Abstract
Background
Neuraminidase (NA) is one of the key surface protein of the influenza virus, and has been established as a primary drug target for anti-influenza therapies. This study aimed to screen bioactive herbal extracts from some medicinal plants traditionally used in Lingnan Chinese Medicines by NA activity high-throughput screening assay.
Methods
One hundred ninety herbal extracts from 95 medicinal plants collected in Guangzhou were screened for their potential inhibitory activities against A (H1N1) influenza neuraminidase, and the most active extracts were further evaluated for their anti-influenza virus activities using virus-induced cytopathic effect (CPE).
Results
Among the tested 190 herbal extracts, 14 extracts inhibited significantly NA activity (IC50 < 40 μg/mL), and the extracts 1–5, which were obtained from Amomurn villosum Lour, Melaphis chinensis (Bell) Baker, Sanguisorba officinalis and Flos Caryophylli, showed potent inhibitory activity against NA with IC50 values ranging from 4.1 to 9.6 μg/mL. Moreover, the most bioactive extracts 1–5 were found to protect MDCK cells from A (H1N1) influenza virus infection with very low cytotoxicity to the host cells (EC50 values ranged from 1.8 to 14.1 μg/mL, CC50 values ranged from 97.0 to 779.2 μg/mL, SI values ranged from 14 to 438). In addition, quantitative RT-PCR analysis showed that the extracts 1–5 inhibited viral RNA synthesis in a dose-dependent manner.
Conclusion
We performed in vitro screening of anti-neuraminidase activities of herbal extracts from medicinal plants used in Lingnan Chinese Medicines, and the results indicate that some bioactive extracts are worth further studies to identify the bioactive components responsible for anti-influenza virus activities, to elucidate their modes of action and finally determine their clinical potentials.
Keywords: A (H1N1) influenza virus, Neuraminidase inhibitor, Anti-influenza agents, Medicinal plant, Lingnan Chinese medicines
Background
Influenza virus causes an acute contagious respiratory tract infection, which is a major contributor to morbidity and mortality among human population. Historically pandemic flu has caused widespread human deaths, most notably the 1918 “Spanish Flu” (A/H1N1) which killed 25–50 million people worldwide [1]. Novel swine-origin influenza A (H1N1 subtype) virus identified in Mexico in 2009 emerges to spread rapidly worldwide via human-human transmission [2] and led to at least 17,798 deaths in 214 countries. Therefore, pandemic influenza A viruses such as the H1N1subtype becomes a serious global public health problem, which calls for more agents of anti-influenza therapies as possible.
Neuraminidase (NA) is an antigenic glycoprotein on the surface of influenza virus, which takes charge of catalyzing the cleavage of neuraminic acid residues to facilitate the detachment from the host cell surface at the end of the viral replication cycle and suppresses their self-aggregation of the virions [3, 4]. NA plays a critical role for virus replication and spread in infected tissues during infection, and has been well established as a primary drug target for anti-influenza therapies [5, 6]. Some potent NA inhibitors, including oseltamivir, zanamivir, laninamivir and peramivir, have been designed and applied in clinical treatments [7, 8]. Unfortunately, resistance to these NA inhibitors has been extensively reported [9–11]. Therefore, there is a continuing need for developing novel NA inhibitors as anti-influenza agents. Medicinal plants may be a probable source for the discovery of natural NA inhibitors and might provide leads to develop the NA inhibitors [12].
In order to search for novel anti-influenza agents from natural resources, a library of 190 extracts of 95 medicinal plants traditionally used in Lingnan Chinese Medicines were screened for in vitro inhibitory activity against A (H1N1) influenza virus neuraminidase using high-throughput assay. The most active five extracts (1–5) were selected to further study their action upon the replication of influenza viruses using cytopathic effect (CPE) reduction assay and quantitative RT-PCR analysis. The results showed that these herbal extracts significantly inhibited the NA activity and the replication of influenza viruses, and exhibited very low cytotoxicity to the host cells.
Methods
Plant materials
Ninety nine medicinal plants traditionally used in Lingnan Chinese Medicines were collected in Guangzhou in 2009. The identity of the plants samples was verified by Dr. Guangtian Peng (Guangzhou University of Chinese Medicine). Voucher specimens of these materials were deposited for references in the Research Center of Medicinal Plants Resource Science and Engineering, Guangzhou University of Chinese Medicine. The samples were stored in the shade at room temperature and pulverized before use.
Standard extraction preparation
Dried powdered plants (100 g) were extracted with ethyl acetate (EtOAc, 250 mL × 3) and methanol (MeOH, 250 mL × 3) by ultrasound wave at 40 kHz and 400 W at 45 °C for 30 min, the filtrates were evaporated under vacuum at 45 °C to give the EtOAc and MeOH extracts, respectively. A total of 190 herbal extracts were obtained. A stock solution for each extract was prepared by dissolution to dimethyl sulfoxide (DMSO), 50 mg of each extract was suspended in 1 ml of DMSO ensuing stock concentration of 50 μg/μL. The solutions were filtered by using 0.22 μm filters, and stored at − 20 °C. The concentration of DMSO in test dilutions was restricted to no more than 0.5% (v/v) to minimize potential effects of the solvent on enzyme activity and cell growth.
Neuraminidase, virus and cells
The human influenza virus strains A/PR/8/34 (H1N1) was kindly provided by China Centers for Disease Control, and was used as the source of NA; Madin-Darby canine kidney (MDCK) and A549 cell lines were obtained from the National Center for Pharmaceutical Screening, Institute of Materia Medica, Chinese Academy of Medical Sciences. Madin-Darby canine kidney (MDCK) cells were grown in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) at 37 °C and 5% CO2 atmosphere. MDCK cells were used for virus infection, and were washed with PBS buffer before infection. 2′-(4-methylunbelliferyl)-α-D-acetyl-neuraminic acid (MUNANA), 2-(N-Morpholino)-ethanesulfonic acid (MES) and 3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma. DMEM, FBS, and 0.25% trypsin-EDTA were purchased from Gibco. Ribavirin with purity more than 98%, and zanamivir with purity more than 98% were purchased from Sigma (Lot#020 M4003) and Full Land international trade company in Shanghai of China (Lot#091209-005LY), respectively. They were used as references in NA and CPE inhibition assays.
In vitro screening of plant extracts for NA activity
Inhibition of influenza virus NA activity was determined by a standard fluorimetric method [13, 14] using4-methylumbelliferyl-α-D-N-acetyl-neuraminate (MUNANA) (Sigma) as substrate, in 96-well microplates. The reaction mixture containing the extracts or compounds, and NA enzyme in MES buffer (32.5 mM) and calcium chloride (4 mM, pH 6.5) was incubated for 60 min. After incubation, the reaction was terminated by adding NaOH (34 mM). Fluorescence intensity (M) was quantified with excitation wavelength at 360 nm and emission wavelength at 450 nm. Percentage inhibition was calculated relative to a blank reaction mixture (solvent control) containing virus NA and solvent (% Inhibition = [1-(Mextract/Mcontrol)] × 100). The 50% inhibitory concentration (IC50) was defined as the concentration of NA inhibitor necessary to reduce NA activity by 50% relative to a blank reaction mixture. IC50 values displayed represent the mean of three individual determinations each performed in triplicate assays. Zanamivir (Sigma) was used as the reference compound.
