Abstract
Glycogen storage disease type Ia (GSD-Ia) is an autosomal recessive metabolic disorder caused by a deficiency in glucose-6-phosphatase-α (G6Pase-α or G6PC) that is expressed primarily in the liver, kidney, and intestine. G6Pase-α catalyzes the hydrolysis of glucose-6-phosphate (G6P) to glucose and phosphate in the terminal step of gluconeogenesis and glycogenolysis, and is a key enzyme for endogenous glucose production. The active site of G6Pase-α is inside the endoplasmic reticulum (ER) lumen. For catalysis, the substrate G6P must be translocated from the cytoplasm into the ER lumen by a G6P transporter (G6PT). The functional coupling of G6Pase-α and G6PT maintains interprandial glucose homeostasis. Dietary therapies for GSD-Ia are available, but cannot prevent the long-term complication of hepatocellular adenoma that may undergo malignant transformation to hepatocellular carcinoma. Animal models of GSD-Ia are now available and are being exploited to both delineate the disease more precisely and develop new treatment approaches, including gene therapy.
Keywords: Glycogen storage disease type Ia (GSD-Ia), Glucose-6-phosphatase-α (G6Pase-α or G6PC), Gene therapy, Hepatocellular adenoma, Recombinant adeno-associated virus vector
1. Introduction
Glycogen storage disease type I (GSD-I), also known as von Gierke disease, consists of two major subtypes, GSD-Ia (MIM232200), caused by a deficiency in glucose-6-phosphatase-α (G6Pase-α or G6PC) and GSD-Ib (MIM232220), caused by a deficiency in the glucose-6-phosphate (G6P) transporter (G6PT).1–3 The incidence of GSD-I is approximately 1 in 100,000, with GSD-Ia being the most prevalent form, representing approximately 80% of cases. G6Pase-α catalyzes the hydrolysis of G6P to glucose and phosphate in the terminal steps of gluconeogenesis and glycogenolysis, and is primarily expressed in the liver, kidney, and intestine.1–3 The active site of G6Pase-α lies on the luminal side of the endoplasmic reticulum (ER), inaccessible to the cytoplasm.4 Therefore, for G6P hydrolysis to occur in vivo, the G6P substrate must be translocated from the cytoplasm into the ER lumen by the G6PT.1–3 Accordingly, G6P transport and hydrolysis are tightly coupled events and the primary function of the G6Pase-α/G6PT complex is to maintain interprandial blood glucose homeostasis.1–3 Patients affected by GSD-Ia are unable to maintain glucose homeostasis and present with fasting hypoglycemia, hepatomegaly, nephromegaly, hyperlipidemia, hyperuricemia, lactic acidemia, and growth retardation.1–3 Untreated GSD-Ia can be lethal to juvenile patients. Dietary therapies have enabled GSD-Ia patients to attain near normal growth and pubertal development.5,6 However, no current therapy is able to address long-term complications of hepatocellular adenoma (HCA) that develop in 75% of GSD-I patients over 25 years-old.1–3,7,8 In 10% of cases, HCA undergoes malignant transformation to hepatocellular carcinoma (HCC).1–3, 8,9 This review focuses on recent developments in GSD-Ia, including gene therapy for the treatment of this disorder.
2. Molecular genetics of GSD-Ia
2.1. The G6PC gene and encoded G6Pase-α enzyme
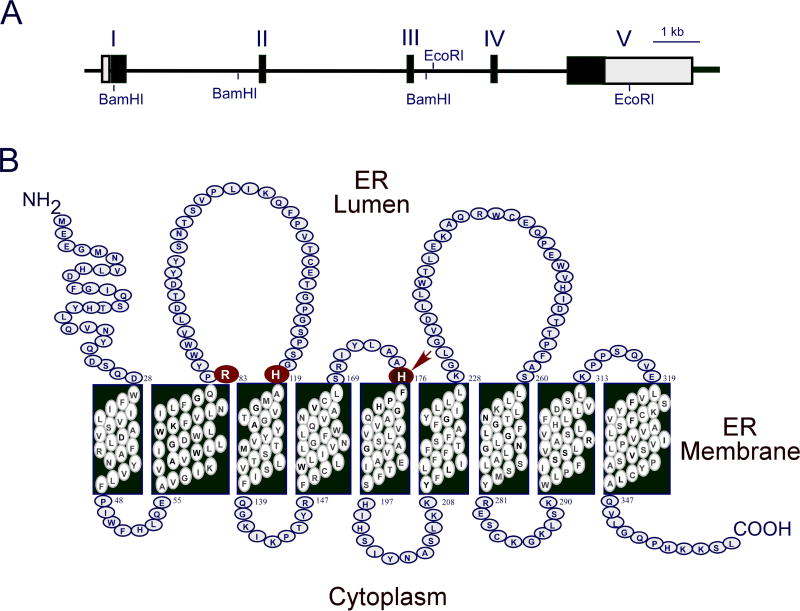
Human G6PC is a single copy gene composed of five exons (Fig. 1A) located on chromosome 17q21.10 The encoded G6Pase-α enzyme is a 357 amino-acid glycoprotein anchored to the ER membrane by nine transmembrane helices (Fig. 1B).10–12 Based on mutational and active site labeling studies, the current paradigm for the G6Pase-α reaction mechanism is that His-176 initiates a nucleophilic attack on the phosphate of G6P to form a phosphohistidine-G6Pase-α intermediate.4 This transition state is stabilized by Arg-83 hydrogen bonding to phosphate, and is resolved by His-119 providing a proton that liberates the glucose moiety. The active site residues of G6Pase-α, Arg-83, His-119, and His-176 are all situated inside the ER lumen (Fig. 1B), inaccessible to G6P in the cytoplasm.
