Summary
Plague is a zoonotic disease (transmitted mainly by fleas and maintained in nature by rodents) that causes severe acute illness in humans. We present a human plague case who became infected by the bite of a wild Gunnison’s prairie dog, and a good practical example of the One Health approach that resulted in a rapid public health response. The exposure occurred while the animal was being transported for relocation to a wildlife refuge after being trapped in a plague enzootic area. This is the first report of a human plague case resulting from the bite of a Gunnison’s prairie dog. Additionally, we present an observation of a longer incubation period for plague in captive prairie dogs, leading to a recommendation for a longer quarantine period for prairie dogs during translocation efforts.
Keywords: Cynomys gunnisoni, disease introduction, plague, prairie dogs, translocation, Yersinia pestis
1 | INTRODUCTION
Plague is a zoonotic disease caused by the Gram-negative bacterium Yersinia pestis that is primarily transmitted by fleas and maintained by rodents (Gage & Kosoy, 2005). Prairie dogs (Cynomys spp.) are more susceptible to plague infection than many other mammals (Biggins, Godbey, Gage, Carter, & Montenieri, 2010; Cully, Barnes, Quan, & Maupin, 1997) with 85%–100% mortality during epizootics (Cully, Johnson, Collinge, & Ray, 2010). Human plague cases have been linked to these events, resulting from either direct contact with infected rodents or bites from infected fleas (Lowell et al., 2009).
Approximately 85% of reported human cases of plague in the United States occur as the result of a flea bite or direct contact with infected rodents (Eisen et al., 2007; Gage et al., 2000; Runfola et al., 2015). Infection is also possible through contact with infected domestic cats (Felis catus) (Eisen et al., 2007; Gage et al., 2000). Dog-to-human transmission of plague was documented in the United States in 2014 (Runfola et al., 2015). Transmission of Y. pestis to a human from a wild prairie dog bite has not been previously documented. In this report, we describe a case of human plague resulting from the bite of a wild Gunnison’s prairie dog (Cynomys gunnisoni) that was trapped in a plague-endemic area and was being translocated to a national wildlife refuge.
2 | TRANSLOCATION OF PRAIRIE DOGS FROM A PLA GUE-ENDEMIC AREA INT O A NATIONAL WILDLIFE REFUGE
On 27 July 2016, a licensed trapper/relocator company contracted by the City of Santa Fe trapped 41 Gunnison’s prairie dogs at the Santa Fe Municipal Airport for relocation. Several dozen animals remained on site for later capture. Trapped animals were transferred in carriers dusted with the insecticide deltamethrin (0.05%) to a holding facility and quarantined for 2 weeks in groups of 20 in containers with 0.05% deltamethrin-treated hay bedding.
On 10 August, the prairie dogs were transferred to a processing facility to be sexed, weighed, microchipped and quarantined for another week prior to relocation to a national wildlife refuge in Socorro County, New Mexico. On 18 August, four animals housed together were found dead and on 22 August were taken to the New Mexico Department of Agriculture’s (NMDA) Veterinary Diagnostic Services (VDS) for necropsy. Gross examination of the carcasses was concerning for plague infection, so liver and spleen tissues of one animal (PD1) were sent to the New Mexico Department of Health’s (NMDOH) Scientific Laboratory Division’s (SLD) microbiology section. The specimens were positive for Y. pestis by direct fluorescent antibody testing and were confirmed by culture on 26 August.
The remaining 37 live animals were transported to the wildlife refuge on 18 August. Upon arrival, one animal (PD5) had a cough and blood-tinged secretions from the nostrils. The animal bit a handler on the right thumb and index finger as it was being placed into a carrier for transport to a veterinary clinic, where it was initially diagnosed with rodenticide poisoning. No additional human exposures were documented. The animal died while returning to the processing facility. It was frozen at −20°C until September 2, when it was submitted to VDS.
On September 2, wildlife refuge staff observed normal activity of the 36 relocated prairie dogs. No sick or dead prairie dogs were observed. Monitoring will continue into the next year.