Cytotoxicity assay
The cytotoxicity of medicinal plant extracts was determined with the MTT (Sigma) method as described previously [15]. Briefly, different concentrations of the extracts and compounds were added to each well of a 96-well culture plate containing a confluent cell monolayer in triplicate, blank medium was used as the control. After incubation at 37 °C in an atmosphere of 5% CO2 for 72 h, 12 μL of MTT solution (5 mg/ml in phosphate buffered saline) was added to each well. The plate was further incubated at 37 °C for 3 h to allow formation of formazan product. After removing the medium, 100 μL of DMSO was added to dissolve the formazan crystals. After 15 min, the contents of the wells were homogenized on a microplate shaker. The optical densities (OD) were then determined by measuring absorbance with a microplate spectrophotometer at a wavelength of 540 nm and a reference wavelength of 620 nm. The median cytotoxic concentration (CC50) was calculated as the concentration of the constituent that reduced the viable cells to 50% of the untreated control. The maximal non-cytotoxic concentration (MNCC) was defined as the maximal concentration of the sample that did not exert a cytotoxic effect and resulted in more than 90% viable cells.
CPE reduction assay
The anti-viral activity of the extracts was measured by a virus-induced cytopathic effect (CPE) reduction assay as described previously [14, 16]. Briefly, 100 μL of virus suspension of 200 tissue culture infective dose (TCID50/mL) was added to each well of a 96-well culture plate containing confluent a MDCK cells monolayer. After incubation at 37 °C for 2 h, the virus solution was removed, and 100 μL of serial dilutions of the extracts and ribavirin were added to each well of the 96-well culture plates, using the maximal non-cytotoxic concentration (MNCC) as the highest concentration. The plates were incubated at 37 °C in a humidified 5% CO2 atmosphere for 48 h, and then the CPE was assessed. The virus-induced CPE was scored as follows: 0 = no CPE, 1 = 0–25% CPE, 2 = 25%–50% CPE, 3 = 50%–75% CPE, and 4 = 75%–100% CPE. Apart from test group, there were control group (treated with FBS-free medium instead of extracts and virus) and model group (treated with FBS-free medium and virus instead of extracts and virus). The CPE inhibition ratios were calculated using the equation: CPE inhibition % = 100 -[(ODtest-ODcontrol) *100/ (ODmodel- ODcontrol)]. The ODtest, ODmodel, and ODcontrol mean the optical density of test group, model group, and control group, respectively. At least three independent experiments with three parallel experiments were performed to determine the mean and SD value.
Measurement of viral RNA synthesis by quantitative and reverse transcription PCR (qPCR)
A549 cells were grown in RPMI1640 to about 90% confluence and were infected with influenza virus A/PR/8/34 (H1N1) influenza virus at 100 TCID50, followed by administration of test extracts for 5 h. To determine the expression level of hemagglutinin (HA) gene mRNA of influenza virus, cells were harvested and the total RNA was extracted by TRIzol (Invitrogen) according to the manufacture’s instruction. The primer sequences which were designed by Primer-BLAST from NCBI for quantitative real-time PCR of influenza virus were 5’-CCTGCTCGAAGACAGCCACAACG-3′ (sense) and 5’-TTCCCAAGAGCCATCCGGCGA-3′ (antisense). The GAPDH were used as internal control of cellular RNAs, with primer sequence of 5′- TGCTCCGAAGGGTGGCCCTTA-3′(sense) and 5′- TGCGTGTTTCCAGAGCCGTGC-3′(antisense). The total RNA was reverse transcribed into cDNA using the TransScript First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China). The cDNA was used as template for real-time PCR conducted by SsoFast EvaGreen PCR 2 × master mix (Bio-Rad) using CFX 96 Realtime PCR system (Bio Rad location) according to the manufacture’s protocol. The data was analyzed using the mode for normalised expression (2-ΔΔCq).
Statistical analysis
Statistical analysis was performed using the Student’s unpaired t-test. The results were presented as mean ± S.D. (n = 3). *p < 0.05 and **p < 0.001 indicate a statistically significant difference as compared to the untreated control.
Results
NA has been validated as one of the most important targets to screen the drugs of anti-influenza virus. We first examined the ability of 190 organic extracts from 95 medicinal plants to inhibit NA activity by in vitro screening assay. Zanamivir was used as a positive control, its IC50 value to NA inhibition was 0.05 μg/mL. 14 extracts were found to effectively inhibit the NA activity at the concentration of 40 μg/mL. Among them, 5 extracts exhibited potent inhibition of NA activity, 9 extracts exhibited moderate NA inhibitory activity with IC50 values ranged from 4.1 to 37.3 μg/mL. The bioactive extracts and their NA inhibition activity were summarized in Table 1. The highest activity was demonstrated by MeOH extracts of Melaphis chinensis (1) and Amomurn villosum Lour (2) with IC50 = 4.1 and 4.9 μg/mL, respectively. Significant activity with IC50 = 5.0–10 μg/mL was also shown by MeOH extract of Sanguisorba officinalis (3), EtOAc extract of Melaphis chinensis (4) and MeOH extract of Flos Caryophylli (5). While other plant extracts (6–14) showed a moderate inhibitory activity on NA with the IC50 values ranging from 20.3 to 37.3 μg/mL. These results demonstrated that these plant extracts possessed significant inhibitory activities against influenza virus NA and the most active extracts 1–5 were then selected to further study their effects on the replication of influenza virus.
Table 1.
