Abstract
Purpose
To evaluate and compare corneal hysteresis (CH) and corneal resistance factor (CRF) in pellucid marginal degeneration (PMD), keratoconus (KCN), and normal eyes using the Ocular Response Analyzer (ORA).
Methods
In this retrospective study, corneal biomechanical parameters were measured in patients with PMD (n = 102) and KCN (n = 202) and normal subjects (n = 208) using the ORA. Data, including full patient history as well as the results of refraction, slit-lamp biomicroscopy, Pentacam HR (Oculus), and ORA (Reichert; Buffalo, New York, USA), were collected from medical records. Also, the data of only one eye per individual were selected for the analysis. The inclusion criteria for PMD and KCN groups were a reliable diagnosis of these ectatic disorders based on the clinical and corneal tomographic findings. CH, CRF, CH–CRF, intraocular pressure (IOP) measurements were assessed for each subject. Data were analyzed with SPSS and MedCalc using the ANOVA, Pearson Correlation, and receiver operating characteristic (ROC) curve analysis.
Results
The mean CH was 8.91 mmHg ± 1.05 [standard deviation (SD)], 8.43 ± 0.78, and 10.89 ± 1.08 in the PMD, KCN, and normal group, respectively. Also, the mean CRF was 8.21 ± 1.35, 7.19 ± 1.11, and 10.69 ± 1.41 in the PMD, KCN, and normal group, respectively. ANOVA showed differences in the mean CH, CRF, and CH–CRF between three groups (P < 0.001). Also, ROC curve analysis showed the cut-off points ≤9.5, ≤9.5, and >1.3 mmHg for CH, CRF, and CH–CRF in the PMD group, respectively. For biomechanical parameters in PMD eyes, CRF had the highest sensitivity (75.49%) while the greatest area under the ROC curve (AUC) was seen for CH (0.903). Moreover, central corneal thickness (CCT) showed no correlation with CH (P = 0.30, r = −0.104) or CRF (P = 0.75, r = 0.033) in the PMD group.
Conclusions
This study presented the values of corneal biomechanics for PMD using the ORA. The results of the ORA were markedly different between PMD, KCN, and normal eyes.
Keywords: Pellucid marginal degeneration, Corneal biomechanics, Corneal hysteresis, Corneal resistance factor, Keratoconus
Introduction
Pellucid marginal degeneration (PMD) is a non-inflammatory and progressive ectatic corneal disease involving the inferior cornea in a crescentic shape.1 PMD is different from other ectatic corneal disorders by its location. The band of thinning usually extends between the 4 and 8 o'clock positions and is apart from the limbus by 1–2 mm of the normal cornea. The area superior to the thinned part is ectatic, and the area between the limbus and thinned part is clear, without any scarring, lipid deposition, or vascularization.2 Although PMD is localized as a bilateral inferior condition, its other features such as superior,3 unilateral4 or a combination of them5 have also been reported. A typical topographic map in PMD shows flattening in the vertical meridian with marked against-the-rule astigmatism.6, 7 Moreover, corrected distance visual acuity usually decreases in the fourth to fifth decades of life.2, 6 Despite the similarities between PMD and keratoconus (KCN), differential diagnosis is based on clinical manifestations and diagnostic modalities.2, 7, 8, 9, 10 On the other hand, it is very important to detect preoperative risk factors before corneal refractive surgery and to rule out corneal ectatic disorders. In eyes with KCN, significant alterations occur in corneal biomechanical properties which make it weaker than normal.11, 12 Considering the time of onset of PMD and KCN,4, 13 decreased corneal biomechanics in older patients probably suggests the onset or existence of PMD. Therefore, early detection of the changes of biomechanical characteristics can minimize the incidence of risk factors for refractive surgery in patients with PMD.14
The Ocular Response Analyzer (ORA), a device to determine corneal hysteresis (CH) and corneal resistance factors (CRF), can be used for evaluation of corneal biomechanics in vivo.15
To our knowledge, two studies have evaluated corneal biomechanical parameters in PMD using the ORA. The first study was conducted by Labiris et al. who evaluated the diagnostic capacity of ectasia specific indices,16 and the second study was performed by Lenk et al. who investigated the diagnostic capacity of corneal biomechanical parameters in a small group of patients with PMD.17 The above-mentioned studies focused on PMD diagnosis with specific software (version 3.01) and did not report the biomechanical outcomes of PMD in a large population.