Fig. 1. The G6PC gene and the encoded G6Pase-α enzyme.
(A) G6PC, located on human chromosome 17q21, is shown as a line diagram with exons marked as boxes I to V. Black boxes represent coding regions, white boxes the 5′ and 3′ untranslated regions of the G6PC transcript. (B) The encoded G6Pase-α enzyme is anchored in the membrane of the ER by nine transmembrane helices. The amino-terminus is located in the ER lumen and the carboxyl-terminus in the cellular cytoplasm. Arg-83, His-119, and His-176, which contribute to the active center, are denoted by large circles. The phosphate acceptor, His-176, is denoted by an arrow.
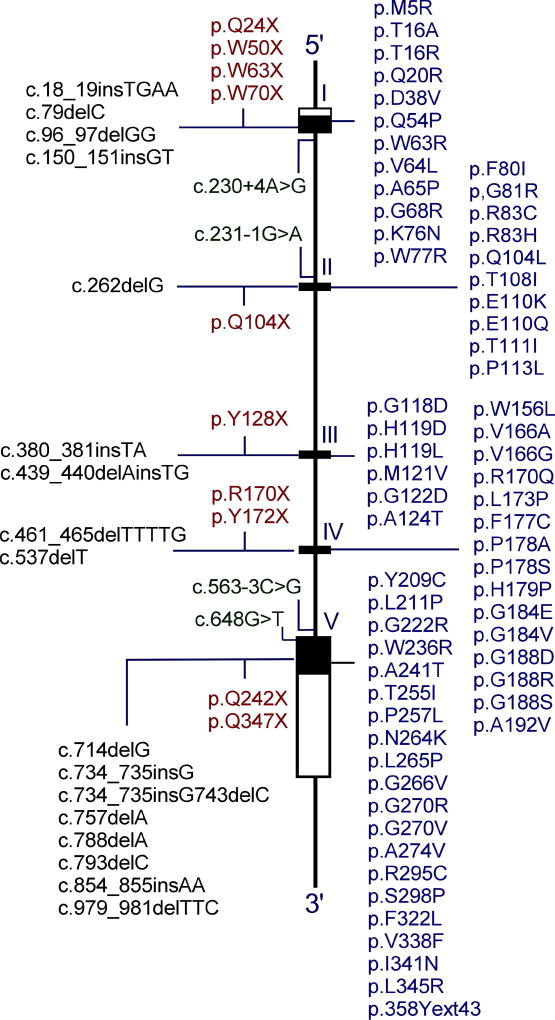
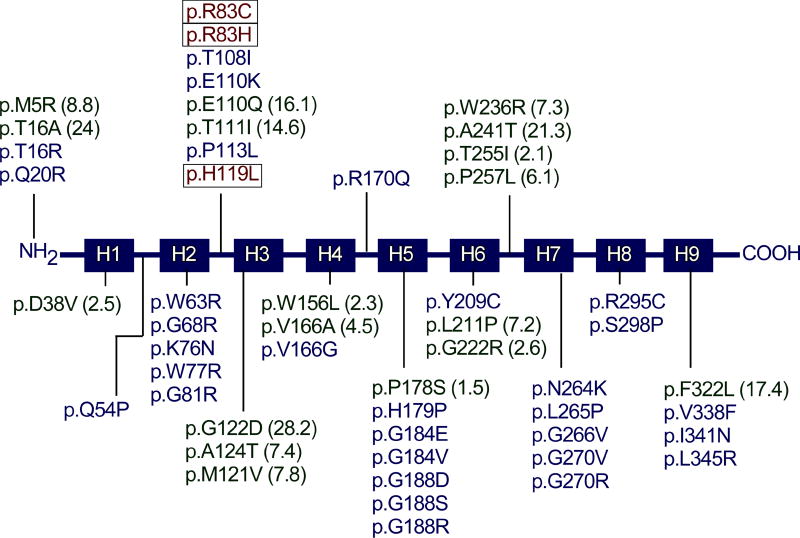
To date, 95 separate G6PC mutations (Fig. 2), including 63 missense, 10 nonsense, 17 insertion/deletion, 4 splicing, and 1 no-stop mutation (c.1074A>C/p.358Yext43) have been identified [http://www.hgmd.cf.ac.uk/ac/gene.php?gene=G6PC]. Of the identified G6PC mutations, 51 missense (Fig. 3), 2 nonsense (p.R170X and p.Q347X), and 2 codon deletion (c.734_735insG743delC and p.F327del) mutations have been confirmed as pathogenic based on site-directed mutagenesis and transient expression assays.13–15 Thirty-three of the G6PC missense mutations completely abolish G6Pase-α activity, and the other 18 retain varying degrees of residual enzymatic activity.13–15 Mutations in two of the proposed G6Pase-α active site residues, namely p.R83C, p.R83H, and p.H119L (Fig. 3), have been identified in GSD-Ia patients and shown to completely abolish G6Pase-α enzymatic activity, consistent with their proposed role.10,14 No mutation has been found in the phosphate acceptor p.H176 in G6Pase-α. However, site-directed mutagenesis has shown that the p.H176A mutation completely abolished G6Pase-α enzymatic activity.16 Four natural occurring G6PC splicing mutations, c.230+4A>G, c.231−1G>A, c.563−3C>G, and c.648G>T have been identified (Fig. 2). The c.231−1G>A mutation causes exon 2 skipping and the c.648G>T mutation results in a 91-nt deletion in exon 5 encoding a severely truncated polypeptide of 201 amino acids.17–19 Both mutations are predicted to inactivate G6Pase-α activity. While GSD-Ia is not predominantly restricted to any one racial or ethnic group, mutations in the G6PC gene unique to Caucasian, Hispanic, Chinese/Japanese/Korean, and Jewish GSD-Ia patients have been described, suggesting separate ethnic founder effects for some mutations.1,2,20,21 GSD-Ia is more prevalent in the Ashkenazi Jewish population, where the carrier frequency for the p.R83C mutation is 1.4%.20 To date, no clear genotype-phenotype correlations have been demonstrated for GSD-Ia.2,21
Fig. 2. Mutations identified in the G6PC gene of GSD-Ia patients.
