Abstract
An immunocompromised patient with a history of multiple hip implant revisions extended courses of empiric antibiotic treatment, and a retained metallic rod in the femoral medullary canal was transferred for diagnostic studies and treatment. A high suspicion of fungal infection and utilization of extended and specific fungal cultures were the diagnostic keys for infection with Trichosporon inkin. The treatment consisted in a debridement surgery with the use of a functional spacer with cement supplemented with voriconazole and vancomycin plus a 6-month systemic treatment with voriconazole. After 2 years of follow-up, the patient is free of symptoms.
Keywords: Hip arthroplasty, Periprosthetic fungal infection, Trichosporon inkin
Introduction
Periprosthetic fungal infections following arthroplasty are extremely rare, constituting only about 1% of all periprosthetic joint infections (PJIs). In the few reported cases, Candida species were the primary infecting fungal organisms [1], [2]. Early knowledge of fungal involvement would aid in selecting the appropriate antimicrobial therapy more quickly and perhaps yield better treatment outcomes [3], [4]. Trichosporon fungi commonly colonize the skin and gastrointestinal tract of humans and are increasingly recognized as a cause of systemic illness in immunocompromised patients [4], [5], [6], [7]. Trichosporon inkin has been long known as the cause of superficial infections such as white piedra, a distal infection of the hair shaft [5]. To our knowledge, this is the first report that describes a case of a revised hip with periprosthetic infection caused by T inkin and its treatment strategy. A review of the literature on the treatment for patients with periprosthetic fungal infection is also presented.
Case history
The patient was a 73-year-old female, with a clinical history of chronic use of corticosteroids for myasthenia gravis, bilateral hip osteonecrosis, chronic hepatitis B, obesity, and non–insulin-dependent diabetes. Informed consent was obtained for this case report.
She underwent a right total hip replacement in 1994. She received a total hip replacement on the left side in 1995. In 2010, she had revision of the left hip implant for a presumptive diagnosis of periprosthetic infection with persistent pain and elevated inflammatory markers (C-reactive protein [CRP] 9 mg/L, erythrocyte sedimentation rate [ESR] 32 mm/h, and an elevated white blood cell count of 4800), but negative cultures. Before the second left hip surgery, she received a course of empirical antibiotic treatment for 3 months. The clinical evolution was with episodes of dislocation and persistent pain.
In 2012, the patient had a second revision surgery. Intraoperative cultures were positive for Klebsiella pneumoniae, and she received antibiotic treatment with trimethoprim/sulfamethoxazole for 1 month, after which the second surgical procedure was performed. One year later the patient developed symptoms of functional impairment and persistent pain associated with signs of prosthetic loosening.
Laboratory studies showed an elevated CRP at 10.1 mg/L, and a high ESR (49 mm/h) for which the prosthetic implant was again removed, but the distal part of a modular long stem in the proximal femur could not be extracted.
Intraoperative cultures were negative. However, the patient again received an empiric course of antibiotics with vancomycin and ceftazidime. She was then referred to our center in 2014 for further evaluation and treatment.
A sample of 250 mL of a cloudy, yellowish, viscous fluid with a pus-like appearance was aspirated from the hip. The laboratory examination of this fluid revealed glucose 24 mg/dL, protein 4.0 mg/dL, lactic dehydrogenase 1535, and a leukocyte count of 100/high power field (Fig. 1). Microscopy also revealed yeast forms and an unusual amount of cholesterol crystals (Figs. 2 and 3).
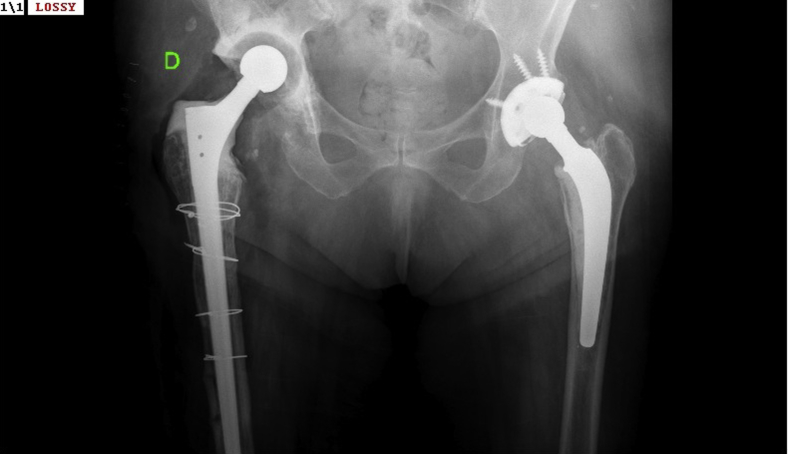
Figure 1.