Therefore, this study was designed to determine in vivo corneal biomechanics (CH and CRF) in PMD using the ORA and to estimate the diagnostic accuracy of these parameters. The secondary objective was to compare these parameters with the corresponding values in patients with KCN and subjects with normal cornea.
Methods
This retrospective, observational case series study was performed at Sedaghat Eye Clinic, Mashhad, Iran, from February 2016 to October 2016. The Institutional Review Board/Ethics Committee of Mashhad University of Medical Sciences approved the study in 2016 (registration number: 940776), and its protocol was in accord with the tenets of the Declaration of Helsinki.
Data were collected from medical records (January 2012–January 2016), including a full patient history as well as the results of uncorrected and corrected distance visual acuity, manifest and cycloplegic refraction (Topcon KR-1, Tokyo, Japan), regularity status of the retinoscopic reflex, non-contact computerized tonometry (Topcon CT-1/CT-1P, Tokyo, Japan), ophthalmoscopy, slit-lamp biomicroscopy, placido disc-based topography (TMS4, Tomey, Erlangen, Germany), Scheimpflug-based tomography (Pentacam HR, Oculus, Optikgerate GmbH, Wetzlar, Germany), and dynamic bidirectional applanation device (ORA; Reichert Ophthalmic Instruments, Buffalo, New York, USA).
Three study groups were PMD and KCN patients and normal subjects within the age range of 20–50 years.
The inclusion criterion for the PMD group was a reliable diagnosis of PMD made by an experienced corneal refractive surgeon (MR.S.) based on the results of slit-lamp biomicroscopy, corneal topography/tomography, with special attention to pachymetry maps.2, 10 On slit-lamp biomicroscopy and corneal tomography, we focused on a clear thinning band in the inferior corneal peripheral zone separated from the corresponding limbus by a 1–2 mm clear zone. Moreover, in corneal topography/tomography patterns, we considered the inferior corneal band of steepening above the band of thinning as well as against-the-rule or irregular astigmatism, while the corneal center is clear without considerable thinning or steepening. In addition to slit-lamp biomicroscopy and corneal tomography, we used clinical manifestations including visual acuity and refractive components of the cases for a diagnosis of PMD.
The patients in the KCN group were selected based on the topographic/tomographic patterns, KCN signs on slit-lamp examination, and an irregular retinoscopic reflex.9, 18, 19, 20, 21 KCN cases were finally confirmed based on the results of corneal topography and tomography.
The normal group comprised individuals with healthy eyes, corrected distance visual acuity of 0.00 logarithm of the minimum angle of resolution (logMAR) or higher (Snellen Equivalent 20/20 or better), normal topography/tomography, and low refractive error (spherical equivalent between plano to −3.00 diopters and astigmatism <1.00 diopter to rule out the effect of high refractive error on corneal biomechanics).22
The exclusion criteria in all groups were previous eye surgery, corneal scarring, vascularization, inflammation, opacity, history of herpetic keratitis, severe dry eye, contact lens use 3 weeks before study, glaucoma or glaucoma suspect, intraocular pressure (IOP) lowering treatment, pregnancy, nursing, and underlying autoimmune or systemic diseases. Eyes diagnosed as KCN suspect were excluded from the study.
Since PMD is a rare condition, we recruited 102 patients with PMD in the study group. After excluding suspect or not true PMD eyes, we had 32 patients with bilateral clinical PMD and 70 patients with unilateral clinical PMD. In order to avoid experimental error, when both eyes were eligible in unilateral PMD cases, the data of only one eye were selected randomly for the study. Then, we selected and compared two participants from the control (normal and KCN) groups (with age matching) per PMD patient in the study group to avoid potential biases.
The number of right and left eyes in PMD group was 62 and 40, respectively. Considering the number of right and left eyes in the study group, we enrolled about the same number of right and left eyes from control groups (two eyes per one PMD eye) in this study.
The ORA has the ability to measure the CH, CRF, corneal compensated IOP (IOPCC), and Goldmann correlated IOP (IOPG). In addition, the difference between CH and CRF (CH–CRF) and the difference between IOPCC and IOPG (IOPCC–IOPG) were calculated for each subject.