G6PC is shown as a line diagram with exons marked as boxes I to V. Black boxes represent coding regions, white boxes the 5′ and 3′ untranslated regions of the G6PC transcript. The positions of all known mutations are listed from left to right as insertion/deletion, nonsense, splicing, and missense mutations.
Fig. 3. A summary of missense protein mutations identified in human GSD-Ia patients that affect phosphohydrolase activity.
The G6Pase-α protein is represented by a line diagram, with the nine helical transmembrane domains marked as boxes H1 to H9. Protein mutations that destroy G6Pase-α activity are listed. Mutants retaining some residual activity are listed with the percent of wild-type enzymatic activity retained in parentheses. Mutations identified in the active site residues that destroy enzymatic activity are bracketed.
2.2. The metabolic phenotype of GSD-Ia
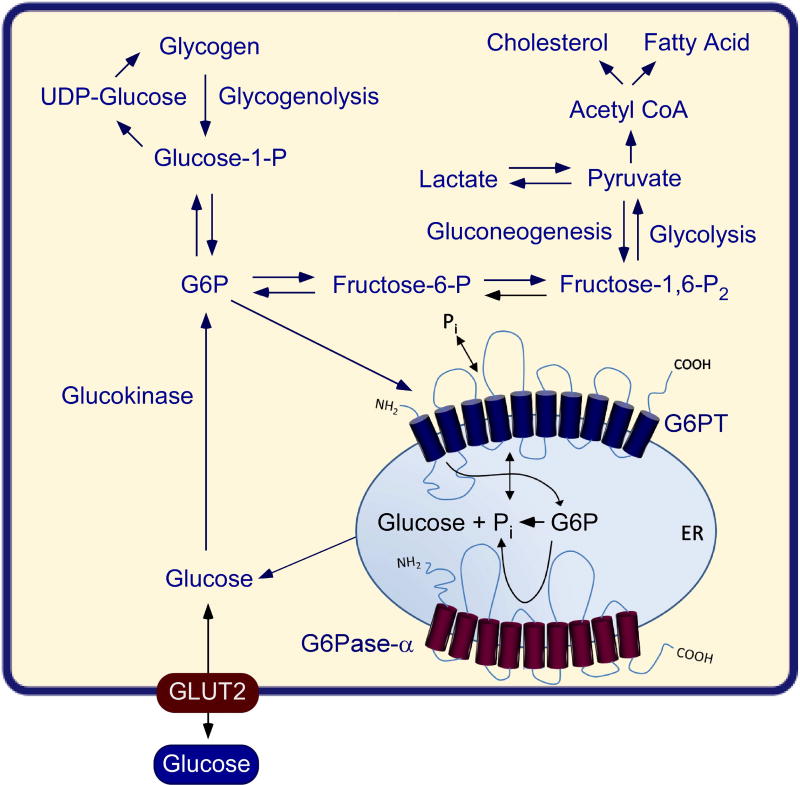
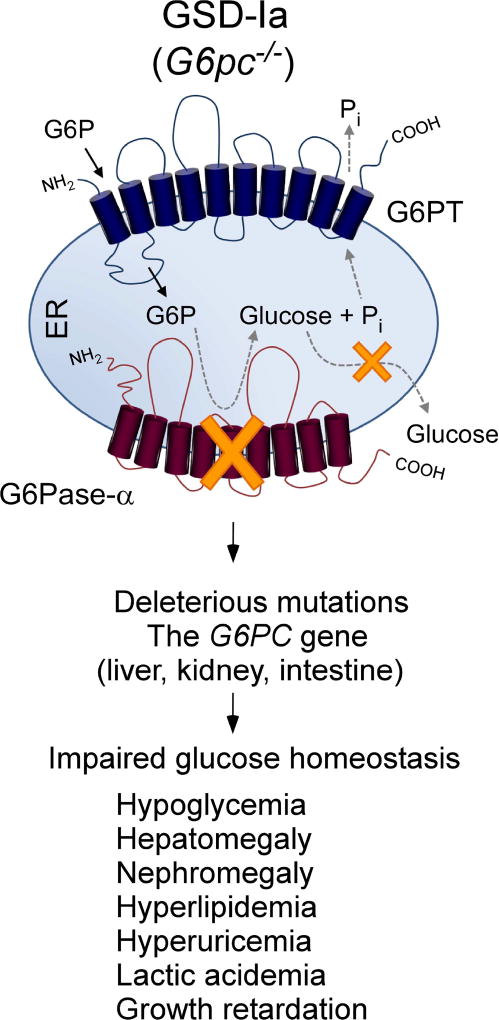
The hallmark of GSD-Ia is hypoglycemia following a short fast of a few hours. The liver, and to a lesser extent, the kidney and intestine, are the primary gluconeogenic organs involved in the regulation of blood glucose homeostasis between meals. As blood glucose levels fall between meals, G6P produced in the terminal step of gluconeogenesis and glycogenolysis in the gluconeogenic organs is transported by G6PT into the ER, where G6P is hydrolyzed by G6Pase-α to glucose for release back into the blood (Fig. 4). The defective G6Pase-α enzyme in the liver, kidney, and intestine of GSD-Ia patients results in impaired blood glucose homeostasis (Fig. 5).1–3 Associated with this is an elevation of G6P in the cytoplasm of the cells, leading to an excessive accumulation of glycogen, which promotes progressive hepatomegaly and nephromegaly seen in GSD-Ia patients. Hepatomegaly is further exacerbated by an accumulation of neutral lipids in the liver. Other major metabolic consequences of elevated liver and kidney cytoplasmic G6P are hypercholesterolemia, hypertriglyceridemia, hyperuricemia, and lactic acidemia that characterize the clinical pathophysiology of GSD-Ia (Fig. 5). Longer-term presentations of GSD-Ia include growth retardation, osteoporosis, gout, pulmonary hypertension, HCA with risk of transformation to HCC, and renal disease.1–3
Fig. 4. The primary anabolic and catabolic pathways of G6P in the liver, kidney, and intestine.