Inhibitory activities of Chinese herbs extract on A(H1N1) influenza virus neuraminidase
| No. | Positive control and Botanical name | Botanical part | Extract | Inhibition (%)a | IC50b | Voucher No. |
|---|---|---|---|---|---|---|
| – | Zanamivir | – | – | 99.8 | 0.05 | – |
| 1 | Melaphis chinensis (Bell)Baker | cecidium | MeOH | 103.6 | 4.1 | MCB091101 |
| 2 | Amomurn villosum Lour. | fruit | MeOH | 92.2 | 4.9 | CG20080829 |
| 3 | Sanguisorba officinalis L. | root | MeOH | 100.8 | 5.1 | SOL091101 |
| 4 | Melaphis chinensis (Bell)Baker | cecidium | EtOAc | 99.3 | 5.3 | MCB091101 |
| 5 | Flos Caryophylli | flowers | MeOH | 94.1 | 9.1 | SA091101 |
| 6 | Areca catechu Linn | fruit | MeOH | 85.1 | 19.3 | ACL091101 |
| 7 | Artemisia capillaries Thunb | whole plant | MeOH | 91.3 | 19.4 | ACT091101 |
| 8 | Terminalia chebula Retz | fruit | EtOAc | 78.4 | 20.3 | TCR091101 |
| 9 | Duchesnea indica (Andr.) Focke | whole plant | EtOAc | 69.1 | 23.3 | DIF091101 |
| 10 | Terminalia chebula Retz. | fruit | MeOH | 68 | 24.3 | TCR091101 |
| 10 | Murraya exotica L. | stem and leaves | MeOH | 65.7 | 28.9 | MEL091101 |
| 11 | Geranium carolinianum L. | whole plant | MeOH | 64.8 | 28.9 | GCL091101 |
| 12 | Polygonum cuspidatum | rhizome | EtOAc | 63.9 | 29.8 | PC091101 |
| 13 | Saposhnikovia divaricata (Turez.) Schischk. | root | EtOAc | 53.1 | 37.3 | SDS091101 |
| 14 | Callicarpa formosana Rolfe | fruit | MeOH | 47.9 | NTd | CFR091103 |
| 15 | Gardenia jasminoides Ellis | fruit | MeOH | 46.6 | NT | GJE091101 |
| 16 | Duchesnea indica (Andr.) Focke | whole plant | EtOAc | 46.1 | NT | DIF091101 |
| 17 | Rosa laevigata Michx. | stem and leaves | EtOAc | 45.8 | NT | RLM091103 |
| 18 | Euphorbia humifusa Willd. ex Schlecht. | whole plant | MeOH | 43.9 | NT | EHW091101 |
| 19 | Litchi chinensis Sonn. | seed | EtOAc | 43.9 | NT | LCS091101 |
| 20 | Punica granatum L. | fruit peel | MeOH | 43.4 | NT | PGL091101 |
| 21 | Scutellaria baicalensis Georgi | root | EtOAc | 41.3 | NT | SBG091101 |
| 22 | Amomum villosum Lour. | fruit | EtOAc | 40.5 | NT | CG20080829 |
| 23 | Geranium carolinianum L. | whole plant | EtOAc | 40.1 | NT | GCL091101 |
| 24 | Isatis indigotica Fort | stem and leaves | EtOAc | 40.1 | NT | IIF091103 |
| 25 | Onosma gmelinii Ledeb | root | EtOAc | 40 | NT | OGL091101 |
| 26 | Houttuynia cordata Thunb | whole plant | EtOAc | 38.5 | NT | HCT091101 |
| 27 | Altingia chinensis (Champ.) Oliver ex Hance | stem and leaves | EtOAc | 37.3 | NT | ACO091103 |
| 28 | Pogostemon cablin (Blanco) Bent. | whole plant | EtOAc | 36.7 | NT | PCB091101 |
| 29 | Polygonum cuspidatum | rhizome | MeOH | 36.1 | NT | PC091101 |
| 30 | Punica granatum L. | fruit peel | EtOAc | 35.5 | NT | PGL091101 |
| 31 | Rosa laevigata Michx. | stem and leaves | MeOH | 34.4 | NT | RLM091103 |
| 32 | Dianella ensifolia (Linn.) Redouté | fruit | EtOAc | 31.5 | NT | DER091103 |
| 33 | Elsholtzia ciliata (Thunb.) Hyland. | whole plant | MeOH | 31.3 | NT | ECH091101 |
| 34 | Atractylodes Lancea (Thunb) DC. | root | EtOAc | 30.4 | NT | ALD091101 |
| 35 | Cynanchum otophyllum Schneid. | root | EtOAc | 29.3 | NT | COS091101 |
| 36 | Homalocladium platycladum (F. Muell.) Bailey | whole plant | MeOH | 29.1 | NT | HPB091101 |
| 37 | Cinnamomum cassia Presl | branch | MeOH | 28.9 | NT | CCP091101 |
| 38 | Elsholtzia ciliata (Thunb.) Hyland. | whole plant | EtOAc | 28.1 | NT | ECP091101 |
| 39 | Sarcandra glabra (Thunb.) Nakai | stem and leaves | EtOAc | 26.8 | NT | SGN091103 |
| 40 | Altingia chinensis (Champ.) Oliver ex Hance | stem and leaves | MeOH | 25.8 | NT | ACO091103 |
| 41 | Litchi chinensis Sonn. | seed | MeOH | 25.5 | NT | LCS091101 |
| 42 | Phellodendron chinense Schneid | bark | EtOAC | 25.4 | NT | PCS091101 |
| 43 | Euphorbia humifusa Willd. ex Schlecht. | whole plant | EtOAc | 23.6 | NT | EHW091101 |
| 44 | Glycyrrhiza uralensis Fisch. | rhizome | EtOAc | 23.1 | NT | GUF091101 |
| 45 | Woodwardia japonica (L. f.) Sm. | rhizome | MeOH | 23 | NT | WJS091101 |
| 46 | Ardisia japonica (Thunb) Blume | whole plant | MeOH | 22.7 | NT | AJB091101 |
| 47 | Cinnamomum cassia Presl | branch | EtOAc | 22.7 | NT | CCP091101 |
| 48 | Equisetum hyemale L. | whole plant | EtOAc | 22.1 | NT | EHL091101 |
| 49 | Fraxinus rhynchophylla Hance | bark | EtOAc | 22.1 | NT | FRH091101 |
| 50 | Ardisia japonica (Thunb.) Blume | whole plant | EtOAc | 21.7 | NT | AJB091101 |
| 51 | Andrographis paniculata (Burm. f.) Nees | whole plant | EtOAc | 20.8 | NT | APN091101 |
| 52 | Punica granatum Linn. | stem | EtOAc | 20.2 | NT | AGL091103 |
| 53 | Syzygium aromaticum | flowers | EtOAc | 19.5 | NT | SA091101 |
| 54 | Artemisia capillaris Thunb. | whole plant | EtOAc | 19.2 | NT | ACT091101 |
| 55 | Nepeta cataria L. | whole plant | MeOH | 18.9 | NT | NCL091101 |
| 56 | Lonicera japonica Thunb. | flowers | MeOH | 18 | NT | AJT091101 |
| 57 | Woodwardia japonica (L. f.) Sm. | rhizome | EtOAc | 17.9 | NT | WJS091101 |
| 58 | Nepeta cataria L. | whole plant | EtOAc | 17.4 | NT | NCL091101 |
| 59 | Dendranthema indicum (L.) Des Moul. | flowers | EtOAc | 16.5 | NT | DID091101 |
| 60 | Senecio scandens Buch. -Ham. ex D. Don | whole plant | MeOH | 16.3 | NT | SSB091101 |
| 61 | Onosma gmelinii Ledeb | root | MeOH | 15.9 | NT | OGL091101 |
