24 h) caused up-regulation in mRNA levels of 58% of 107 genes analysed. Regulated genes included 54% of inflammatory genes (cytokines, chemokines, C3 and s100β), 52% of purine genes (AdoR2A; AdoR2B; P2RY1, P2RY2, P2RY6, P2RX3, P2RX7; AMPD3; ENTPD2; ENTPD3; and NADSYN1), other channels, vesicular transporters, antioxidant enzymes, growth factors and transcription factors. ATP release increased 5-fold with LPS. Treatment also disrupted calcium signalling, ATP and mechanically – evoked Ca2+responses. Inflammation induced a purinergic switch from ATP to other types of purinergic signalling, i.e. A2AAR signalling. Studies have implications for GI diseases and disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Shi Q, Zhu Q, Zou T, Li G, Huang A, Wu B, Peng L, Song M, Wu Q, Xie Q, Lin W, Xie W, Wen S, Zhang Z, Lv Q, Zou L, Zhang X, Ying M, Li G, Liang S. P2X7 receptor of rat dorsal root ganglia is involved in the effect of moxibustion on visceral hyperalgesia. Purinergic Signal. 2015;11:161–169. doi: 10.1007/s11302-014-9439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhi MS, Moss A, Jiang ZG, Robson SC. Purinergic signaling during intestinal inflammation. J Mol Med. 2017 doi: 10.1007/s00109-017-1545-1. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Feng C, Wu J, Wu Y, Liu D, Wu J, Dai F, Zhang J. P2Y1, P2Y2, and TRPV1 receptors are increased in diarrhea-predominant irritable bowel syndrome and P2Y2 correlates with abdominal pain. Dig Dis Sci. 2016;61:2878–2886. doi: 10.1007/s10620-016-4211-5. [DOI] [PubMed] [Google Scholar]
- Ma B, Jiang Q, Li W, Li Z. Estrogen receptor beta (ERβ) activation plays a therapeutic role in murine models of inflammatory bowel disease (IBD) via inhibiting P2X3 and P2X7 receptors. The FASEB Journal. 2016;30:1023. [Google Scholar]
- Matsukawa T, Izawa K, Isobe M, Takahashi M, Maehara A, Yamanishi Y, Kaitani A, Okumura K, Teshima T, Kitamura T, Kitaura J. Ceramide-CD300f binding suppresses experimental colitis by inhibiting ATP-mediated mast cell activation. Gut. 2016;65:777–787. doi: 10.1136/gutjnl-2014-308900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CE, Jacobson KA. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim. Biophys. Acta - Biomembranes. 2011;1808:1290–1308. doi: 10.1016/j.bbamem.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves AR, Castelo-Branco MT, Figliuolo VR, Bernardazzi C, Buongusto F, Yoshimoto A, Nanini HF, Coutinho CM, Carneiro AJ, Coutinho-Silva R, de Souza HS. Overexpression of ATP-activated P2X7 receptors in the intestinal mucosa is implicated in the pathogenesis of Crohn’s disease. Inflamm Bowel Dis. 2014;20:444–457. doi: 10.1097/01.MIB.0000441201.10454.06. [DOI] [PubMed] [Google Scholar]
- Ochoa-Cortes F, Liñán-Rico A, Jacobson KA, Christofi FL. Potential for developing purinergic drugs for gastrointestinal diseases. Inflamm Bowel Dis. 2014;20:1259–1287. doi: 10.1097/MIB.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Jin-Hee, Kim Yong-Chul. P2X7 receptor antagonists: a patent review (2010– 2015) Exp Opin Therap Patents. 2017;27:3, 257–267. doi: 10.1080/13543776.2017.1246538. [DOI] [PubMed] [Google Scholar]
- Rech JC, Bhattacharya A, Letavic MA, Savall BM. The evolution of P2X7 antagonists with a focus on CNS indications. Bioorg Med Chem Lett. 2016;26:3838–3845. doi: 10.1016/j.bmcl.2016.06.048. [DOI] [PubMed] [Google Scholar]