All corneal imaging and measurements have been obtained in a consistent manner based on the manufacturers' instructions. The manufacturers' representatives routinely check the calibration of the device every six months. The mechanism of the ORA has been already described in other studies23 and the repeatability and reproducibility of the ORA have been reported acceptable.24, 25 As for the results of ORA available in patients' records, the system monitors the entire process and produces a specific waveform. The ORA results consist of three consecutive measurements. If the measurements are of high quality according to the waveform score, only the reading with a better quality is included in the analysis. Also, the measurements of the Pentacam HR were used for keratometry and pachymetry in this study.
Statistical analysis
Data were analyzed with SPSS version 23 (IBM Inc., Chicago, Illinois, USA) and MedCalc software version 15.8.X86 (bvba, Ostend, Belgium). The normality of the data was assessed using the Kolmogorov–Smirnov test; then parametric tests were applied accordingly. The one-way analysis of variance (ANOVA) with Tukey adjustment for multiple comparisons was used to determine the mean of variables in normal, KCN, and PMD groups. The Independent Samples t Test was used to compare the means of parameters between male and female in PMD group. Pearson correlation test was employed to assess the correlation between CH, CRF, CH–CRF, and central corneal thickness (CCT) in PMD group. The best differential diagnostic cut-off point for each corneal biomechanical parameter was determined using the receiver operating characteristic (ROC) curve in PMD group. The cut-off points were the values which yielded the maximum value of the sum of sensitivity and specificity.26 P-values less than 0.05 were considered significant.
Results
The normal, KCN, and PMD group included 208 eyes [male: 106 (50.96%)], 202 eyes [male: 107 (52.97%)], and 102 eyes [male 74 (72.55%)], respectively. Considering the method of this study, the data of only one eye per individual were selected for analyses.
The baseline data and the corneal biomechanical measurements of the three groups are displayed in Table 1. One-way ANOVA showed significant differences in the mean of the variables (P < 0.05) except for age (P = 0.78) between all three groups. Multiple comparisons using the Tukey test showed differences in CH, CRF, IOPCC, IOPG, CH–CRF, and IOPCC–IOPG between all groups (all P < 0.001), but no significant difference was seen in maximum keratometry (P = 0.63) and CCT (P = 0.48) between KCN and PMD groups.
Table 1.
Baseline data of all subjects by groups and outcomes of ocular response analyzer (ORA).
| Variables | Groups |
P-valuea | ||
|---|---|---|---|---|
| Normal (Mean ± SD) (n = 208) | KCN (Mean ± SD) (n = 202) | PMD (Mean ± SD) (n = 102) | ||
| Age (years) | 30.29 ± 5.33 | 28.62 ± 3.88 | 31.48 ± 6.75 | 0.78 |
| SE (D) | −2.50 ± 0.43 | −2.81 ± 2.18 | −2.24 ± 2.51 | 0.011 |
| KMAX (D) | 45.07 ± 1.53 | 51.59 ± 5.19 | 51.32 ± 4.44 | <0.001 |
| CCT (μm) | 541.45 ± 29.25 | 477.68 ± 37.33 | 492.09 ± 38.72 | <0.001 |
| TPP (μm) | 536.48 ± 29.64 | 469.18 ± 42.16 | 471.89 ± 59.76 | <0.001 |
| CH (mmHg) | 10.89 ± 1.08 | 8.43 ± 0.78 | 8.91 ± 1.05 | <0.001 |
| CRF (mmHg) | 10.69 ± 1.41 | 7.19 ± 1.11 | 8.21 ± 1.35 | <0.001 |
| CH–CRF (mmHg) | 0.22 ± 0.87 | 1.24 ± 0.86 | 0.69 ± 1.06 | <0.001 |
| IOPCC (mmHg) | 15.03 ± 1.62 | 15.57 ± 1.42 | 15.86 ± 1.55 | <0.001 |
| IOPG (mmHg) | 14.84 ± 2.02 | 12.86 ± 1.07 | 13.64 ± 1.53 | <0.001 |
| IOPCC–IOPG (mmHg) | 0.19 ± 1.53 | 2.80 ± 1.55 | 2.20 ± 1.70 | <0.001 |
KCN: Keratoconus, PMD: Pellucid marginal degeneration, SD: Standard deviation, SE: Spherical equivalent, KMAX: Maximum keratometry, CCT: Central corneal thickness, TPP: Thinnest point pachymetry, CH: Corneal hysteresis, CRF: Corneal resistance factor, IOPCC: Corneal compensated intraocular pressure, IOPG: Goldmann correlated intraocular pressure.