3 | POSTMORTEM EXAMINA TION OF PD5
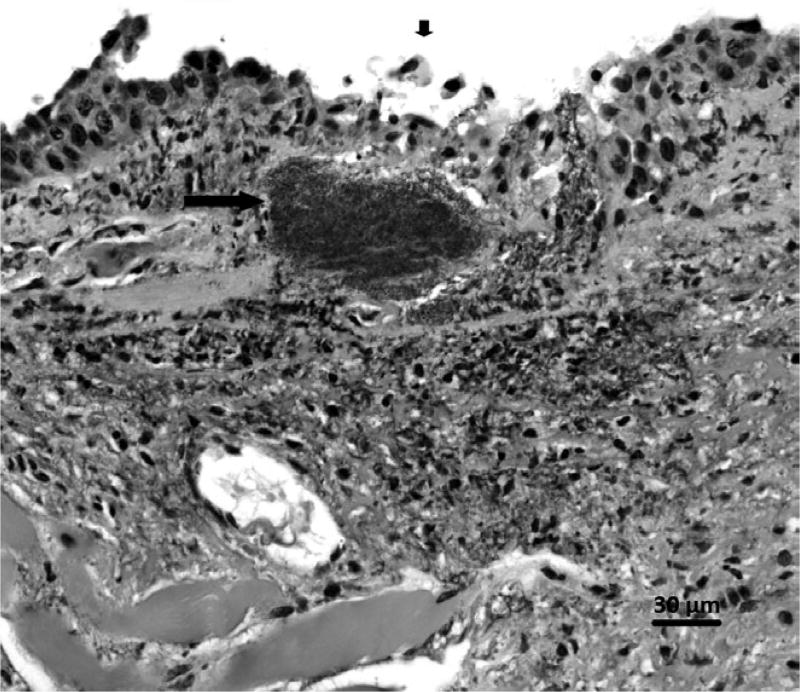
Postmortem examination was consistent with septicaemic plague caused by Y. pestis. There was moderate-to-severe decomposition and freezing artefact in all tissues examined. Except for the mandibular lymph nodes being red, gross examination was unremarkable. Microscopically, there was necrotizing hepatitis, splenitis and lymphadenitis of the mandibular lymph node, with coccobacilli that were positive for Y. pestis by immunohistochemistry in all three. The lung contained intravascular bacteria often associated with fibrinocellular thrombi. There was also ulcerative nasopharyngitis associated with Y. pestis (Figure 1). There was no evidence of tonsillitis or ulceration of the oral epithelium. On September 9, a lung/liver/spleen composite tissue culture tested positive for Y. pestis.
FIGURE 1.
Yersinia pestis bacteria (long arrow) subjacent to necrotic, ulcerating nasal epithelium (short arrow) in a Gunnison’s prairie dog. Haematoxylin and eosin. 400×
4 | GUNNISON’S PRAIRIE DOG DIE-OFF
On 10 August, the relocation contractor returned to the Santa Fe Airport to trap any remaining prairie dogs, but none were observed; it was assumed that all remaining prairie dogs had died.
5 | PUBLIC HEALTH RESPONSE
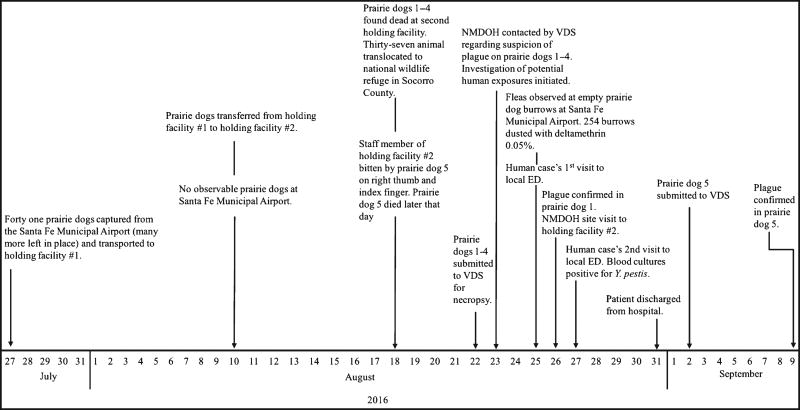
The on-call epidemiologist of the NMDOH was contacted on 23 August by VDS regarding suspicion of plague in four dead prairie dogs (Figure 2). Three people at the processing facility had close contact with the dead prairie dogs. No bites, or direct contact, with infectious material were reported, and one individual failed to report a bite from PD5 at this time. The individuals were categorized low-to-moderate risk and advised to check their temperatures twice daily and to look for other signs and symptoms consistent with plague infection for 7 days after their last exposure. One individual reported feeling ill at day 6 post-exposure (25 August). The NMDOH ensured that he received appropriate medical care and isolation.
FIGURE 2.
Timeline of events leading to the exposure of a human from a bite from a prairie dog being translocated from an area endemic for plague
On 25 August, the NMDOH visited the processing facility. No ill animals were observed. NMDOH also evaluated plague risk at the airport. Fleas were observed in empty prairie dog burrows, and each of the 254 identified burrows in the area was treated with deltamethrin by City of Santa Fe staff.