Preoperative anteroposterior (AP) radiograph of the right hip before revision surgery. As a result of several failed revisions attributed to a presumptive diagnosis of periprosthetic infection, the hip displayed a severe bone defect, osteolysis, and foreign materials including the distal part of a modular osteointegrated stem.
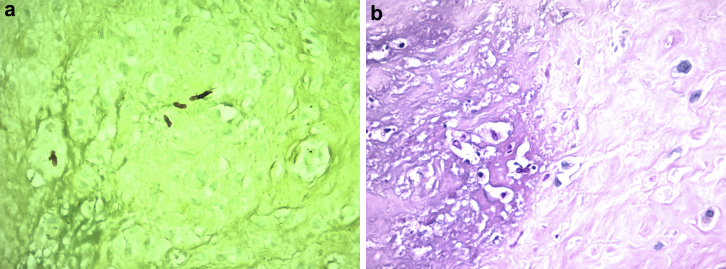
Figure 2.
(a) Grocott 400×. Highlights of yeasts with the Grocott technique (the yeast are in black). (b) Past 400×. Polymorphonuclear leukocytes, histoid cells, necrosis, and fungi.
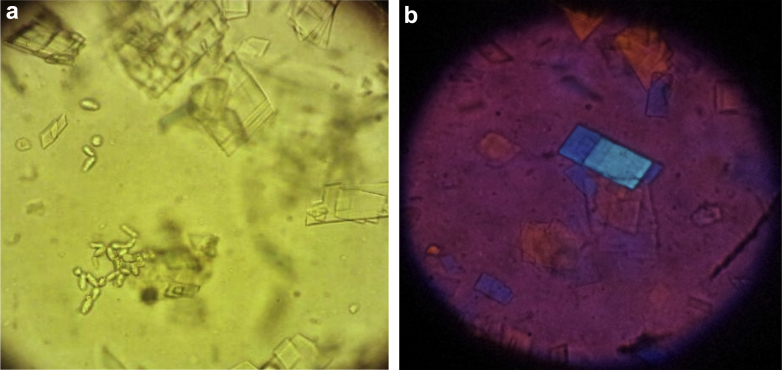
Figure 3.
(a) Optical microscopy showed the presence of abundant crystals of cholesterol, some iron particles, and yeast. (b) Optical microscopy with polarized light.
Cultures in enriched Sabouraud agar showed no bacterial growth, but after 3 weeks a fungus was isolated and identified as T inkin. This isolate was sensitive to itraconazol, amphotericin B, fluconazole, and voriconazole (Fig. 2b).
A 2-stage revision procedure was planned. The first stage consisted of extensive debridement, with the removal of the distal part of the modular long-stem implant with a functional spacer and a partially fixed cemented cup. The cement of the spacer was supplemented with voriconazole and vancomycin (Fig. 4). The intraoperative cultures confirmed the presence of T inkin.
Figure 4.
Postoperative AP radiograph of functional spacer after 2 years of follow-up.
The patient was treated with a 6-month course of treatment with voriconazole.
At 2-year follow-up, the CRP was negative and ESR was normal at 22. The patient is asymptomatic, walking with a cane and has declined further procedures.
Discussion
Few case reports and limited clinical series have been published in the literature about fungal periprosthetic hip infections. The lack of evidence concerning fungal PJI, as well as the lack of diagnostic criteria presents clinicians with a dilemma when presented with a failed prosthetic joint due to a presumed infection [8]. Fungal pathogens, other than Candida, have been reported previously only in the case of periprosthetic hip infection caused by Rhodotorula minuta [3], [9]. Trichosporon species occasionally are part of the gastrointestinal and oral cavity microbiota and can transiently colonize the respiratory tract and skin [5], [6]. T inkin is the etiologic agent of white piedra, a rare hair infection commonly seen in tropical climates [5].
Invasive Trichosporon spp. have been documented mostly in cancer patients and in critically ill patients following multiple invasive medical procedures. In addition, there is an increased risk of fungal infection after use of antibiotics for a prolonged period, indwelling catheters, and in patients in the intensive care unit [4], [5], [6], [10].
In this particular patient, several factors like multiple surgeries, the repetition of extended courses of empiric antibiotic, and the presence of indwelling catheters for long periods led to a high risk for fungal infection [1], [5]. It is possible that the ability of Trichosporon strains to adhere to form biofilms on implanted devices may account for the process of invasive trichosporonosis, because this ability promotes resistance to antimicrobial drugs and the host immune response [5], [7], [11]. It is rare to observe cholesterol crystals in a synovial fluid sample of the hip. In this case, their presence simulated purulent fluid.
It is not clear what led to the presence of cholesterol crystals in the synovial fluid, but local factors, including defective drainage, increased permeability, intra-articular bleeding, and local destruction, seem relevant [5].
Detection of T inkin was successful following aspiration, immediate inoculation in Sabouraud agar, and incubation for more than 3 weeks. This method led to a final diagnosis, especially given the unexplained signs of infection.