One-way ANOVA test. Bold values are significant. No missing data are available. A P-value < 0.05 was considered statistically significant.
There was no correlation between age and CH (P = 0.40, r = −0.084), CRF (P = 0.36, r = −0.091), or CH–CRF (P = 0.82, r = 0.023), nor between CCT and CH (P = 0.30, r = −0.104), CRF (P = 0.75, r = 0.033), or CH–CRF (P = 0.17, r = −0.137) in the PMD group.
There was no difference in CH (9.02 ± 1.01 vs 8.63 ± 1.10 mmHg, P = 0.124) and CH–CRF (0.58 ± 1.03 vs 1.00 ± 1.10 mmHg, P = 0.073) between men and women in the PMD group. Notably, women had a lower mean CRF than men (7.60 ± 1.27 vs 8.45 ± 1.32 mmHg, P = 0.012) in the PMD group.
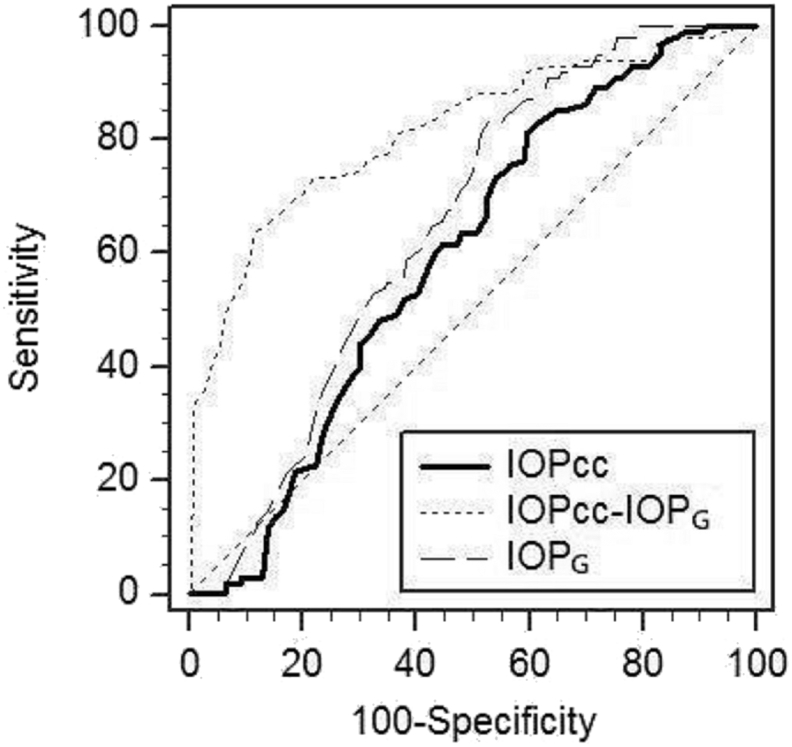
As for the ROC curve, the sensitivity was plotted as a function of the 100 − Specificity for different values of the studied parameters. According to ROC analysis, the cut-off points of all parameters in the results of ORA in the PMD group are presented in Fig. 1, Fig. 2, and Table 2. Despite the highest specificity of CH–CRF, the diagnostic accuracy of CH–CRF [sensitivity: 32.35%, specificity: 93.27%, area under the ROC curve (AUC): 0.627] at the cut point >1.3 was relatively poor for PMD detection (Table 2).
Fig. 1.
The receiver operating characteristic (ROC) curve analysis for determination of the cut-off point of corneal biomechanical parameters include: corneal hysteresis (CH), corneal resistance factor (CRF), and difference between CH and CRF (CH–CRF) in pellucid marginal degeneration using the Ocular Response Analyzer (ORA).
Fig. 2.
The receiver operating characteristic (ROC) curve analysis for determination of the cut-off point of corneal-compensated intraocular pressure (IOPCC), Goldmann-correlated intraocular pressure (IOPG), and difference between IOPCC and IOPG (IOPCC–IOPG) in pellucid marginal degeneration using the Ocular Response Analyzer (ORA).