Intracellular glucose is produced via hydrolysis of G6P by G6Pase-α in the terminal rate-limiting step of gluconeogenesis and glycogenolysis. The G6Pase-α and G6PT components of the G6Pase-α/G6PT complex are shown embedded within the membrane of the ER. The GLUT2 transporter, responsible for the transport of glucose in and out of the cell, is shown embedded in the plasma membrane. Abbreviations: G6P, glucose-6-phosphate; UDP-Glucose, uridine 5'-diphosphate glucose.
Fig. 5. GSD-Ia is caused by deleterious mutations in the G6PC gene and manifests a phenotype of impaired glucose homeostasis.
The G6Pase-α and G6PT components of the G6Pase-α/G6PT complex are shown embedded within the membrane of the ER. Abbreviations: G6PC, Glucose-6-phosphatase-α.
3. GSD-Ia animal models
Several GSD-Ia animal models exist, including a global G6pc knock-out mouse model (G6pc−/−),22 a naturally occurring dog model23, and two conditional G6pc-null mouse models.24,25 The G6pc−/− mouse model manifests all known symptoms of human GSD-Ia, making it an excellent model of the human disease.22 However, G6pc−/− mice exhibit relatively mild lactic acidemia compared with human GSD-Ia patients. The GSD-Ia dog model is based on a naturally occurring p.M121I G6PC mutation identified in the Maltese breed that was cross-bred with beagles to overcome size, neonatal survival, and small litter size limitations of the carrier Maltese background.23 This Maltese-Beagle hybrid GSD-Ia dog manifests all of the typical symptoms of the human disorder, including lactic acidosis typical of human GSD-Ia patients. These animal models have been widely used to understand the biology, pathophysiology, and long-term complications of GSD-Ia, and have been utilized to develop gene and cell therapies for GSD-Ia.26,27 The G6pc−/− mice and G6pc−/− dogs rarely survive longer than three weeks, even under intensive dietary therapy regimes.22,23 Consequently, HCA development has not been reported in the untreated global G6pc knockout animal models. However, liver-specific G6pc-null (L-G6pc−/−) mice survive to adulthood and develop multiple HCA,25,28 which can be used to study the etiology of HCA/HCC associated with GSD-Ia. In addition, kidney-specific G6pc knockout and intestine-specific knockout mice are available to delineate molecular mechanisms underlying other long-term complications of GSD-Ia.29,30
4. GSD-I-associated HCA
4.1. Hepatic tumors identified in human GSD-I
One severe long-term complication of GSD-I is HCA, a benign liver tumor that develops in 75% of GSD-I patients over 25 years-old.1–3 HCAs in GSD-I patients are small, multiple, non-encapsulated, and produce excess hepcidin that contributes to anemia.31 Additional complications from GSD-I-associated HCA include intratumoral hemorrhage and, in 10% of cases, malignant transformation to HCC can occur. Previously, molecular analysis classified HCA into four major subgroups: hepatocyte nuclear factor 1A mutated HCA (HHCA), inflammatory HCA (IHCA), β-catenin (CTNNB1 exon 3) mutated HCA (bex3HCA), and unclassified HCA (UHCA).32 More recently, a new HCA subgroup, shHCA, caused by activation of sonic hedgehog signaling, was identified.33 shHCA represents 4% of all HCAs, and is associated with obesity and bleeding. HCAs are now classified into eight subgroups: HHCA, IHCA, bex3HCA with an increased risk of malignant transformation to HCC, bex7,8HCA without an increased risk of malignant transformation, bex3IHCA, bex7,8 IHCA, shHCA, and UHCA.33 The bex3HCA and bex7,8HCA are β-catenin-mutated HCAs in CTNNB1 exon 3 and exon 7 or 8, respectively. The bex3IHCA and bex7,8IHCA exhibited both inflammatory phenotype and β-catenin activating mutations.33
Calderaro et al.34 characterized 25 HCAs that developed in 14 GSD-Ia and 1 GSD-Ib patients using gene expression and DNA sequence of mutated genes in sporadic HCAs. They classified GSD HCAs as IHCA (52%), bHCA (28%), or UHCA (20%). The bHCA seen in GSD-Ia patients were characterized by high expression of two β-catenin target genes, glutamate-ammonia ligase (GLUL), and leucine-rich repeat containing G protein-coupled receptor 5 (LGR5).34 The IHCA were characterized by a constitutive activation of signal transducer and activator of transcription (STAT3), a cancer-promoting transcription factor.34,35 Interestingly, while HHCA was the major sporadic HCA identified, no HHCA was observed in GSD-I patients.34 Calderaro et al.34 showed that GSD-I livers without tumors and the HHCA exhibited similar metabolic defects characterized by gluconeogenesis repression, glycolysis activation, and fatty acid synthesis activation, providing one clue why HHCA had not been identified in GSD-I patients. Based on the new HCA classification,33 the seven bHCA cases identified in human GSD-I patients are: bex3HCA (n = 3), bex3IHCA (n = 1), bex7,8HCA (n = 2), and bex7,8IHCA (n = 1). Presently, 6 HCA subgroups (IHCA, bex3HCA, bex3IHCA, bex7,8HCA, bex7,8IHCA, and UHCA) have been identified in GSD-I patients. The underlying etiology of HCA in GSD-Ia is unknown.