| 62 | Evodia rutaecarpa (Juss.) Benth. | fruit | MeOH | 15.5 | NT | ERB091101 |
| 63 | Ligusticum chuanxiong Hort. | root | MeOH | 15.5 | NT | LCH091101 |
| 64 | Atractylodes Lancea (Thunb.) DC. | root | MeOH | 15.2 | NT | ALD091101 |
| 65 | Punica granatum L. | leaves | MeOH | 15 | NT | PGL091101 |
| 66 | Artemisia indices Willd. | leaves | MeOH | 14.8 | NT | AIW091101 |
| 67 | Serissa japonica (Thunb.) Thunb. | stem and leaves | EtOAc | 14.8 | NT | SJT091101 |
| 68 | Prunella vulgaris L. | whole plant | MeOH | 14.1 | NT | PVL091101 |
| 69 | Dicliptera chinensis (L.) Juss. | whole plant | MeOH | 14 | NT | DCJ091101 |
| 70 | Glycyrrhiza uralensis Fisch. | rhizome | MeOH | 13.7 | NT | GUF091101 |
| 71 | Platycladus orientalis (L.) Franco | leaves | EtOAc | 13.4 | NT | POF091101 |
| 72 | Angelica dahurica (Fisch. ex Hoffm.) Benth. | root | MeOH | 13.3 | NT | ADB091101 |
| 73 | Sarcandra glabra (Thunb.) Nakai | stem and leaves | MeOH | 13.3 | NT | SGN091101 |
| 74 | Cynanchum otophyllum Schneid. | root | MeOH | 13 | NT | COS091101 |
| 75 | Clerodendrum fortunatum Linn. | stem and leaves | EtOAc | 12.5 | NT | CFL091101 |
| 76 | Scutellaria baicalensis Georgi | root | MeOH | 12.2 | NT | SBG091101 |
| 77 | Sophora flavescens Alt. | root | MeOH | 11.6 | NT | SFA091101 |
| 78 | Paris verticillata M.Bieb. | rhizome | EtOAc | 11.4 | NT | PVM091101 |
| 79 | Semiaquilegia adoxoides (DC.) Makino | whole plant | EtOAc | 11.4 | NT | SAM091101 |
| 80 | Magnolia liliflora Desr. | flowers | EtOAc | 11.3 | NT | MLD091101 |
| 81 | Albizia julibrissin Durazz. | flowers | MeOH | NAc | NT | AJD091101 |
| 82 | Albizia julibrissin Durazz. | flowers | EtOAc | NA | NT | AJD091101 |
| 83 | Andrographis paniculata (Burm. f.) Nees | whole plant | MeOH | NA | NT | APN091101 |
| 84 | Angelica dahurica (Fisch. ex Hoffm.) Benth. | root | EtOAc | NA | NT | ADB091101 |
| 85 | Arctium lappa L. | seed | MeOH | NA | NT | ALL091101 |
| 86 | Arctium lappa L. | seed | EtOAc | NA | NT | ALL091101 |
| 87 | Areca catechu Linn | fruit | EtOAc | NA | NT | ACL091101 |
| 88 | Artemisia argyi Levl. et Van. | leaves | MeOH | NA | NT | AAL091101 |
| 89 | Artemisia argyi Levl. et Van. | leaves | EtOAc | NA | NT | AAL091101 |
| 90 | Artemisia carvifolia Buch. -Ham. ex Roxb. | whole plant | EtOAc | NA | NT | ACB091101 |
| 91 | Artemisia carvifolia Buch. -Ham. ex Roxb. | whole plant | MeOH | NA | NT | ACB091101 |
| 92 | Artemisia indices Willd. | leaves | EtOAc | NA | NT | AIW091103 |
| 93 | Bidens pilosa Linn. | whole plant | EtOAc | NA | NT | BPL091103 |
| 94 | Bidens pilosa Linn. | whole plant | MeOH | NA | NT | BPL091103 |
| 95 | Bupleurum tenue Buch-Ham. ex D. Don | root | EtOAc | NA | NT | BTB091101 |
| 96 | Bupleurum tenue Buch-Ham. ex D. Don | root | MeOH | NA | NT | BTB091101 |
| 97 | Callicarpa formosana Rolfe | fruit | EtOAc | NA | NT | CFR091103 |
| 98 | Clerodendrum fortunatum Linn. | stem and leaves | MeOH | NA | NT | CFL091103 |
| 99 | Clinopodium megalanthum | seed | EtOAc | NA | NT | CMC091101 |
| 100 | Clinopodium megalanthum | seed | MeOH | NA | NT | CMC091101 |
| 101 | Crataegus pinnatifida Bge. | fruit | MeOH | NA | NT | CPB091101 |
| 102 | Crataegus pinnatifida Bge. | fruit | EtOAc | NA | NT | CPB091101 |
| 103 | Dendranthema indicum (L.) Des Moul. | flowers | MeOH | NA | NT | DID091101 |
| 104 | Dendranthema morifolium (Ramat.) Tzvel. | flowers | EtOAc | NA | NT | DMT091101 |
| 105 | Dendranthema morifolium (Ramat.) Tzvel. | flowers | MeOH | NA | NT | DMT091101 |
| 106 | Dianella ensifolia (Linn.) Redouté | fruit | MeOH | NA | NT | DER091103 |
| 107 | Dicliptera chinensis (L.) Juss. | whole plant | EtOAc | NA | NT | DCJ091103 |
| 108 | Duchesnea indica (Andr.) Focke | whole plant | MeOH | NA | NT | DIF091103 |
| 109 | Epaltes australis Less. | whole plant | EtOAc | NA | NT | EAL091101 |
| 110 | Epaltes australis Less. | whole plant | MeOH | NA | NT | EAL091101 |
| 111 | Equisetum hyemale L. | whole plant | MeOH | NA | NT | EHL091101 |
| 112 | Euchresta japonica Hook. f. ex Regel | root | EtOAc | NA | NT | EJH091101 |
| 113 | Euchresta japonica Hook. f. ex Regel | root | MeOH | NA | NT | EJH091101 |
| 114 | Eupatorium catarium Veldkamp | whole plant | MeOH | NA | NT | ECV091103 |
| 115 | Eupatorium catarium Veldkamp | whole plant | EtOAc | NA | NT | ECV091103 |
| 116 | Eupatorium fortunei Turcz. | whole plant | EtOAc | NA | NT | EFT091101 |
| 117 | Eupatorium fortunei Turcz. | whole plant | MeOH | NA | NT | EFT091101 |
| 118 | Eupolyphaga seu Steleophaga | insect | EtOAc | NA | NT | ESS091101 |
| 119 | Eupolyphaga seu Steleophaga | insect | MeOH | NA | NT | ESS091101 |
| 120 | Evodia rutaecarpa (Juss.) Benth. | fruit | EtOAc | NA | NT | ERB091101 |
| 121 | Ficus hirta Vahl | leaves | MeOH | NA | NT | FHV091101 |
| 122 | Ficus hirta Vahl | leaves | EtOAc | NA | NT | FHV091101 |
| 123 | Forsythia suspensa (Thunb.) Vahl | fruit | MeOH | NA | NT | FSV091101 |
| 124 | Forsythia suspensa (Thunb.) Vahl | fruit | EtOAc | NA | NT | FSV091101 |
| 125 | Fraxinus rhynchophylla Hance | bark | MeOH | NA | NT | FRH091101 |
| 126 | Gardenia jasminoides Ellis | fruit | EtOAc | NA | NT | GJE091101 |
| 127 | Homalocladium platycladum (F. Muell.) Bailey | whole plant | EtOAc | NA | NT | HPB091103 |
