Abstract
Purpose
To quantify normal corneal transparency by anterior segment optical coherence tomography (AS-OCT) by measuring the average pixel intensity. To analyze the variation in the average pixel intensity in mild and severe grades of corneal opacities.
Methods
This is an observational, cross-sectional study of 38 eyes from 19 patients with mild or severe grades of corneal opacities greater than 3 mm and a normal contralateral cornea. AS-OCT was performed centered on the opacity with a 3 mm cruciate protocol. A similar image is taken of the contralateral clear cornea in the same quadrant. The average pixel intensity was calculated in a standardized manner using MATLAB software.
Result
The average pixel intensity of the normal cornea was 99.6 ± 10.9 [standard deviation (SD)]. The average pixel intensity of the mild and severe corneal opacities was 115.5 ± 9.1 and 141.1 ± 10.3, respectively. The differences were statistically significant.
Conclusions
AS-OCT images can be used to quantify corneal transparency. Average pixel intensity is a measure that varies significantly with varying corneal opacification.
Keywords: Corneal transparency, Anterior segment optical coherence tomography, Mild and severe grades of corneal opacities, Pixel intensity
Introduction
Optical coherence tomography (OCT) is a tool which is commonly used to assess the retina and choroid. It is based on the principle of low coherence interferometry and is capable of taking high resolution, cross-sectional images of the retina. An anterior attachment to the OCT machine allows imaging of the anterior segment, and this is referred to as anterior segment OCT (AS-OCT).1 The transparent corneal dome can be assessed by different imaging modalities.2, 3, 4, 5 The AS-OCT creates cross-sectional images of the cornea and anterior segment. It is a standard diagnostic modality of imaging the cornea and anterior segment.6 Corneal transparency can be assessed qualitatively by slit-lamp photography, and opacities can be graded into Nebula, Macula and Leucoma depending on the density of the opacity and the clarity of the iris details seen through the opacity.7 Opacities in the cornea have been graded by qualitative scales.8 The lack of quantification greatly limits the clinical ability to notice subtle changes in corneal transparency, and can induce potential errors of documentation because of inter-observer variability. In research, qualitative scales struggle with issues of standardization and the inability to apply statistics to the data collected. Corneal transparency ex vivo has been qualitatively assessed by passing a defined beam of light through the tissue and detecting how much is transmitted without absorption or scattering. The commonly used techniques to measure corneal transparency ex vivo, quantitatively are tungsten light-based or laser-based bench-top optical systems.
One of the early attempts to measure light scatter in vivo was based on the use of an optical fiber, Scheimpflug photography, video pachometry using a slit-lamp, confocal microscopy and OCT.9 Various techniques have been used for segmentation of three important layers of the cornea in normal eyes using AS-OCT.10
We designed an innovative method of quantifying corneal transparency. The standardized AS-OCT photographs were processed by image analysis, and the average pixel intensity was calculated. In this pilot, cross-sectional study we analyzed the distribution of average pixel intensity in clinically normal corneas and compared the values in patients with mild and severe grades of corneal opacities.
Methods
The institutional review board approved the study (IRB Min. No: 9786). Informed consent was obtained from all participants. The study was undertaken from January 2016 to June 2016. A cross-sectional observational study design was used to acquire data. Consecutive patients over 18 years of age that presented to the outpatient clinic of the department of ophthalmology who fitted the inclusion criteria after a comprehensive eye examination were chosen. Consecutive patients that had mild or severe grades of corneal opacity of more than 3 mm in diameter with a clear contralateral cornea were recruited. The clinical grade and inclusion criteria were assessed by two independent observers before the subject was included in the study. Since there is no study prior to this that has used the AS-OCT as a quantification tool in humans, an arbitrary sample size of 20 was chosen to pilot the concept of the study.
A line segment (vertical and horizontal) AS-OCT (DRI OCT, Swept source, Triton plus, Topcon) scan using a 3 mm cruciate protocol was used to take an image of the cornea centered on the opacity (n = 19). A similar protocol was used to take an image of the contralateral clear cornea (n = 19) in the same corresponding quadrant.