6 | ASSOCIATED HUMAN CASE
The sixty-six-year-old male who was bitten by PD5 on 18 August experienced chills, dizziness and tenderness under his right armpit the night of 24 August. He presented to a local emergency department (ED) on 25 August. He reported the prairie dog bite and was admitted and placed on droplet and contact isolation.
The patient was febrile (37.8°C), had elevated blood pressure (152/92) and slightly decreased haematocrit (40%; normal range: 42%–52%). Complete blood count and chemistry were unremarkable. Liver function tests were elevated with ALT: 385 U/L (normal range: 7–55 U/L) and AST: 194 U/L (normal range: 8–48 U/L). No other symptoms were noted. The patient denied cough or shortness of breath. Radiographs showed no abnormalities or pneumonia.
Isolation was discontinued, and the patient was discharged the same day with a 10-day course of oral doxycycline against NMDOH’s recommendations. He was instructed to check his temperature twice daily, follow up with his primary care provider the next business day, and return to the ED if symptoms worsened.
The patient re-presented to the ED on 27 August with increased swelling of the right axilla. Examination revealed a firm right axillary lymphadenopathy with surrounding erythema and cellulitis extending mid-torso. The patient was admitted, placed on contact and droplet isolation and treated with intravenous gentamicin and doxycycline. His symptoms improved; intravenous gentamicin and isolation precautions were discontinued after 3 days. The patient was discharged on 31 August with another 10-day course of oral doxycycline. He experienced a complete recovery.
Blood cultures collected on 25 August grew Gram-negative rods at day 2. Y. pestis was confirmed by culture and phage lysis at SLD.
7 | GENOMIC ANALYSIS OF DNA FR OM THE YERSINIA PESTIS IS OLATES FR OM THE HUMAN AND PRAIRIE DOG CASES
DNA from 24-hr-old Y. pestis bacterial isolates grown from both the human blood sample and the PD5 lung/liver/spleen pool was extracted using the Qiagen QIAamp DSP DNA Blood Mini Kit, followed by filtering through a 0.1-µm filter and inoculation of a plate using 10 µl of eluate to confirm sterility (no growth after 7 days in blood agar plate). The two purified DNA samples were sent to the Centers for Disease Control and Prevention, Fort Collins, CO, where whole-genome libraries were prepared using Illumina Nextera XT library prep reagents and sequence data generated using the Illumina MiSeq and the Illumina V2 300 cycle kit. The two genomes were sequenced to an average coverage depth of >200× with an average quality score of 38. Genomes were assembled and analysed using whole-genome multilocus sequence typing (wgMLST) (Kingry et al., 2016). Sequences were compared against 3,716 genes (74% of the genome) from the reference strain CO92 and single nucleotide polymorphisms (SNPs) or insertion/deletions (INDELS) identified. By wgMLST, the isolates from the human patient and prairie dog did not differ from one another (Figure 3). In contrast, the two genome sequences were distinct from 10 other non-epidemiologically linked Y. pestis isolates from Santa Fe County (Figure 3). See Table S1 for sequence data.
FIGURE 3.
wgMLST analysis of the PD5 and human isolates and non-epidemiologically linked isolates from Santa Fe County, NM. Cluster analysis of genome sequences from 12 Yersinia pestis isolates from Santa Fe County, NM. 3,617 genes (74% of genome) were compared. The tree was constructed in BioNumerics 7.5 using categorical coefficients and Unweighted Paired Group Method with Averages. Strains labelled SF_2011A or B, 2013 and 2015 were sequenced as part of this investigation. The numbering corresponds to the year they were isolated. The two NM02 strains were isolated in 2002 and genome-sequenced previously (Kingry et al., 2016). The four AGJ strains were isolated in 2009 and genome sequences previously reported (Gibbons et al., 2012)
8 | DISCUSSION
Timely and appropriate diagnosis and treatment of plague are imperative, particularly in this case as infections acquired through direct animal contact are associated with higher mortality than those caused by a flea bite (Kugeler, Staples, Hinckley, Gage, & Mead, 2015) and misdiagnosis or delays in treatment of plague can result in death (Gage et al., 2000). A rapid public health intervention was possible because of the direct line of communication that exists between VDS and SLD (both located in the same building) and the epidemiologists at the NMDOH, and because SLD does all testing for animal and human specimens, permitting a rapid monitoring of human-animal disease interactions. In the case presented, the NMDOH contacted the individual who handled PD5 as soon as suspicion of plague was reported by VDS and the NMDOH ensured that he received appropriate care. Overall, this was a good practical example of the One Health approach towards public health (Kahn, 2011; Rabinowitz et al., 2013).