| 128 | Homalomena occulta (Lour.) Schot | rhizome | MeOH | NA | NT | HOS091101 |
| 129 | Homalomena occulta (Lour.) Schot | rhizome | EtOAc | NA | NT | HOS091101 |
| 130 | Houttuynia cordata Thunb | whole plant | MeOH | NA | NT | HCT091101 |
| 131 | Ilex cornuta Lindl | stem | MeOH | NA | NT | ICL091103 |
| 132 | Ilex cornuta Lindl | stem | EtOAc | NA | NT | ICL091103 |
| 133 | Inula japonica Thunb. | flowers | MeOH | NA | NT | IJT091101 |
| 134 | Inula japonica Thunb. | flowers | EtOAc | NA | NT | IJT091101 |
| 135 | Isatis indigotica Fort | stem and leaves | MeOH | NA | NT | IIF091103 |
| 136 | Ligusticum chuanxiong Hort. | root | EtOAc | NA | NT | LCH091101 |
| 137 | Lobelia chinensis Lour. | whole plant | MeOH | NA | NT | LCH091101 |
| 138 | Lobelia chinensis Lour. | whole plant | EtOAc | NA | NT | LCL091101 |
| 139 | Lonicera confusa (Sweet) DC. | stem and leaves | MeOH | NA | NT | LCD091103 |
| 140 | Lonicera confusa (Sweet) DC. | stem and leaves | EtOAc | NA | NT | LCD091103 |
| 141 | Lonicera japonica Thunb. | flowers | EtOAc | NA | NT | LJT091101 |
| 142 | Lonicera japonica Thunb. | stem and branch | MeOH | NA | NT | LJT091101 |
| 143 | Lonicera japonica Thunb. | stem and branch | EtOAc | NA | NT | LJT091101 |
| 144 | Lycium chinense Mill. | root bark | MeOH | NA | NT | LCM091101 |
| 145 | Lycium chinense Mill. | Root bark | EtOAc | NA | NT | LCM091101 |
| 146 | Magnolia liliflora Desr. | flowers | MeOH | NA | NT | MLD091101 |
| 147 | Melia azedarach L. | bark | EtOAc | NA | NT | MAL091103 |
| 148 | Melia azedarach L. | bark | MeOH | NA | NT | MAL091103 |
| 149 | Murraya exotica L. | stem and leaves | EtOAc | NA | NT | MEL091103 |
| 150 | Mussaenda pubescens Ait. f. | stem and leaves | EtOAc | NA | NT | MPA091103 |
| 151 | Mussaenda pubescens Ait. f. | stem and leaves | MeOH | NA | NT | MPA091103 |
| 152 | Paris verticillata M.Bieb. | rhizome | MeOH | NA | NT | PVM091101 |
| 153 | Perilla frutescens (L.) Britt. | flowers | EtOAc | NA | NT | PFB091103 |
| 154 | Perilla frutescens (L.) Britt. | flowers | MeOH | NA | NT | PFB091103 |
| 155 | Peucedanum praeruptorum Dunn | root | EtOAc | NA | NT | PPD091101 |
| 156 | Peucedanum praeruptorum Dunn | root | MeOH | NA | NT | PPD091101 |
| 157 | Phellodendron chinense Schneid | bark | MeOH | NA | NT | PCS091101 |
| 158 | Phytolacca acinosa Roxb. | root | EtOAc | NA | NT | PAR091101 |
| 159 | Phytolacca acinosa Roxb. | root | MeOH | NA | NT | PAR091101 |
| 160 | Pinellia ternata (Thunb.) Breit. | stem | MeOH | NA | NT | PTB091101 |
| 161 | Pinellia ternata (Thunb.) Breit. | stem | EtOAc | NA | NT | PTB091101 |
| 162 | Platycladus orientalis (L.) Franco | leaves | MeOH | NA | NT | POF091101 |
| 163 | Pogostemon cablin (Blanco) Bent. | whole plant | MeOH | NA | NT | PCB091101 |
| 164 | Prunella vulgaris L. | whole plant | EtOAc | NA | NT | PVL091101 |
| 165 | Punica granatum L. | leaves | EtOAc | NA | NT | PGL091103 |
| 166 | Punica granatum Linn. | stem | MeOH | NA | NT | PGL091103 |
| 167 | Sanguisorba officinalis L. | root | EtOAc | NA | NT | SOL091101 |
| 168 | Saposhnikovia divaricata (Trucz.) Schischk. | root | MeOH | NA | NT | SDS091101 |
| 169 | Scaphium wallichii Shott & Endl. | seed | MeOH | NA | NT | SWS091101 |
| 170 | Scaphium wallichii Shott & Endl. | seed | EtOAc | NA | NT | SWS091101 |
| 171 | Semiaquilegia adoxoides (DC.) Makino | whole plant | MeOH | NA | NT | SAM091101 |
| 172 | Senecio scandens Buch-Ham. ex D. Don | whole plant | EtOAc | NA | NT | SSB091101 |
| 173 | Serissa japonica (Thunb.) Thunb. | stem and leaves | MeOH | NA | NT | SJT091103 |
| 174 | Sophora flavescens Alt. | root | EtOAc | NA | NT | SFA091101 |
| 175 | Stemona japonica (Bl.) Miq. | root | MeOH | NA | NT | SJM091101 |
| 176 | Stemona japonica (Bl.) Miq. | root | EtOAc | NA | NT | SJM091101 |
| 177 | Strobilanthes cusia (Ness) W. Ktze. | stem and leaves | MeOH | NA | NT | SCW091101 |
| 178 | Strobilanthes cusia (Ness) W. Ktze. | stem and leaves | EtOAc | NA | NT | SCW091101 |
| 179 | Thlaspi arvense L. | whole plant | MeOH | NA | NT | TAL091103 |
| 180 | Thlaspi arvense L. | whole plant | EtOAc | NA | NT | TAL091103 |
| 181 | Turczaninovia fastigiata (Fisch.) DC. | flowers | MeOH | NA | NT | TFD091101 |
| 182 | Turczaninovia fastigiata (Fisch.) DC. | flowers | EtOAc | NA | NT | TFD091101 |
| 183 | Vitex trifolia L. | stem and leaves | EtOAc | NA | NT | VTL091103 |
| 184 | Vitex trifolia L. | stem and leaves | MeOH | NA | NT | VTL091103 |
| 185 | Wikstroemia indica (Linn.) C. A. Mey. | whole plant | MeOH | NA | NT | WIC091103 |
| 186 | Wikstroemia indica (Linn.) C. A. Mey. | whole plant | EtOAc | NA | NT | WIC091103 |
| 187 | Xanthium sibiricum Patrin ex Widder | fruit | EtOAc | NA | NT | XSP091103 |
| 188 | Xanthium sibiricum Patrin ex Widder | fruit | MeOH | NA | NT | XSP091103 |
| 189 | Zanthoxylum nitidum (Roxb.) DC. | root | MeOH | NA | NT | ZND091101 |
| 190 | Zanthoxylum nitidum (Roxb.) DC. | root | EtOAc | NA | NT | ZND091101 |
aPercentage inhibition was calculated relative to a blank group containing virus NA but no inhibitors, final concentration at 40 μg/mL; bIC50 values represent the concentration that caused 50% NA enzyme activity loss, the average of at least three independent assays, IC50 values are in μg/mL. c: not active; d: not test