Table 2.
Results of the receiver operating characteristic (ROC) curve analysis for outcomes of ocular response analyzer (ORA) in eyes with pellucid marginal degeneration.
| Variablesa | AUC value (at 95% CI) | P | Cut points (sensitivity–specificity) |
|---|---|---|---|
| CH | 0.903 | <0.0001 | ≤9.5 (71.57%–91.83%) |
| CRF | 0.896 | <0.0001 | ≤9.0 (75.49%–84.62%) |
| CH–CRF | 0.627 | <0.0003 | >1.3 (32.35%–93.27%) |
| IOPCC | 0.596 | 0.0030 | >14.3 (81.40%–40.40%) |
| IOPG | 0.649 | <0.0001 | ≤15.0 (83.30%–47.10%) |
| IOPCC–IOPG | 0.816 | <0.0001 | >1.8 (63.73%–88.46%) |
AUC: Area under the ROC curve, CI: Confidence interval, CH: Corneal hysteresis, CRF: Corneal resistance factor, CH–CRF: Difference between CH and CRF, IOPCC: Corneal-compensated intraocular pressure, IOPG: Goldmann correlated intraocular pressure, IOPCC–IOPG: Difference between and IOPCC and IOPG.
Unit of all variables: mmHg.
Discussion
Since PMD has a great effect on visual acuity and outcomes of refractive surgery, detection of this corneal ectatic disorder is critically necessary.2, 6, 14, 27 According to the published articles, CH and CRF are lower in KCN than their corresponding values in the normal cornea.11, 15 Furthermore, corneal refractive surgery in cases with decreased CH and CRF significantly increases the risk of iatrogenic ectasia, and PMD is one of the risk factors of this condition.14, 28 Multiple studies have reported the biomechanical measurements of the ORA in normal cornea, KCN, glaucoma, Fuchs' Endothelial Dystrophy, diabetes, post-refractive surgery, iatrogenic ectasia, etc.12, 15 However, to the best of our knowledge, no specific study has evaluated and compared the corneal biomechanics and results of ORA in a large population of PMD, KCN, and normal individuals.
In a large population of PMD patients, we found that CH [mean ± standard deviation (SD)] was 8.91 ± 1.05 and CRF was 8.21 ± 1.35. Other findings of ORA in PMD were evaluated in this study, as well (Table 1). Our findings showed that the percentage of men in the PMD group was higher than women. This may be the result of the higher prevalence of this condition in males.10
According to the results of this study, it can be concluded that CH and CRF were lower in the PMD group than the normal group but close to the KCN group. Additionally, there was no correlation between age and CH, CRF, or CH–CRF. The poor correlation between age and corneal biomechanics in this study may be the result of the similarity of age between all groups. We also found no correlation between CCT and CH, CRF, or CH–CRF in the PMD group. As shown in Table 1, the difference in CH–CRF was more considerable between PMD and normal groups than its difference between PMD and KCN groups. Also, we evaluated the sensitivity and specificity of ORA outcomes for PMD, but the values of biomechanical parameters (CH, CRF, and CH–CRF) were not very strong indicators for diagnostic ability and clinical application.
As for the biomechanics of PMD, Labiris et al. studied PMD with the purpose of evaluating the sensitivity of the ORA software version 3.01 in order to assess the diagnostic capacity of this software for PMD.16 However, the main focus of their study was not to determine the values of CH and CRF specifically. Although Labiris et al. showed significant differences between CH and CRF in PMD (8.39 ± 1.50 and 7.83 ± 1.97, respectively) and normal eyes (10.80 ± 1.77 and 10.18 ± 2.08, respectively), their reported values for PMD and normal eyes were different from our findings (Table 1). Meanwhile, their control and study groups comprised 40 normal and 40 PMD eyes, but we conducted our study on 208 normal and 102 PMD eyes. As a matter of fact, Labiris et al. reported lower CH and CRF values in the study group in comparison with the control group and similar results were obtained in our study. It also should be noted that, Labiris et al. compared neither CH nor CRF between KCN and PMD groups. They checked the sensitivity of keratoconus match index (KMI) and keratoconus match probability (KMP) in PMD cases with considerable false-positive and false-negative results in a small number of PMD cases.16
In another study, Lenk et al. investigated the diagnostic capacity of corneal biomechanics in a group of patients with PMD,17 but they did not compare their results with KCN group. Lenk et al. included only 29 eyes in the PMD group and focused on diagnostic accuracy of the ORA for PMD. They used the ORA software version 3.01, and this specific software has many sophisticated results. Also, Lenk et al. analyzed the adjusted values of IOP and CCT which is not routine in daily clinical practice. Our retrospective study was performed on PMD, KCN, and normal groups. We created three groups that were matched for ethnicity,29 age,30 and diurnal variations31 (all measurements have been done in the afternoon).