Triazoles seem to have better in vitro and in vivo antifungal activity against Trichosporon spp. than amphotericin B [5]. Voriconazole has excellent in vitro activity against Trichosporon spp. and may be useful for treating patients with trichosporonosis [5], [12]. Some recent in vitro studies document a very good elution of voriconazole from polymethyl methacrylate and activity on bioassay [1]. Some authors describe a general phenomenon of coexisting or disproportionate history of bacterial PJI in fungal PJI [1]. The combination of systemic and local antifungals mixed in bone cement can be an effective adjuvant to a rigorous debridement surgery to treat severe fungal joint infections, as is local application of vancomycin to treat and/or prevent bacterial infections [13], [14].
The findings in the present report suggest that in cases with a confounding clinical and laboratory picture in which the diagnosis is in doubt, fungal infection must be considered. Such cases warrant a high degree of suspicion for the presence of rare microorganisms, specific culture conditions, and an extended culture time, with an aggressive 2-stage treatment protocol with removal of all foreign materials [1], [10].
Even if this is the first report of a hip PJI caused by T inkin, after combining all the reported cases of trichosporonosis, one can suggest that reports of Trichosporon spp. are increasing as agents of invasive mycoses in contemporary medicine.
Summary
An immunocompromised patient with a history of multiple hip implant revisions, extended courses of empiric antibiotic treatment, and a retained metallic rod in the femoral medullary canal was transferred for diagnostic studies and treatment.
A high suspicion of fungal infection and utilization of extended and specific fungal cultures were the diagnostic keys for infection with T inkin.
The treatment consisted in a debridement surgery with the use of a functional spacer with cement supplemented with voriconazole and vancomycin plus a 6-month systemic treatment with voriconazole. After 2 years of follow-up, the patient is free of symptoms.
Footnotes
No author associated with this paper has disclosed any potential or pertinent conflicts which may be perceived to have impending conflict with this work. For full disclosure statements refer to http://dx.doi.org/10.1016/j.artd.2017.05.005.
Appendix A. Supplementary data
References
- 1.Schoof B., Jakobs O., Schmidl S. Fungal periprosthetic joint infection of the hip: a systematic review. Orthop Rev. 2015;7:5748. doi: 10.4081/or.2015.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ueng S.W., Lee C.Y., Hu C.C., Hsieh P.H., Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471:3002. doi: 10.1007/s11999-013-3007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artiaco S., Ferrero A., Boggio F., Colzani G. Pseudotumor of the hip due to fungal prosthetic joint infection. Case Rep Orthop. 2013;2013:502728. doi: 10.1155/2013/502728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzam K., Parvizi J., Jungkind D. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: a multi-institutional experience. J Bone Joint Surg Am. 2009;91:142. doi: 10.2106/JBJS.I.00574. [DOI] [PubMed] [Google Scholar]
- 5.Colombo A., Padovan A., Chaves G. Current knowledge of Trichosporon spp. and Trichosporonosis. Clin Microbiol Rev. 2011;24(4):682. doi: 10.1128/CMR.00003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vazquez J.A. Trichosporon infection. Curr Fungal Infect Rep. 2010;4:52. [Google Scholar]
- 7.Zuo Q., Dong L., Mu W. Trichosporon asahii infection after total knee arthroplasty: a case report and review of the literature. Can J Infect Dis Med Microbiol. 2015;26(1):47. doi: 10.1155/2015/458670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colten D., Bracken B.S., Elie F. Systemic inflammatory markers and aspiration cell count may not differentiate bacterial from fungal prosthetic infections. Clin Orthop Relat Res. 2014;472:3291. doi: 10.1007/s11999-014-3631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutrona A.F., Shah M., Himes M.S. Rhodotorula minuta: an unusual fungal infection in hip-joint prosthesis. Am J Orthop. 2002;31:137. [PubMed] [Google Scholar]
- 10.Gebauer M., Frommelt L., Pramond A. Management of fungal or atypical periprosthetic joint infections. J Orthop Res. 2014;32:S147. doi: 10.1002/jor.22559. [DOI] [PubMed] [Google Scholar]
- 11.Chandra J., Kuhn D.M., Mukherjee P.K. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller R.B., McLaren A.C., Pauken C. Voriconazole is delivered from antifungal loaded bone cement. Clin Orthop Relat Res. 2013;471:195. doi: 10.1007/s11999-012-2463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deelstra J., Neut D., Jutte P. Successful treatment of Candida albicans-infected total hip prosthesis with staged procedure using an antifungal-loaded cement spacer. J Arthroplasty. 2013;28:35. doi: 10.1016/j.arth.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Harmsen S., McLaren A.C., Pauken C., McLemore R. Amphotericin B is cytotoxic at locally delivered concentrations. Clin Orthop Relat Res. 2011;469:3016. doi: 10.1007/s11999-011-1890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.