4.2. Hepatic tumors identified in murine GSD-Ia
Using magnetic resonance imaging, Mutel et al.25 showed that L-G6pc−/− mice developed hepatic nodules nine months after gene deletion and all mice developed multiple HCAs 18 months after gene deletion, but they did not characterize the tumor subtypes. Of the three rAAV-treated G6pc−/− mice that developed hepatic tumors at age 71–72 weeks, one harbored a HCA nodule and the other two each harbored a single HCC lesion.36 The non-tumor liver regions of the two HCC-bearing rAAV-treated G6pc−/− mice had 1.5–2.2 units of G6Pase-α activity. However, the HCC lesions of the rAAV-treated G6pc−/− mice had non-detectable G6Pase-α activity.36 HCC-1 expressed increased levels of mRNA for Glul and Lgr5, suggesting that HCC-1 was derived from a bHCA. HCC-2 displayed increased levels of the active p-STAT3-Y705 protein, suggesting that HCC-2 was derived from an IHCA.36
5. Treatment
5.1. Conventional treatment
Metabolic disruption present in GSD-Ia patients can be adequately managed with strong adherence to dietary therapies.1–3 GSD-Ia infants typically receive nocturnal nasogastric infusion of glucose to avoid hypoglycemia.5 GSD-Ia patients 3 years or older are prescribed uncooked cornstarch, a slow release carbohydrate, to prolong the length of euglycemia between meals.6 These dietary therapies enable GSD-Ia patients to maintain normoglycemia, but the underlying pathological processes remain uncorrected. Consequently, HCA/HCC and renal disease, two major causes of morbidity and mortality in patients with GSD-Ia, are common.1–3
5.2. Gene therapy
G6Pase-α is an extremely hydrophobic transmembrane protein that is difficult to purify and protein replacement therapy is not an option,11,12 but somatic gene therapy is a promising approach. A variety of gene transfer vectors, including adenovirus vectors,37 helper-dependent adenovirus vectors,38 lentivirus vectors,39–41 and recombinant adeno-associated virus (rAAV) vectors,26,27,36,42–47 have been developed using animal models of GSD-Ia. The rAAV-mediated gene therapy in both mouse and canine models of GSD-Ia has led to long-term correction of metabolic abnormalities with no detectable toxicity.36,42–47 The most promising results come from GSD-Ia studies using rAAV vectors directed by the human G6PC promoter/enhancer (GPE).43,44 rAAV8-miGPE and rAAV8-GPE are human G6Pase-α-expressing rAAV2/8 vectors driven by 382-bp of the minimal (mi) GPE (rAAV8-miGPE) and approximately 3 kb GPE (rAAV8-GPE),43,44 respectively. Both vectors demonstrated efficacy in treating G6pc−/− mice and the rAAV8-miGPE vector also showed efficacy in GSD-Ia dogs.43–45 While a cell-mediated immune response of hepatic CD8+ lymphocyte infiltration was observed in G6pc−/− mice infused with a G6Pase-α-expressing rAAV8 vector driven by the CBA/CMA promoter/enhancer, no such immune response was observed with rAAV8-GPE under the same conditions.44
To select the best vector for clinical translation, a direct comparison of rAAV8-GPE and rAAV8-miGPE vectors was conducted using G6pc−/− mice.46 The results show that the rAAV8-GPE vector directs significantly higher levels of hepatic G6Pase-α expression, achieves greater reduction in hepatic glycogen accumulation, and leads to a better tolerance of fasting, than the rAAV8-miGPE vector.46 This suggests that the rAAV8-GPE vector is the best vector to take forward into clinical trials for GSD-Ia. In a long-term dose-ranging study, Lee et al.45 further showed that rAAV8-GPE-mediated gene transfer showed that restoring ≥ 3% (5 units) of normal hepatic G6Pase-α activity in G6pc−/− mice was sufficient to maintain glucose homeostasis. The treated G6pc−/− mice displayed normal hepatic fat storage, normal blood metabolite and glucose tolerance profiles, reduced fasting blood insulin levels, and had no evidence of hepatic abnormalities or HCA. Fasting hypoglycemia is the hallmark of GSD-Ia. Promisingly, the rAAV8-GPE-treated G6pc−/− mice were able to sustain a 24-hour fast, which is a stress test of the liver’s ability to maintain blood normoglycemia through glycogenolysis and gluconeogenesis catalyzed by G6Pase-α in the absence of dietary glucose (Fig. 6). Lee et al.45 further showed that the ability of rAAV-GPE-treated G6pc−/− mice expressing low levels of hepatic G6Pase-α to produce sufficient glucose to maintain interprandial glucose homeostasis correlated with an increase in hepatic G6PT mRNA expression and a corresponding increase in microsomal G6P uptake activity (Fig. 6).
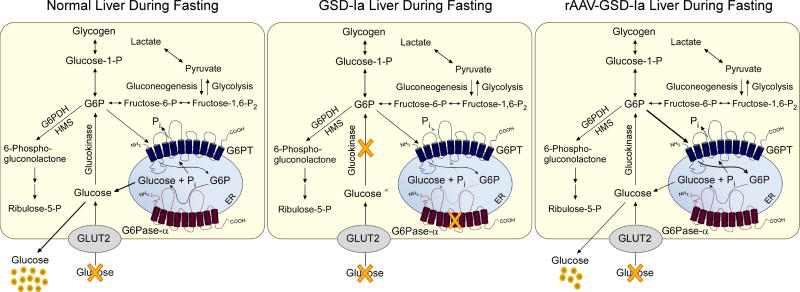
Fig. 6. Pathways for G6P metabolism in the livers of normal, GSD-Ia, and rAAV8-GPE-treated GSD-Ia mice during fasting.