Touboul et al. showed that CH–CRF was negative in the normal population, but this value was positive in our study.23 Touboul used both eyes of the participants in their study that is a confounding factor. We evaluated the results of ORA in 208 eyes of 208 healthy samples.
Galletti et al. showed that CH–CRF was positive in healthy and KCN eyes and not significantly correlated with CCT in either group. In addition, they demonstrated that the CH–CRF was considerably different between healthy and KCN eyes.32 Our findings are in agreement with their results for KCN and healthy eyes, but they did not investigate these observations for eyes with PMD.
Ruisenor Vazquez et al. reported that additional biomechanical descriptors such as CH–CRF could serve as an indicator for understanding corneal biomechanics.33 According to our results, the diagnostic accuracy of CH–CRF for detection of PMD was relatively poor in the clinical setting.
Although other ORA results in PMD patients were evaluated in our research, the focus of this study was on CH and CRF, as the most important viscoelastic biomechanical parameters of the cornea, measured by the ORA.34 The present study showed that biomechanical properties of the PMD were weaker than normal. Understanding the biomechanics of the PMD is critical for its diagnosis16, 17 and long-term monitoring,17 as well as designing treatment strategies (intracorneal ring implantation35 and corneal cross-linking36).
Based on the published literature, true PMD is clinical PMD with typical inferior corneal thinning.4, 5, 6, 7, 8, 37 This thinning process is absolutely time-consuming and does not occur suddenly. The PMD cases presented in the published articles are severe cases of PMD, fail to describe the classification for PMD,4, 5, 6, 7, 8, 37 and now the question is what is the topographic/tomographic pattern in the early stage of PMD. This is the reason why we conducted this study with true clinical PMD cases. Belin et al37 explained the definition and diagnosis of PMD in details but presented no classification or grading of PMD (especially early stages).37 The staging and classification of PMD are controversial, and nobody really knows subclinical or clinical presentations of pre-advanced or moderate PMD.16, 37 Considering the absence of PMD classification, we could not compare the early stages of this condition with different grades of KCN. Furthermore, we could not investigate the correlation between different stages of PMD and KCN.
In conclusion, this research evaluated biomechanical properties of the cornea in PMD and compared them with normal and KCN corneas. Also, we specially focused on investigation of diagnostic ability of ORA in eyes with PMD; therefore, we did not present diagnostic information for KCN and normal eyes.
PMD and KCN can cause distinctive differences in the corneal biomechanical properties, possibly due to the difference in the position of the corneal apex relative to the entrance pupil. However, in PMD patients, it is better to determine the biomechanical properties of the inferior cornea instead of the central cornea because it is the main location of clinical manifestations, while the ORA mainly evaluates the center of the cornea. It should be mentioned as a limitation of our study. The retrospective nature and lack of matching in some demographic data are other limitations of this study.