Both the case and control images were of 1024 × 900 pixels in resolution and saved in the portable network graphics (png) format. This format saves image data with no loss of information. These images were then imported, processed, and analyzed using MATLAB software. Only the pixels in the corneal region of the image were considered for analysis, and the background pixels were excluded. This was done by using thresholding and dilation image processing techniques.11, 12
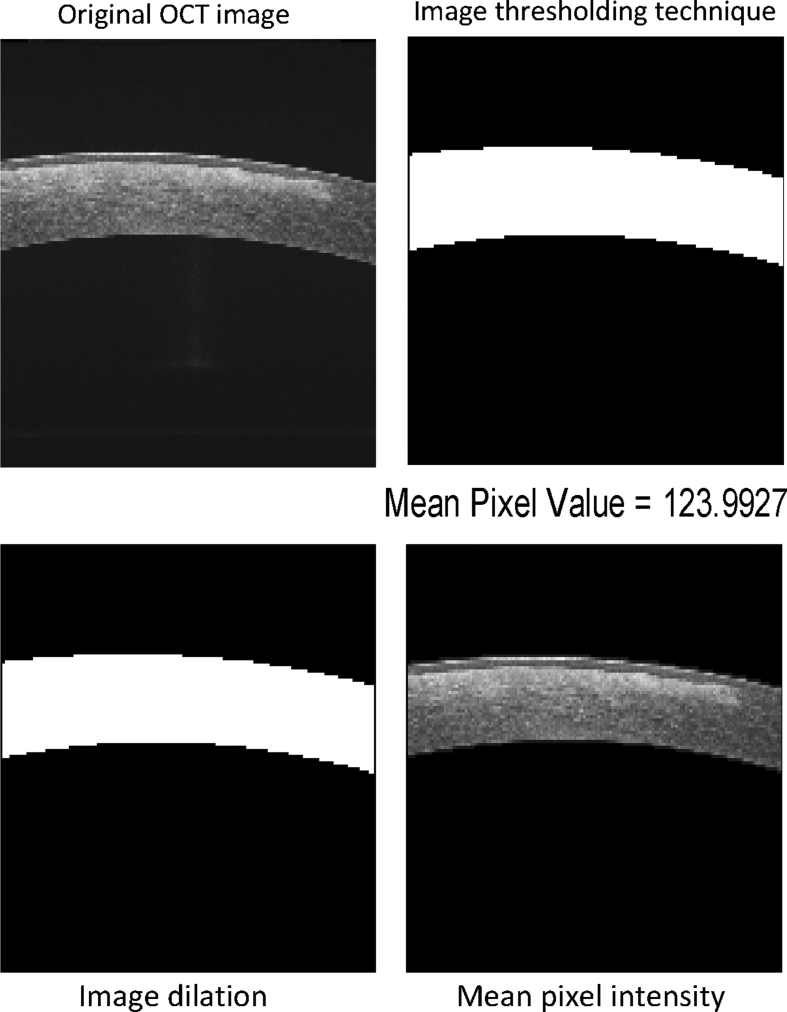
By thresholding, pixels inside the corneal boundary were made equal to 1 (white) whereas all the background pixels were made 0 (black). However, the same pixels inside the boundary may still be black. Therefore, dilation was applied to remove these black spots and produce a white corneal area. This, when multiplied to the original image, selects only the pixels inside the boundary and makes the background 0. The number of non zero pixels is now equal to the pixels in the corneal area. The process is shown in Fig. 1. The average pixel intensity for normal, mild, and severe grades of corneal opacities was calculated (Fig. 2).
Fig. 1.
Figure showing the techniques used in image analysis. OCT: Optical coherence tomography.
Fig. 2.