The prairie dog bite was the most likely cause of the human plague infection as there was no history of a flea bite, the animals had been housed in deltamethrin-treated cages prior to the bite exposure, the axillary bubo was proximal to the bite on the right arm and the potential for the presence of plague bacilli in nasal secretions and saliva of the animal was demonstrated through histopathology (Figure 1). Additionally, the genomic sequences of the Y. pestis isolates from the human and prairie dog specimens did not differ by wgMLST (Figure 3). Previous studies have demonstrated that Y. pestis wgMLST alleles are stable between epidemiologically linked isolates and can be used for identification of the most likely source of Y. pestis infection (Danforth et al., 2016; Kingry et al., 2016).
This case resulted because of the relocation and handling of wild prairie dogs from an endemic area for plague. The City of Santa Fe ensures a prairie dog ecosystem management plan, which includes protection of colonized open spaces and the translocation of nuisance prairie dogs from city-administered land. A limitation found was that the incubation period for plague was longer than the 2-week quarantine period required by the City’s ordinances (the animals died 22 days post-trapping). Transmission of plague among the captive prairie dogs was not thought to be maintained by fleas during this time due to the use of insecticides in the holding tanks. A 2-week quarantine was thought to be sufficient based on observed incubation periods in domestic animals and humans; however, experimental infection of black-tailed prairie dogs (Cynomys ludovicianus) has resulted in mortality of animals from four to 16 and even as late as 23 days post-subcutaneous inoculation with a wild-type isolate of Y. pestis (Rocke, Kingstad-Bakke, Berlier, & Osorio, 2014; Rocke et al., 2012, 2015). The incubation period of plague in wild prairie dogs is not fully understood and a 2-week quarantine period is insufficient.
Historically, only five cases of plague have been identified in Socorro County: two feline cases in 1993 and 1994, two human cases in 1980 and one in 1986 (https://nmhealth.org/data/view/infectious/1013/). Introduction of disease by translocation of animals is common (Woodford & Rossiter, 1993) and can have catastrophic consequences on established mammals. Intensive monitoring of the prairie dogs in the Socorro County wildlife refuge did not reveal any further mortality in the weeks following the translocation event.
The situation presented highlights the necessity of communities in areas with enzootic plague and with prairie dogs near human habitation to (i) investigate large die-offs in prairie dog colonies and potentially test fleas and dead prairie dogs if resources permit; (ii) educate the public about plague and what prevention steps they can take for themselves and their family and pets to decrease risk of being infected; (iii) avoid translocation of plague-susceptible rodents from enzootic areas to minimize the risk of human exposure and introducing plague into disease-free areas; (iv) ensure that local jurisdiction staff are trained and licensed in pesticide application if it is deemed necessary to apply to decrease risk to the community; and (v) educate healthcare providers in plague-endemic areas to regard wild rodent bites, particularly from prairie dogs and other ground squirrels, as high-risk of plague.
Supplementary Material
Impacts.
Healthcare providers in plague-endemic areas should consider wild rodent bites as high risk of plague and admit patients presenting with classical plague symptoms for close observation.
Translocation of wild prairie dogs or other rodents from plague-endemic areas into areas with low or inexistent plague transmission could result in the introduction of the disease with potentially devastating consequences for the existing fauna and humans.
Incubation period for plague in prairie dogs captured from the wild is longer than previously thought. Trapped animals should be quarantined for at least 3 weeks prior to relocation.
Acknowledgments
The authors would like to thank Jon Erz with the Fish and Wildlife Services for his assistance in monitoring prairie dog activity at the wildlife refuge in Socorro County, Tonie Rocke with the United States Geological Survey for her valuable input and shared expertise in plague incubation periods for captive prairie dogs, and City of Santa Fe staff for assisting with flea flagging and insecticide treatment of prairie dog burrows at the Santa Fe Municipal Airport. Lastly, the authors would like to thank Chad Smelser, Amy Drake and Kelly Fitzpatrick-Cuoco with the New Mexico Department of Health for their valuable input in reviewing this manuscript.