To validate whether these extracts 1–5 that exhibited NA inhibitory activity could protect host cells from influenza virus A (H1N1) infections, the CPE reduction assay was carried out in MDCK cells. The human influenza virus A/PR/8/34 (H1N1) strain was used to infect MDCK cells. Cells were incubated in the presence or absence of the extracts 1–5, after 48 h of incubation, their CPE reduction activity on virus multiplication was then examined. As shown in Table 2, the extracts 1–5 could protect MDCK cells from the infection of influenza virus A (H1N1), exhibited a drastic reduction of influenza virus-induced CPE. The EC50 values of the extracts 1–5 ranged from 1.8 to 14.1 μg/mL, similar to the results obtained in NA assays. Among the five extracts, the MeOH extract (2) from the fruits of Amomurn villosum had excellent CPE activity with very low EC50 values of 1.8 μg/mL, this is comparable to that of the positive compound ribavirin (3.2 μg/mL). The viability of MDCK cells incubated in the presence or absence of the extracts was evaluated by MTT assay, the CC50 values of the extracts 1–5 was found to be from 97.0 to 779.2 μg/mL, suggesting that the extracts protected significantly host cells from influenza virus infection and did not exhibit considerable cytotoxicity against MDCK cells. The maximal non-cytotoxic concentration (MNCC) of the extracts 1–5 were found to be from 30 to 300 μg/mL in MDCK cells. Their therapeutic selective index (SI) in MDCK cells ranged from 14 to 438, and among of them, the SI value of A. villosum was highest on basis of its low cytotoxicity and its high CPE effect. These data demonstrated that the extracts 1–5 protected MDCK host cells from viral damage with very low toxicity. Thus, in agreement with that these extracts inhibited NA activities, the extracts 1–5 reduced host cell damage caused by the influenza virus A (H1N1) infection.
Table 2.
Inhibitory activity of Chinese herbs extracts (1–5) on A(H1N1) influenza virus by CPE assay
| Sample No. | EC50a | CC50b | MNCCc | SId |
|---|---|---|---|---|
| 1 | 7.7 | 184.3 | 30 | 24 |
| 2 | 1.8 | 779.2 | 300 | 438 |
| 3 | 8.1 | 478.4 | 100 | 59 |
| 4 | 7.2 | 97.0 | 30 | 14 |
| 5 | 14.1 | 744.3 | 300 | 53 |
| Ribavirin | 3.2 | > 100 | —e | > 31 |
| Zanamivir | > 90.4 | > 1506.0 | > 301.2 | 17 |
aEC50: Effective concentration required to protect 50% of cells; b CC50: Median (50%) cytotoxic concentration in MDCK cells; c MNCC: Maximal non-cytotoxic concentration in MDCK cells, values in μg/mL; d SI:Selectivity index, CC50/EC50.e: not test
To further examine whether the protective effect of the extracts1–5 is related with the inhibition of influenza viral replication, total RNA was extracted and subjected to quantitative reverse-transcription PCR in the A/H1N1 virus-infected A549 cells. Our results showed that treatment with the extracts 1–5 for 5 h resulted in a substantial reduction in viral RNA expression level in a dose-dependent manner (Fig. 1). All extracts 1–5 at the high concentration (30 μg/mL) had significant inhibitory effects on viral RNA expression as compared with untreated control, even more powerful than ribavirin (Fig. 1). The extracts 2–5 at medium concentration (10 μg/mL) also demonstrated significant inhibitory effects on viral RNA synthesis. Interestingly, the extracts 3 and 4 at low concentration of 3 μg/mL still significantly inhibited RNA synthesis of influenza viruses. These data indicate that the extracts 1–5 could inhibit significantly the replication of influenza viruses in cultures by RT-PCR analysis, which validated their anti-influenza viral activity obtained by CPE reduction assay.
Fig. 1.
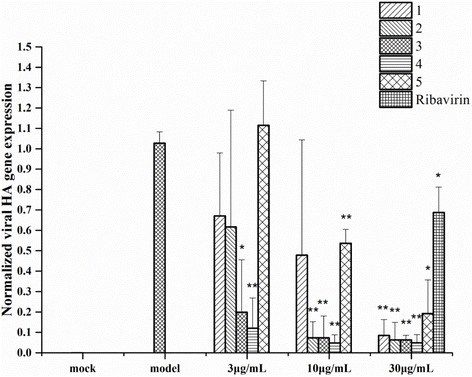
Dose-dependent inhibitory effect of the extracts 1–5 on viral RNA synthesis. A549 cells were infected with 100 TCID50 influenza H1N1 viruses and treated with different concentrations of the extracts 1–5 (3, 10 or 30 μg/mL) and the DMSO (0.03%) for 5 h. The total RNA was extracted and followed by qPCR analysis. To quantify the changes in gene expression, the 2-ΔΔC(q) method was used to calculate relative changes which were normalized to the GAPDH gene and the untreated control (model group, which was set to 1). Value calculated as Mean ± SD of three independent tests, with * p < 0.05 and **p < 0.001, respectively
Discussion
In the course of our screening of NA inhibitors for influenza virus A (H1N1), a total of 190 extracts of 95 medicinal plants traditionally used in Lingnan Chinese Medicines were submitted to in vitro screening for their NA inhibitory activities. Among of them, the organic extracts 1–5, obtained from Melaphis chinensis, Amomurn villosum, Sanguisorba officinalis and Flos Caryophylli, were found to significantly inhibit the NA activity (IC50 < 10 μg/mL, Table 1) and the replication of influenza virus in a dose-dependent manner (Fig. 1), and exhibited very low cytotoxicity to the host cells with the high selective index (SI) values ranging 14 to 438 (Table 2). Therefore, these Chinese herb extracts might contain bioactive components responsible for anti-influenza virus activity at non-toxic concentration and they could be a promising source of natural NA inhibitors.