Footnotes
Financial disclosure: None of the authors has a financial or proprietary interest in any material or method mentioned.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Krachmer J.H. Pellucid marginal corneal degeneration. Arch Ophthalmol. 1978;96(7):1217–1221. doi: 10.1001/archopht.1978.03910060051009. [DOI] [PubMed] [Google Scholar]
- 2.Sridhar M.S., Mahesh S., Bansal A.K., Nutheti R., Rao G.N. Pellucid marginal corneal degeneration. Ophthalmology. 2004;111(6):1102–1107. doi: 10.1016/j.ophtha.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Sridhar M.S., Mahesh S., Bansal A.K., Rao G.N. Superior pellucid marginal corneal degeneration. Eye. 2004;18(4):393–399. doi: 10.1038/sj.eye.6700643. [DOI] [PubMed] [Google Scholar]
- 4.Basak S.K., Hazra T.K., Bhattacharya D., Sinha T.K. Unilateral pellucid marginal degeneration. Indian J Ophthalmol. 2000;48(3):233–234. [PubMed] [Google Scholar]
- 5.Dundar H., Kara N., Kaya V., Bozkurt E., Yazici A.T., Hekimhan P.K. Unilateral superior pellucid marginal degeneration in a case with ichthyosis. Contact Lens Anterior Eye. 2011;34(1):45–48. doi: 10.1016/j.clae.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Karabatsas C.H., Cook S.D. Topographic analysis in pellucid marginal corneal degeneration and keratoglobus. Eye. 1996;10(Pt 4):451–455. doi: 10.1038/eye.1996.99. [DOI] [PubMed] [Google Scholar]
- 7.Maguire L.J., Klyce S.D., McDonald M.B., Kaufman H.E. Corneal topography of pellucid marginal degeneration. Ophthalmology. 1987;94(5):519–524. doi: 10.1016/s0161-6420(87)33416-5. [DOI] [PubMed] [Google Scholar]
- 8.Jabbarvand M., Hashemian H., Khodaparast M., Hassanpour N., Mohebbi M. Intrastromal lamellar keratoplasty in patients with pellucid marginal degeneration. J Cataract Refract Surg. 2015;41(1):2–8. doi: 10.1016/j.jcrs.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Gomes J.A., Tan D., Rapuano C.J. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359–369. doi: 10.1097/ICO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 10.Jinabhai A., Radhakrishnan H., O'Donnell C. Pellucid corneal marginal degeneration: a review. Cont Lens Anterior Eye. 2011;34(2):56–63. doi: 10.1016/j.clae.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Ortiz D., Pinero D., Shabayek M.H., Arnalich-Montiel F., Alio J.L. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. J Cataract Refract Surg. 2007;33(8):1371–1375. doi: 10.1016/j.jcrs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Porta N., Fernandes P., Queiros A., Salgado-Borges J., Parafita-Mato M., Gonzalez-Meijome J.M. Corneal biomechanical properties in different ocular conditions and new measurement techniques. ISRN Ophthalmol. 2014;2014:724546. doi: 10.1155/2014/724546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vazirani J., Basu S. Keratoconus: current perspectives. Clin Ophthalmol. 2013;7:2019–2030. doi: 10.2147/OPTH.S50119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatar M.G., Aylin Kantarci F., Yildirim A. Risk factors in post-LASIK corneal ectasia. J Ophthalmol. 2014;2014:204191. doi: 10.1155/2014/204191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinero D.P., Alcon N. In vivo characterization of corneal biomechanics. J Cataract Refract Surg. 2014;40(6):870–887. doi: 10.1016/j.jcrs.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Labiris G., Giarmoukakis A., Sideroudi H. Diagnostic capacity of biomechanical indices from a dynamic bidirectional applanation device in pellucid marginal degeneration. J Cataract Refract Surg. 2014;40(6):1006–1012. doi: 10.1016/j.jcrs.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Lenk J., Haustein M., Terai N., Spoerl E., Raiskup F. Characterization of ocular biomechanics in pellucid marginal degeneration. Cornea. 2016;35(4):506–509. doi: 10.1097/ICO.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 18.Rabinowitz Y.S., Rasheed K. KISA% index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg. 1999;25(10):1327–1335. doi: 10.1016/s0886-3350(99)00195-9. [DOI] [PubMed] [Google Scholar]