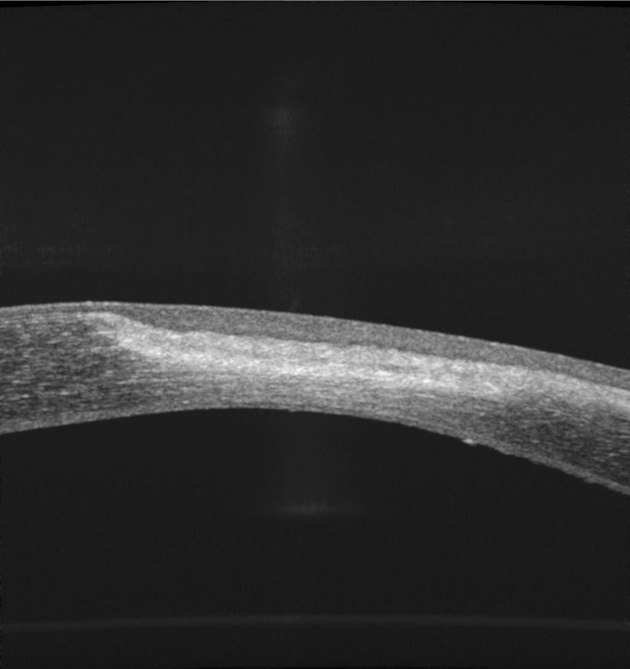
Image of severe scar.
An Excel (Microsoft Corp., Redmond, WA, USA) spreadsheet was used to collect the data, which was then transferred to statistical package for social studies (SPSS 22; IBM Corp., Armonk, NY, USA). Independent t test was performed to compare the mean and standard deviations for normal, mild, and severe grades corneal opacities.
Results
The baseline parameters of all controls showed no statistically significant difference with respect to meridian, age, or sex (Table 1). The average pixel intensity of normal corneas was 99.58 ± 21.8 [standard deviation (SD)] (Table 2). There was a statistically significant difference in average pixel intensity between control (98.2) and mild (115.4) corneas (P < 0.001) (Tables 3 and 4). The significance persisted between control (100.8) and severe (141.1) corneas (P < 0.001). There was a significant difference between mild (115.4) and severe (141.1) corneas (P < 0.001).
Table 1.
Profile of normal corneas (n = 19).
| Pixel intensity (mean ± SD) | ±SD | P value | |
|---|---|---|---|
| Meridian | |||
| Vertical | 98.4 | 12.2 | 0.4 |
| Horizontal | 100.7 | 12.6 | |
| Age | |||
| <60= | 97.6 | 10.2 | 0.2 |
| >60= | 105.0 | 12.0 | |
| Gender | |||
| Male | 101.5 | 12.2 | 0.4 |
| Female | 97.4 | 11.3 | |
SD: Standard deviation.
Table 2.
Distribution of pixel intensity of normal corneas (n = 19).
| Distribution of pixel intensity | No. of controls |
|---|---|
| 70–79 | 1 |
| 80–89 | 2 |
| 90–99 | 7 |
| 100–109 | 5 |
| 110–119 | 4 |
Table 3.
Profile of cases.
| Pixel intensity | P value | |
|---|---|---|
| Meridian – mild (n = 9) | ||
| Vertical | 122.4 | 0.007 |
| Horizontal | 108.5 | |
| Meridian – severe (n = 10) | ||
| Vertical | 145.5 | 0.180 |
| Horizontal | 136.8 | |
Table 4.
Comparison of cases and controls.
| Average pixel intensities | Controls | ±SD | Cases | ±SD | P value |
|---|---|---|---|---|---|
| Mild (n = 9) | 98.2 | 10.07 | 115.4 | 9.05 | 0.001 |
| Severe (n = 10) | 100.8 | 12.06 | 141.1 | 10.32 | 0.001 |
| Mild versus severe | 115.4 | 141.1 | 0.001 |
SD: Standard deviation.
Discussion
The study highlights an added utility of the AS-OCT as a quantification tool for corneal transparency. Normative data was derived from the normal control corneas. The distribution of normal followed a Gaussian distribution as would be expected in most biological parameters.
This can be used as a guideline for other clinical studies involving corneal opacification and as a possible clinical tool to monitor corneal scarring.