During fasting, G6P, the end product of gluconeogenesis and glycogenolysis, is transported from the cytoplasm into the lumen of the ER by G6PT. Inside the ER, G6P is hydrolyzed by G6Pase-α and the resulting glucose is transported back into the cytoplasm then released into circulation. In the GSD-Ia liver, which lacks a functional G6Pase-α, the ER-localized G6P cannot be converted to glucose, leading to hypoglycemia following a short fast. The rAAV8-GPE-treated GSD-Ia (rAAV-GSD-Ia) liver, which expresses reduced levels of G6Pase-α, but increased levels of G6PT compared with a normal liver, generates reduced levels of endogenous glucose and maintains interprandial glucose homeostasis. The GLUT2 transporter, responsible for the transport of glucose in and out of the cell, is shown embedded in the plasma membrane. G6PT and G6Pase-α are shown embedded in the ER membrane.
More recently, Kim et al.36 conducted a second long-range study to examine the minimum dosage of rAAV-GPE vector required to prevent HCA formation in G6pc−/− mice. The authors characterized 11 rAAV-GPE-treated G6pc−/− mice harboring 0.9%–2.4% (1.5–4.1 units) of normal hepatic G6Pase-α activity, and showed that three mice expressing 0.9%–1.3% of normal hepatic G6Pase-α activity developed HCA/HCC and eight did not. The results established a threshold of hepatic G6Pase-α activity required to prevent HCA/HCC, and showed that GSD-Ia mice harboring less than 2% of normal hepatic G6Pase-α activity are at risk of tumor development.36
Correction of renal disease in GSD-Ia has been less extensively studied. rAAV8-mediated gene transfer results in little or no renal G6Pase-α expression, and the abnormal renal pathology persists. This has been attributed to poor kidney transduction mediated by the AAV2/8 serotype.43,46,48 Different AAV serotypes have different tissue transduction efficiencies and more recent data suggest that rAAV2/9 may be the preferred choice for future renal gene delivery.49–52 However, rAAV2/9-mediated transgene expression in the kidney is still significantly lower than that in the liver. Using a retrograde renal vein injection method, Rocca et al.52 showed that rAAV2/9-mediated kidney transduction could be markedly improved. Identification of viral serotypes that effectively transduce all affected tissue types remains to be further explored. Notably, serotypes can have very different targeting efficiencies in different species. Only a small number of serotypes have been used in clinical trials to date. Therefore, there is a need to understand more about the primate specificity of the many serotypes that appear promising in rodents.
6. Conclusions
Metabolic abnormalities in GSD-Ia are currently being treated by dietary therapies that have enabled patients to maintain euglycemia and remove early symptomatic signs of the disease. However, dietary therapies leave the patient vulnerable to severe long-term complications of renal disease and HCA/HCC. The effective use of gene therapies to correct the disease in GSD-Ia animal models is very promising, with efforts to initiate clinical trials on the horizon. While early interventions of gene therapy prevent HCA development, it is unclear whether gene therapy can abrogate pre-existing hepatic tumors. Delineating the molecular mechanisms underlying HCA/HCC in GSD-Ia remains to be explored. The L-G6pc−/− mice that survive to adulthood and develop HCA/HCC offer a suitable model to study the etiology of hepatic tumors and their treatment of GSD-Ia. It is important to identify potential targets to guide the development of therapies, not only to correct metabolic abnormalities, but to eradicate the long-term complications of this disorder.
Acknowledgments
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Chou JY, Matern D, Mansfield BC, Chen YT. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr Mol Med. 2002;2:121–143. doi: 10.2174/1566524024605798. [DOI] [PubMed] [Google Scholar]
- 2.Chou JY, Jun HS, Mansfield BC. Glycogen storage disease type I and G6Pase-β deficiency: etiology and therapy. Nat Rev Endocrinol. 2010;6:676–688. doi: 10.1038/nrendo.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou JY, Jun HS, Mansfield BC. Type I glycogen storage diseases: Disorders of the glucose-6-phosphatase/glucose-6-phosphate transporter complexes. J Inherit Metab Dis. 2015;38:511–519. doi: 10.1007/s10545-014-9772-x. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh A, Shieh JJ, Pan CJ, Sun MS, Chou JY. The catalytic center of glucose-6-phosphatase: His176 is the nucleophile forming the phosphohistidine-enzyme intermediate during catalysis. J Biol Chem. 2002;277:32837–32842. doi: 10.1074/jbc.M201853200. [DOI] [PubMed] [Google Scholar]
- 5.Greene HL, Slonim AE, O'Neill JA, Jr, Burr IM. Continuous nocturnal intragastric feeding for management of type 1 glycogen-storage disease. N Engl J Med. 1976;294:423–425. doi: 10.1056/NEJM197602192940805. [DOI] [PubMed] [Google Scholar]
- 6.Chen YT, Cornblath M, Sidbury JB. Cornstarch therapy in type I glycogen-storage disease. N Engl J Med. 1984;310:171–175. doi: 10.1056/NEJM198401193100306. [DOI] [PubMed] [Google Scholar]