- 19.Orucoglu F., Toker E. Comparative analysis of anterior segment parameters in normal and keratoconus eyes generated by Scheimpflug tomography. J Ophthalmol. 2015;2015:925414. doi: 10.1155/2015/925414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matalia H., Swarup R. Imaging modalities in keratoconus. Indian J Ophthalmol. 2013;61(8):394–400. doi: 10.4103/0301-4738.116058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belin M.W., Ambrosio R. Scheimpflug imaging for keratoconus and ectatic disease. Indian J Ophthalmol. 2013;61(8):401–406. doi: 10.4103/0301-4738.116059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bueno-Gimeno I., Espana-Gregori E., Gene-Sampedro A., Lanzagorta-Aresti A., Pinero-Llorens D.P. Relationship among corneal biomechanics, refractive error, and axial length. Optom Vis Sci. 2014;91(5):507–513. doi: 10.1097/OPX.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 23.Touboul D., Roberts C., Kerautret J. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J Cataract Refract Surg. 2008;34(4):616–622. doi: 10.1016/j.jcrs.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 24.David V.P., Stead R.E., Vernon S.A. Repeatability of ocular response analyzer metrics: a gender-based study. Optom Vis Sci. 2013;90(7):691–699. doi: 10.1097/OPX.0b013e318297da45. [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Montanes J., Maldonado M.J., Garcia N., Mendiluce L., Garcia-Gomez P.J., Segui-Gomez M. Reproducibility and clinical relevance of the ocular response analyzer in nonoperated eyes: corneal biomechanical and tonometric implications. Invest Ophthalmol Vis Sci. 2008;49(3):968–974. doi: 10.1167/iovs.07-0280. [DOI] [PubMed] [Google Scholar]
- 26.Akobeng A.K. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007;96(5):644–647. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 27.Fuchihata M., Maeda N., Toda R., Koh S., Fujikado T., Nishida K. Characteristics of corneal topographic and pachymetric patterns in patients with pellucid marginal corneal degeneration. Jpn J Ophthalmol. 2014;58(2):131–138. doi: 10.1007/s10384-013-0291-3. [DOI] [PubMed] [Google Scholar]
- 28.Ambrosio R., Jr., Dawson D.G., Salomao M., Guerra F.P., Caiado A.L., Belin M.W. Corneal ectasia after LASIK despite low preoperative risk: tomographic and biomechanical findings in the unoperated, stable, fellow eye. J Refract Surg. 2010;26(11):906–911. doi: 10.3928/1081597X-20100428-02. [DOI] [PubMed] [Google Scholar]
- 29.Aghaian E., Choe J.E., Lin S., Stamper R.L. Central corneal thickness of Caucasians, Chinese, Hispanics, Filipinos, African Americans, and Japanese in a glaucoma clinic. Ophthalmology. 2004;111(12):2211–2219. doi: 10.1016/j.ophtha.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Elsheikh A., Wang D., Brown M., Rama P., Campanelli M., Pye D. Assessment of corneal biomechanical properties and their variation with age. Curr Eye Res. 2007;32(1):11–19. doi: 10.1080/02713680601077145. [DOI] [PubMed] [Google Scholar]
- 31.Shen M., Wang J., Qu J. Diurnal variation of ocular hysteresis, corneal thickness, and intraocular pressure. Optom Vis Sci. 2008;85(12):1185–1192. doi: 10.1097/OPX.0b013e31818e8abe. [DOI] [PubMed] [Google Scholar]
- 32.Galletti J.G., Pfortner T., Bonthoux F.F. Improved keratoconus detection by ocular response analyzer testing after consideration of corneal thickness as a confounding factor. J Refract Surg. 2012;28(3):202–208. doi: 10.3928/1081597X-20120103-03. [DOI] [PubMed] [Google Scholar]
- 33.Ruisenor Vazquez P.R., Delrivo M., Bonthoux F.F., Pfortner T., Galletti J.G. Combining ocular response analyzer metrics for corneal biomechanical diagnosis. J Refract Surg. 2013;29(9):596–602. doi: 10.3928/1081597X-20130710-01. [DOI] [PubMed] [Google Scholar]
- 34.Roberts C.J. Concepts and misconceptions in corneal biomechanics. J Cataract Refract Surg. 2014;40(6):862–869. doi: 10.1016/j.jcrs.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Pinero D.P., Alio J.L., Morbelli H. Refractive and corneal aberrometric changes after intracorneal ring implantation in corneas with pellucid marginal degeneration. Ophthalmology. 2009;116(9):1656–1664. doi: 10.1016/j.ophtha.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Spadea L. Corneal collagen cross-linking with riboflavin and UVA irradiation in pellucid marginal degeneration. J Refract Surg. 2010;26(5):375–377. doi: 10.3928/1081597X-20100114-03. [DOI] [PubMed] [Google Scholar]
- 37.Belin M.W., Asota I.M., Ambrosio R., Jr., Khachikian S.S. What's in a name: keratoconus, pellucid marginal degeneration, and related thinning disorders. Am J Ophthalmol. 2011;152(2):157–162. doi: 10.1016/j.ajo.2011.03.028. e1. [DOI] [PubMed] [Google Scholar]