- Rodrigues L, Miranda IM, Andrade GM, Mota M, Cortes L, Rodrigues AG, Cunha RA, Goncalves T. Blunted dynamics of adenosine A2A receptors is associated with increased susceptibility to Candida albicans infection in the elderly. Oncotarget. 2016;7:62862–62872. doi: 10.18632/oncotarget.11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbatko A, Foletti D, Poulsen K, Strop P, Zhu G, Hasa-Moreno A, Melton WJ, Loo C, Krimm S, Pios A, Yu J, Brown C, Lee JK, Stroud R, Rajpal A, Shelton D. Modulation of P2X3 and P2X2/3 receptors by monoclonal antibodies. J Biol Chem. 2016;291:12254–12270. doi: 10.1074/jbc.M116.722330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, et al. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med. 2012;18(4):595–9. doi: 10.1038/nm.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz TH, Dubyak GR, Chen BK. Purinergic Receptors: Key mediators of HIV-1 Infection and Inflammation. Front Immunol. 2015 doi: 10.3389/fimmu.2015.00585. https://doi.org/10.3389/fimmu.2015.00585. [DOI] [PMC free article] [PubMed]
- **.Tian T, Zhou Y, Feng X, Ye S, Wang H, Wu W, Tan W, Yu C, Hu J, Zheng R, Chen Z, Pei X, Luo H. MicroRNA-16 is putatively involved in the NF-κB pathway regulation in ulcerative colitis through adenosine A2a receptor (A2aAR) mRNA targeting. Sci Rep. 2016;6:30824. doi: 10.1038/srep30824. MicroRNAs are post-transcriptional regulators of gene expression. MicroRNA-16 is overexpressed and A2AAR is down regulated in the colonic mucosa of active ulcerative colitis (UC) patients. Mechanistic analysis suggests that miR-16 regulates immune and inflammatory responses in ulcerative colitis by inhibiting the expression of A2AAR protein at the post-transcriptional level to promote activation of the NF-kB pro-inflammatory signalling pathway. It was claimed that miR-16 and A2AAR are potential therapeutic targets in ulcerative colitis since A2AAR activation is known to inhibit the pro-inflammatory NF-kB signalling pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HX, Hu JH, Xie R, Yang SM, Dong H. Important roles of P2Y receptors in the inflammation and cancer of digestive system. Oncotarget. 2016a;7:28736–28747. doi: 10.18632/oncotarget.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan P, Liu X, Xiong Y, Ren Y, Chen J, Lu N, Guo Y, Bai A. Extracellular ATP mediates inflammatory responses in colitis via P2 x 7 receptor signaling. Sci Rep. 2016b;6:19108. doi: 10.1038/srep19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng ZJ, Wu LY, Zhou CL, Dou CZ, Shi Y, Liu HR, Wu HG. Effect of electroacupuncture on P2X3 receptor regulation in the peripheral and central nervous systems of rats with visceral pain caused by irritable bowel syndrome. Purinergic Signal. 2015;11:321–329. doi: 10.1007/s11302-015-9447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, He Y, Feng X, Ye S, Wang H, Tan W, Yu C, Hu J, Zheng R, Zhou Y. MicroRNA-206 is involved in the pathogenesis of ulcerative colitis via regulation of adenosine A3 receptor. Oncotarget. 2017;8:705–721. doi: 10.18632/oncotarget.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Ren J, Chen G, Wu L, Song X, Li G, Deng Y, Wang G, Gu G, Li J. Systemic blockade of P2X7 receptor protects against sepsisinduced intestinal barrier disruption. Scientific Reports. 2017;7:4364. doi: 10.1038/s41598-017-04231-5. http://doi.org/10.1038/s41598-017-04231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Xie Z, Wang H, Shen Z, Guo Y, Gao Y, et al. Bacterial Diversity of Intestinal Microbiota in Patients with Substance Use Disorders Revealed by 16S rRNA Gene Deep Sequencing. Sci Rep. 2017;7(1):3628. doi: 10.1038/s41598-017-03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante A, Borgoni R, Falzoni S, D’Incà R, Sturniolo GC, Cifalà V, Di Virgilio F. Inflammasome activation P2X7-dependent in Crohns disease. Journal of Gastrointestinal & Digestive System. 2015;5:316. [Google Scholar]
- Zhang B, Cheng B, Li FS, Ding JH, Feng YY, Zhuo GZ, Wei HF, Zhao K. High expression of CD39/ENTPD1 in malignant epithelial cells of human rectal adenocarcinoma. Tumour Biol. 2015;36:9411–9419. doi: 10.1007/s13277-015-3683-9. [DOI] [PubMed] [Google Scholar]