- 7.Labrune P, Trioche P, Duvaltier I, Chevalier P, Odièvre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr. 1997;24:276–279. doi: 10.1097/00005176-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I) Eur J Pediatr. 2002;161:S20–S34. doi: 10.1007/s00431-002-0999-4. [DOI] [PubMed] [Google Scholar]
- 9.Franco LM, Krishnamurthy V, Bali D, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005;28:153–162. doi: 10.1007/s10545-005-7500-2. [DOI] [PubMed] [Google Scholar]
- 10.Lei KJ, Shelly LL, Pan CJ, Sidbury JB, Chou JY. Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type 1a. Science. 1993;262:580–583. doi: 10.1126/science.8211187. [DOI] [PubMed] [Google Scholar]
- 11.Pan CJ, Lei KJ, Annabi B, Hemrika W, Chou JY. Transmembrane topology of glucose-6-phosphatase. J Biol Chem. 1998;273:6144–6148. doi: 10.1074/jbc.273.11.6144. [DOI] [PubMed] [Google Scholar]
- 12.Pan CJ, Lei KJ, Chou JY. Asparagine-linked oligosaccharides are localized to a luminal hydrophilic loop in human glucose-6-phosphatase. J Biol Chem. 1998;273:21658–21662. doi: 10.1074/jbc.273.34.21658. [DOI] [PubMed] [Google Scholar]
- 13.Chou JY, Mansfield BC. Mutations in the glucose-6-phosphatase-alpha (G6PC) gene that cause type Ia glycogen storage disease. Hum Mutat. 2008;29:921–930. doi: 10.1002/humu.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shieh JJ, Terizioglu M, Hiraiwa H, et al. The molecular basis of glycogen storage disease type 1a: structure and function analysis of mutations in glucose-6-phosphatase. J Biol Chem. 2002;277:5047–5053. doi: 10.1074/jbc.M110486200. [DOI] [PubMed] [Google Scholar]
- 15.Shieh JJ, Lu YH, Huang SW, et al. Misdiagnosis as steatohepatitis in a family with mild glycogen storage disease type 1a. Gene. 2012;509:154–157. doi: 10.1016/j.gene.2012.07.057. [DOI] [PubMed] [Google Scholar]
- 16.Lei KJ1, Pan CJ, Liu JL, Shelly LL, Chou JY. Structure-function analysis of human glucose-6-phosphatase, the enzyme deficient in glycogen storage disease type 1a. J Biol Chem. 1995;270:11882–11886. doi: 10.1074/jbc.270.20.11882. [DOI] [PubMed] [Google Scholar]
- 17.Akanuma J, Nishigaki T, Fujii K, et al. Glycogen storage disease type Ia: molecular diagnosis of 51 Japanese patients and characterization of slicing mutations by analysis of ectopically transcribed mRNA from lymphoblastoid cells. Am J Med Genet. 2000;91:107–112. [PubMed] [Google Scholar]
- 18.Kajihara S, Matsuhashi S, Yamamoto K, et al. Exon redefinition by a point mutation within exon 5 of the glucose-6-phosphatase gene is the major cause of glycogen storage disease type 1a in Japan. Am J Hum Genet. 1995;57:549–555. [PMC free article] [PubMed] [Google Scholar]
- 19.Okubo M, Aoyama Y, Kishimoto M, Shishiba Y, Murase T. Identification of a point mutation (G727T) in the glucose-6-phosphatase gene in Japanese patients with glycogen storage disease type 1a, and carrier screening in healthy volunteers. Clin Genet. 1997;51:179–183. doi: 10.1111/j.1399-0004.1997.tb02449.x. [DOI] [PubMed] [Google Scholar]
- 20.Ekstein J, Rubin BY, Anderson SL, et al. Mutation frequencies for glycogen storage disease Ia in the Ashkenazi Jewish population. Am J Med Genet A. 2004;129A:162–164. doi: 10.1002/ajmg.a.30232. [DOI] [PubMed] [Google Scholar]
- 21.Matern D, Seydewitz HH, Bali D, Lang C, Chen YT. Glycogen storage disease type I: diagnosis and phenotype/genotype correlation. Eur J Pediat. 2002;161(Suppl 1):S10–S19. doi: 10.1007/s00431-002-0998-5. [DOI] [PubMed] [Google Scholar]
- 22.Lei KJ, Chen H, Pan CJ, et al. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse. Nat Genet. 1996;13:203–209. doi: 10.1038/ng0696-203. [DOI] [PubMed] [Google Scholar]
- 23.Kishnani PS, Bao Y, Wu JY, Brix AE, Lin JL, Chen YT. Isolation and nucleotide sequence of canine glucose-6-phosphatase mRNA: identification of mutation in puppies with glycogen storage disease type 1a. Biochem Mol Med. 1997;61:168–177. doi: 10.1006/bmme.1997.2600. [DOI] [PubMed] [Google Scholar]
- 24.Peng WT, Pan CJ, Lee EJ, Westphal H, Chou JY. Generation of mice with a conditional allele for G6pc. Genesis. 2009;47:590–594. doi: 10.1002/dvg.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutel E, Abdul-Wahed A, Ramamonjisoa N, et al. Targeted deletion of liver glucose-6 phosphatase mimics glycogen storage disease type 1a including development of multiple adenomas. J Hepatol. 2011;54:529–537. doi: 10.1016/j.jhep.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Chou JY, Mansfield BC. Gene therapy for type I glycogen storage diseases. Curr Gene Ther. 2007;7:79–88. doi: 10.2174/156652307780363152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou JY, Mansfield BC. Recombinant AAV-directed gene therapy for type I glycogen storage diseases. Expert Opin Biol Ther. 2011;11:1011–1024. doi: 10.1517/14712598.2011.578067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Resaz R, Vanni C, Segalerba D, et al. Development of hepatocellular adenomas and carcinomas in mice with liver-specific G6Pase-α deficiency. Dis Model Mech. 2014;7:1083–1091. doi: 10.1242/dmm.014878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clar J, Gri B, Calderaro J, et al. Targeted deletion of kidney glucose-6 phosphatase leads to nephropathy. Kidney Int. 2014;86:747–756. doi: 10.1038/ki.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajas F, Clar J, Gautier-Stein A, Mithieux G. Lessons from new mouse models of glycogen storage disease type 1a in relation to the time course and organ specificity of the disease. J Inherit Metab Dis. 2015;38:521–527. doi: 10.1007/s10545-014-9761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 32.Bioulac-Sage P, Laumonier H, Couchy G, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481–489. doi: 10.1002/hep.22995. [DOI] [PubMed] [Google Scholar]