To prove further the validity of the test, we have shown how a change in the clinical grade of the opacity as assessed by clinical examination, the current gold standard, produces a statistically significant difference in average pixel intensity with worsening corneal opacification. Corneal opacification has been graded clinically by subjective methods into mild and severe scarring. These are prone to bias, subjectivism and are unamenable to statistical analysis in a research setting. The results of this analysis shows that AS-OCT with image analysis is a reliable measure to quantify corneal transparency in vivo.
The study describes the standardized procedure of image acquisition and analysis that needs to be followed for acquiring accurate information.
The study lends proof of principle evidence to estimate corneal opacification on a nominal scale. A follow-up study with a larger sample size is underway to prove this principle in a larger population including leucoma scars.
AS-OCT imaging of the cornea along with image analysis can be used to quantify corneal transparency. Average pixel intensity for the normal cornea is 99.58 ± 10.9 (SD). The average pixel intensity varied significantly with increasing clinical grades of corneal opacification (115.4 and 141.1 for mild and severe, respectively).
The limitations of the study include the small sample size, no correlation with any specific aetiological diagnosis, analysis of only two directional scans for a given image, and the lack of comparisons with any other instruments that can measure corneal transparency.
Footnotes
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Burns M.E., Levine E.S., Miller E.B. New developments in murine imaging for assessing photoreceptor degeneration in vivo. Adv Exp Med Biol. 2016;854:269–275. doi: 10.1007/978-3-319-17121-0_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner H., Barr J.T., Zasnik K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study: methods and findings to date. Corneal scarring and vision in keratoconus. Cont Lens Anterior Eye. 2007 Sep;30(4):223–232. doi: 10.1016/j.clae.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu S., Hertsenberg A.J., Funderburgh M.L. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med. 2014 Dec 10;6(266):266ra172. doi: 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandali O., El Sanharawi M., Temstet C. Fourier-domain optical coherence tomography imaging in keratoconus: a corneal structural classification. Ophthalmology. 2013 Dec;120(12):2403–2412. doi: 10.1016/j.ophtha.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 5.Coulson-Thomas V.J., Caterson B., Kao W.W. Transplantation of human umbilical mesenchymal stem cell cures the corneal defects of mucopolysaccharidosis VII mice. Stem Cells. 2013 Oct;31(10):2116–2126. doi: 10.1002/stem.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izatt J.A., Hee M.R., Swanson E.A. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994 Dec;112(12):15849. doi: 10.1001/archopht.1994.01090240090031. [DOI] [PubMed] [Google Scholar]
- 7.Parsons J.H. Diseases of the cornea. In: Sihota Ramanjit, Tandon Radhika., editors. Parson's Diseases of the Eye. 21st ed. Elsevier, A Division of Reed Elsevier India Private Limited; New Delhi: 2011. p. 190. [Google Scholar]
- 8.Dolin P.G., Faal H., Johnson G.J., Ajewole J., Mohammed A.A., Lee P.S. Trachoma in the Gambia. Br J Ophthalmol. 1998 August;82:930–933. doi: 10.1136/bjo.82.8.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meek K.M., Knupp C. Corneal structure and transparency. Prog Retin Eye Res. 2015 Nov;49:1–16. doi: 10.1016/j.preteyeres.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabbani H., Kafieh R., Jorjandi S. Obtaining thickness maps of corneal layers using optimal algorithm for intracorneal layer segmentation. Int J Biomed Imaging. 2016;2016:1420230. doi: 10.1155/2016/1420230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gayzik F.S., Hoth J.J., Stitzel J.D. Quantitative histology of contused lung tissue with comparison to computed tomography. Biomed Sci Instrum. 2008;44:225–230. [PubMed] [Google Scholar]
- 12.Rieder M.J., O'Drobinak D.M., Greene A.S. A computerized method for determination of microvascular density. Microvasc Res. 1995 Mar;49(2):180–189. doi: 10.1006/mvre.1995.1014. [DOI] [PubMed] [Google Scholar]