It was demonstrated previously that the aqueous extracts of barks, leaves and galls of Melaphis chinensis have anti-influenza virus activity and some compounds such as gallotannins isolated from M. chinensis are responsible for the anti-influenza virus effect [17]. The presence of such compounds in our EtOAc and MeOH extracts of galls of M. chinensis may explain the biological activities seen in our screenings.
Flos Caryophylli also known as cloves, is considered acrid, warm and aromatic in Traditional Chinese Medicines for the treatment of stomachache, diarrhea and dental pain [18]. It was reported that the hot water extract of Flos Caryophylli have been shown to have anti-herpes virus, anti-hepatitis C virus and anti-cytomegalovirus activities in vitro and in vivo, and compounds such as ellagitannin and eugeniin were identified as the bioactive components with anti-virus properties [19]. In the present study, the MeOH extract of Flos Caryophylli showed IC50 value of 9.1 μg/mL towards NA and EC50 value of 14.1 μg/mL against influenza virus. In our latest phytochemical study on the MeOH extract of Flos Caryophylli [14], a bioassay-guided isolation led to identification of ten flavonoids, seven tannins and two chromones as NA inhibitors with IC50 values ranging from 8.4 to 94.1 μM. These polyphenolic constituents were found to protect MDCK cells from A(H1N1) influenza infections (EC50 = 1.5–84.7 μM) with very low cytotoxicity to the host cells (CC50 = 374.3–1266.9 μM)), with selective index (SI) ranging from 7 to 297 [14].
The roots of S. officinalis (Rosaceae) are well-known Chinese herbs officially listed in the Chinese Pharmacopeia and have been used for the treatment of bleeding, diarrhea and burns. Early chemical studies showed that S. officinalis synthesize a variety of secondary metabolites, particularly polyphenols, triterpenoids, saponins and flavonoids with specific biological activities such as anti-asthmatic, anti-bacterial, anti-cancer and anti-inflammation [20–25]. A variety of flavonoids, saponins and polyphenols isolated from medicinal plant have been studied extensively and exhibited anti-influenza activities [12]. The MeOH extract of S. officinalis showed strong activities towards NA (IC50: 5.1 μg/mL) and against influenza virus (EC50: 8.1 μg/mL). The anti-influenza activity may be due to the presence of flavonoids and polyphenols in the MeOH fraction.
The fruits of A. villosum (Zingiberaceae) were consumed widely as popular cooking spices in East Asian countries and have been traditionally used as a medicine to treat various digestive disorders [26]. The volatile oils of the fruits of A. villosum were shown to be the major components and suggested to be responsible for the different biological activities such as analgesic, anti-oxidation and anti-inflammation [27]. In this study, the MeOH extract of the fruits of A. villosumwas shown to significantly inhibit NA activities (IC50: 4.9 μg/mL) and protect the host cells from CPE damage (EC50: 1.8 μg/mL) without cytotoxicity, and its therapeutic selective index (SI) is 439 in MDCK cell culture.
In this study, we limit our study on EtOAc and MeOH extracts of medical plants since bioassay-guided isolation of neuraminidase inhibitors in aqueous extracts remains a challenging task for us. However, this may decrease the risk of false-positive results in the enzyme-based screening caused by some interfering components present within aqueous extracts. Future study will try to improve the screening methods on aqueous extracts that may also contain active components with anti-neuraminidase activity.
Conclusion
We carried out the in vitro screening of anti-neuraminidase activity of 190 herbal extracts from 95 medicinal plants traditionally used in Lingnan Chinese Medicines. Among the tested extracts, 5 extracts, obtained from Amomurn villosum, Melaphis chinensis, Sanguisorba officinalis and Flos Caryophylli, showed potent NA inhibitory activity. Comprehensive literature survey revealed that no study has been reported on the effects of the organic extracts of A. villosum and S. officinalis on anti-influenza virus activities and small-molecule NA inhibitors from these extracts have not been chemically identified yet. Further studies are underway to isolate bioactive components of these extracts by bioassay-guided fractionation, and to explore their antiviral mechanisms and finally determine their clinical potentials.
Acknowledgements
The authors would like to acknowledge all the fellows in Research Center of Medicinal Plants Resource Science and Engineering, Guangzhou University of Chinese Medicine for their great support and encouragement. We also thanks for their assistance in collecting medicinal plants from Jinxing Qiu, Guozheng He and Honghui Huang.
Funding
This work was supported by China National Natural Science Foundation Grant (No.81373432), Guangzhou Science and Technology Program Grant (No. 2014 J4100118) to JL, National Great Science and Technology Major Projects (2012ZX09301002-2013HXW-11) and Beijing Natural Science Foundation Grant (No. 7152103) to AL.
Availability of data and materials
The data sets used and /or analysed during the current study available from the corresponding authors on reasonable request.
Abbreviations
- CPE
Cytopathic effect
- HA
Haemagglutinin
- HHDP
Hexahydroxydiphenoyl
- MDCK
Madin-Darby canine kidney
- MNCC
Maximal non-cytotoxic concentration
- MTT
3-[4,5-dimethyl-thiazol-2-yl]-2,5- diphenyl tetrazolium bromide
- MUNANA
methylumbelliferyl-α-D-N-acetylneuraminate
- NA
Neuraminidase
- SI
Selectivity index.
Authors’ contributions
JL and AL conceived and designed the study. JL, KC and HM collected the herbs and prepared the herbal extracts. MZ, LG, WZ and AL carried out herbal screening and anti-influenza virus studies. JL, MZ and AL analyzed data. JL wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiawei Liu, Phone: +86-20-3935-8547, Email: jiawei.liu@ymail.com.
Mian Zu, Email: rabbitzumian@outlook.com.
Kaotan Chen, Email: 24446630@qq.com.
Li Gao, Email: gaoli5945lily@yeah.net.
Huan Min, Email: minhuan8681@163.com.
Weiling Zhuo, Email: sdnj20060867@126.com.
Weiwen Chen, Email: chenww@gzucm.edu.cn.
Ailin Liu, Phone: +86-10-8315-0885, Email: liuailin@imm.ac.cn.