- 33.Nault JC, Couchy G, Balabaud C, et al. Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology. 2017;152:880–894. doi: 10.1053/j.gastro.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 34.Calderaro J, Labrune P, Morcrette G, et al. Molecular characterization of hepatocellular adenomas developed in patients with glycogen storage disease type I. J Hepatol. 2013;58:350–357. doi: 10.1016/j.jhep.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 35.Pilati C, Zucman-Rossi J. Mutations leading to constitutive active gp130/JAK1/STAT3 pathway. Cytokine Growth Factor Rev. 2015;26:499–506. doi: 10.1016/j.cytogfr.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Kim GY, Lee YM, Kwon JH, et al. Glycogen storage disease type Ia mice with less than 2% of normal hepatic glucose-6-phosphatase-α activity restored are at risk of developing hepatic tumors. Mol Genet Metab. 2017;120:229–234. doi: 10.1016/j.ymgme.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zingone A, Hiraiwa H, Pan CJ, et al. Correction of glycogen storage disease type 1a in a mouse model by gene therapy. J Biol Chem. 2000;275:828–832. doi: 10.1074/jbc.275.2.828. [DOI] [PubMed] [Google Scholar]
- 38.Crane B, Luo X, Demaster A, et al. Rescue administration of a helper-dependent adenovirus vector with long-term efficacy in dogs with glycogen storage disease type Ia. Gene Ther. 2012;19:443–452. doi: 10.1038/gt.2011.86. [DOI] [PubMed] [Google Scholar]
- 39.Salani B, Damonte P, Zingone A, et al. Newborn liver gene transfer by an HIV-2-based lentiviral vector. Gene Ther. 2005;12:803–814. doi: 10.1038/sj.gt.3302473. [DOI] [PubMed] [Google Scholar]
- 40.Grinshpun A, Condiotti R, Waddington SN, et al. Neonatal gene therapy of glycogen storage disease type Ia using a feline immunodeficiency virus based vector. Mol Ther. 2010;18:1592–1598. doi: 10.1038/mt.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clar J, Mutel E, Gri B, et al. Hepatic lentiviral gene transfer prevents the long-term onset of hepatic tumours of glycogen storage disease type 1a in mice. Hum Mol Genet. 2015;24:2287–2296. doi: 10.1093/hmg/ddu746. [DOI] [PubMed] [Google Scholar]
- 42.Ghosh A, Allamarvdasht M, Pan CJ, et al. Long-term correction of murine glycogen storage disease type Ia by recombinant adeno-associated virus-1-mediated gene transfer. Gene Ther. 2006;13:321–329. doi: 10.1038/sj.gt.3302650. [DOI] [PubMed] [Google Scholar]
- 43.Koeberl DD, Pinto C, Sun B, et al. AAV vector-mediated reversal of hypoglycemia in canine and murine glycogen storage disease type Ia. Mol Ther. 2008;16:665–672. doi: 10.1038/mt.2008.15. [DOI] [PubMed] [Google Scholar]
- 44.Yiu WH, Lee YM, Peng WT, et al. Complete normalization of hepatic G6PC deficiency in murine glycogen storage disease type Ia using gene therapy. Mol Ther. 2010;18:1076–1084. doi: 10.1038/mt.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee YM, Jun HS, Pan CJ, et al. Prevention of hepatocellular adenoma and correction of metabolic abnormalities in murine glycogen storage disease type Ia by gene therapy. Hepatology. 2012;56:1719–1729. doi: 10.1002/hep.25717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YM, Pan CJ, Koeberl DD, Mansfield BC, Chou JY. The Upstream enhancer elements of the G6PC promoter are critical for optimal G6PC expression in murine glycogen storage disease type Ia. Mol Genet Metab. 2013;110:275–280. doi: 10.1016/j.ymgme.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee YM, Kim GY, Pan CJ, Mansfield BC2, Chou JY. Minimal hepatic glucose-6-phosphatase-α activity required to sustain survival and prevent hepatocellular adenoma formation in murine glycogen storage disease type Ia. Mol Genet Metab Rep. 2015;3:28–32. doi: 10.1016/j.ymgmr.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akache B, Grimm D, Pandey K, et al. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michelfelder S, Trepel M. Adeno-associated viral vectors and their redirection to cell-type specific receptors. Adv Genet. 2009;67:29–60. doi: 10.1016/S0065-2660(09)67002-4. [DOI] [PubMed] [Google Scholar]
- 50.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 51.Schievenbusch S, Strack I, Scheffler M, et al. Combined paracrine and endocrine AAV9 mediated expression of hepatocyte growth factor for the treatment of renal fibrosis. Mol Ther. 2010;18:1302–1309. doi: 10.1038/mt.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rocca CJ, Ur SN, Harrison F, Cherqui S. rAAV9 combined with renal vein injection is optimal for kidney-targeted gene delivery: conclusion of a comparative study. Gene Ther. 2014;21:618–628. doi: 10.1038/gt.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]