Footnotes
CONFLICT OF INTEREST
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- Biggins DE, Godbey JL, Gage KL, Carter LG, Montenieri JA. Vector control improves survival of three species of prairie dogs (Cynomys) in areas considered enzootic for plague. Vector-Borne and Zoonotic Diseases. 2010;10(1):17–25. doi: 10.1089/vbz.2009.0049. [DOI] [PubMed] [Google Scholar]
- Cully JF, Jr, Barnes AM, Quan TJ, Maupin G. Dynamics of plague in a Gunnison’s prairie dog colony complex from New Mexico. Journal of Wildlife Diseases. 1997;33(4):706–719. doi: 10.7589/0090-3558-33.4.706. [DOI] [PubMed] [Google Scholar]
- Cully JF, Johnson TL, Collinge SK, Ray C. Disease limits populations: Plague and black-tailed prairie dogs. Vector-borne and Zoonotic Diseases. 2010;10(1):7–15. doi: 10.1089/vbz.2009.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth M, Novak M, Petersen J, Mead P, Kingry L, Weinburke M, Jackson B. Investigation of and response to 2 plague cases, Yosemite National Park, California, USA, 2015. Emerging Infectious Diseases. 2016;22(12):2045. doi: 10.3201/eid2212.160560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Enscore RE, Biggerstaff BJ, Reynolds PJ, Ettestad P, Brown T, Gage K. Human plague in the southwestern United States, 1957–2004: Spatial models of elevated risk of human exposure to Yersinia pestis. Journal of Medical Entomology. 2007;44(3):530–537. doi: 10.1603/0022-2585(2007)44[530:hpitsu]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gage KL, Dennis DT, Orloski KA, Ettestad P, Brown TL, Reynolds PJ, Stein JD. Cases of cat-associated human plague in the western US, 1977–1998. Clinical Infectious Diseases. 2000;30:893–900. doi: 10.1086/313804. [DOI] [PubMed] [Google Scholar]
- Gage KL, Kosoy MY. Natural history of plague: Perspectives from more than a century of research. Annual Review of Entomology. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- Gibbons HS, Krepps MD, Ouellette G, Karavis M, Onischuk L, Leonard P, Donarum G. Comparative genomics of 2009 seasonal plague (Yersinia pestis) in New Mexico. PLoS One. 2012;7(2):e31604. doi: 10.1371/journal.pone.0031604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn LH. The need for one health degree programs. Infection Ecology and Epidemiology. 2011;1:7919. doi: 10.3402/iee.v1i0.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingry LC, Rowe LA, Respicio-Kingry LB, Beard CB, Schriefer ME, Petersen JM. Whole genome multilocus sequence typing as an epidemiologic tool for Yersinia pestis. Diagnostic Microbiology and Infectious Disease. 2016;84(4):275–280. doi: 10.1016/j.diagmicrobio.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugeler KJ, Staples E, Hinckley AF, Gage KL, Mead PS. Epidemiology of human plague in the United States, 1900–2012. Emerging Infectious Diseases. 2015;21(1):16–22. doi: 10.3201/eid2101.140564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell JL, Eisen RJ, Schotthoefer AM, Xiaocheng L, Montenieri JA, Tanda D, Gage K. Colorado animal-based plague surveillance systems: Relationships between targeted animal species and prediction efficacy of areas at risk for humans. Journal of Vector Ecology. 2009;34(1):22–31. doi: 10.1111/j.1948-7134.2009.00004.x. [DOI] [PubMed] [Google Scholar]
- Rabinowitz PM, Kock R, Kachani M, Kunkel R, Thomas J, Gilbert J, Rubin C. Toward proof of concept of a one health approach to disease prediction and control. Emerging Infectious Diseases. 2013;19(12):e130265. doi: 10.3201/eid1912.130265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocke TE, Kingstad-Bakke B, Berlier W, Osorio JE. A recombinant raccoon poxvirus vaccine expressing both Yersinia pestis F1 and truncated V antigens protects animals against lethal plague. Vaccines. 2014;2:772–784. doi: 10.3390/vaccines2040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocke TE, Tripp D, Lorenzsonn F, Falendysz E, Smith S, Williamson J, Abbott R. Age at vaccination may influence response to sylvatic plague vaccine (SPV) in Gunnison’s prairie dogs (Cynomys gunnisoni) EcoHealth. 2015;12(2):278–287. doi: 10.1007/s10393-014-1002-3. [DOI] [PubMed] [Google Scholar]
- Rocke TE, Williamson J, Cobble KR, Busch JD, Antolin MF, Wagner DM. Resistance to plague among black-tailed prairie dog populations. Vector-borne and Zoonotic Diseases. 2012;12(2):111–116. doi: 10.1089/vbz.2011.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runfola JK, House J, Miller L, Colton L, Hite D, Hawley A, Douglas JM., Jr Outbreak of human pneumonic plague with dog-to-human and possible human-to-human transmission – Colorado, June–July 2014. MMWR. Morbidity and Mortality Weekly Report. 2015;64(16):429–434. [PMC free article] [PubMed] [Google Scholar]
- Woodford MH, Rossiter PB. Disease risks associated with wildlife translocation projects. Scientific and Technical Review of the Office International des Epizooties. 1993;12(1):115–135. doi: 10.20506/rst.12.1.667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.