References
- 1.Ansart S, Pelat C, Boelle PY, Carrat F, Flahault A, Valleron AJ. Mortality burden of the 1918-1919 influenza pandemic in Europe. Influenza Other Respir Viruses. 2009;3(3):99–106. doi: 10.1111/j.1750-2659.2009.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Emergence of a novel swine-origin influenza a (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 3.Gong J, Xu W, Zhang J. Structure and functions of influenza virus neuraminidase. Curr Med Chem. 2007;14(1):113–122. doi: 10.2174/092986707779313444. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Liu S, Du L, Jiang S. A new role of neuraminidase (NA) in the influenza virus life cycle: implication for developing NA inhibitors with novel mechanism of action. Rev Med Virol. 2016;26(4):242–250. doi: 10.1002/rmv.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jagadesh A, Salam AA, Mudgal PP, Arunkumar G. Influenza virus neuraminidase (NA): a target for antivirals and vaccines. Arc Virol. 2016;161(8):2087–2094. doi: 10.1007/s00705-016-2907-7. [DOI] [PubMed] [Google Scholar]
- 6.Air GM, Ghate AA, Stray SJ. Influenza neuraminidase as target for antivirals. Adv Virus Res. 1999;54:375–302. doi: 10.1016/S0065-3527(08)60372-3. [DOI] [PubMed] [Google Scholar]
- 7.Shobugawa Y, Saito R, Sato I, Kawashima T, Dapat C, et al. Clinical effectiveness of neuraminidase inhibitors-oseltamivir, zanamivir, laninamivir, and peramivir for treatment of influenza a(H3N2) and a(H1N1) infection: an observational study in the 2010–2011 influenza season in Japan. J Infect Chemother. 2012;18:858–864. doi: 10.1007/s10156-012-0428-1. [DOI] [PubMed] [Google Scholar]
- 8.Von Itzstein M. The war against influenza: discovery and development of sialidase inhibitors. Natr Rev Drug Discov. 2007;6(12):967–974. doi: 10.1038/nrd2400. [DOI] [PubMed] [Google Scholar]
- 9.Subbarao K, Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat Rev Immunol. 2007;7:267–278. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorlund K, Awad T, Boivin G, Thabane L. Systematic review of influenza resistance to the neuraminidase inhibitors. BMC Infec Dis. 2011;11:134. doi: 10.1186/1471-2334-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamura D, DeBiasi RL, Okomo-Adhiambo M, Mishin VP, Campbell AP, et al. Emergence of multidrug-resistant influenza a(H1N1)virus variants in an immunocompromised child treated with oseltamivir and Zanamivir. J Infec Dis. 2015;212(8):1209–1213. doi: 10.1093/infdis/jiv245. [DOI] [PubMed] [Google Scholar]
- 12.Grienke U, Schmidtke M, von Grafenstein S, Kirchmair J, Liedl KR, Rollinger JM. Influenza neuraminidase: a druggable target for natural products. Nat Prod Rep. 2012;29(1):11–36. doi: 10.1039/C1NP00053E. [DOI] [PubMed] [Google Scholar]
- 13.Chen KT, Zhou WL, Liu JW, Zu M, He ZN, Du GH, et al. Active neuraminidase constituents of Polygonum cuspidatum against influenza a(H1N1) influenza virus. Zhongguo Zhong Yao Za Zhi. 2012;37(20):3068–3073. [PubMed] [Google Scholar]
- 14.He Z, Lian W, Liu J, Zheng R, Xu H, Du G, Liu A. Isolation, structural characterization and neuraminidase inhibitory activities of polyphenolic constituents from Flos Caryophylli. Phytochem Lett. 2017;19:160–167. doi: 10.1016/j.phytol.2016.12.031. [DOI] [Google Scholar]
- 15.Liu AL, Liu B, Qin HL, Lee SM, Wang YT, Du GH. Anti-influenza virus activities of flavonoids from the medicinal plant Elsholtzia rugulosa. Planta Med. 2008;74(8):847–851. doi: 10.1055/s-2008-1074558. [DOI] [PubMed] [Google Scholar]
- 16.Liu AL, Wang HD, Lee SM, Wang YT, Du GH. Structure-activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorg Med Chem. 2008;16(15):7141–7147. doi: 10.1016/j.bmc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Park,CJ. Composition for prevention of influenza viral infection comprising tannic acid, air filter comprising the same and air cleaning device comprising the filter. US Patent 9,119,814; 2015.
- 18.Chen G, Shen Y, Zhang M, Zhu Z, Wang D, Liu X, Ma S. Studies on warming the middle-jiao and analgesic effect of flos Caryophylli. Zhongguo Zhong Yao Za Zhi. 1991;16(7):429–432. [PubMed] [Google Scholar]
- 19.Kurokawa M, Hozumi T, Basnet P, Nakano M, Kadota S, Namba T, et al. Purification and characterization of eugeniin as an anti-herpesvirus compound from Geum japonicum and Syzygium aromaticum. J Pharmacol Exp Ther. 1998;284(2):728–735. [PubMed] [Google Scholar]
- 20.Kang SY, Seo JK, Lim JW. Antiviral pentacyclic triterpenoids isolated from Sanguisorba officinalis roots against viral hemorrhagic septicemia virus and simultaneous quantification by LC-MS/MS. Planta Med. 2016;81(Suppl 1):1–81. [Google Scholar]
- 21.Yu T, Lee YJ, Yang HM, Han S, Kim JH, Lee Y, et al. Inhibitory effect of Sanguisorba officinalis ethanol extract on NO and PGE2 production is mediated by suppression of NF-kappaB and AP-1 activation signaling cascade. J Ethnopharmacol. 2011;134(1):11–17. doi: 10.1016/j.jep.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 22.Lee NH, Lee MY, Lee JA, Jung DY, Seo CS, Kim JH, et al. Anti-asthmatic effect of Sanguisorba officinalis L. and potential role of heme oxygenase-1 in an ovalbumin-induced murine asthma model. Int J Mol Med. 2010;26(2):201–208. doi: 10.3892/ijmm_00000453. [DOI] [PubMed] [Google Scholar]
- 23.Kim TG, Kang SY, Jung KK, Kang JH, Lee E, Han HM, et al. Antiviral activities of extracts isolated from terminalis chebula Retz., Sanguisorba officinalis L., Rubus coreanus Miq. And Rheum palmatum L. against hepatitis B virus. Phytother Res. 2001;15(8):718–720. doi: 10.1002/ptr.832. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Shang F, Meng Y, Li L, Cui Y, Zhang M, et al. Ethanol extract of Sanguisorba officinalis L. inhibits biofilm formation of methicillin-resistant Staphylococcus aureus in an Ica-dependent manner. J Dairy Sci. 2015;98(12):8486–8491. doi: 10.3168/jds.2015-9899. [DOI] [PubMed] [Google Scholar]
- 25.Liu MP, Liao M, Dai C, Chen JF, Yang CJ, Liu M, et al. Sanguisorba officinalis L synergistically enhanced 5-fluorouracil cytotoxicity in colorectal cancer cells by promoting a reactive oxygen species-mediated, mitochondria-caspase-dependent apoptotic pathway. Sci Rep. 2016;6:34245. doi: 10.1038/srep34245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng JM, Zhang LX, Ma J, Guan ZB. The review of Amomum villosum in Xishuangbanna. Zhongguo Zhong Yao Za Zhi. 2006;31(2):97–91. [PubMed] [Google Scholar]
- 27.Wu Y, Ge F, Shi Q, Tan X, Wu H. Study of supercritical-CO2 fluid extraction in extracting essential oils of Amomun tsao-ko. Zhong Yao Cai. 1997;20(5):240–241. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and /or analysed during the current study available from the corresponding authors on reasonable request.


