Abstract
Parental bonding and oxytocin receptor (OXTR) gene genotype each influences social abilities in adulthood. Here, we hypothesized an interaction between the two – environmental experience (parental bonding history) and genetic factors (OXTR gene genotype) – in shaping adults’ social sensitivity (physiological response to distress). We assessed heart rate and peripheral temperature (tip of the nose) in 42 male adults during presentation of distress vocalizations (distress cries belonging to female human infants and adults as well as bonobo). The two physiological responses index, respectively, state of arousal and readiness to action. Participants’ parental bonding in childhood was assessed through the self-report Parental Bonding Instrument. To assess participants’ genetic predispositions, buccal mucosa cell samples were collected, and region rs2254298 of the oxytocin receptor gene was analyzed: previous OXTR gene findings point to associations between the G allele and better sociality (protective factor) and the A allele and poorer sociality (risk factor). We found a gene * environment interaction for susceptibility to social distress: Participants with a genetic risk factor (A carriers) with a history of high paternal overprotection showed higher heart rate increase than those without this risk factor (G/G genotype) to social distress. Also, a significant effect of the interaction between paternal care and genotype on nose temperature changes was found. This susceptibility appears to represent an indirect pathway through which genes and experiences interact to shape mature social sensitivity in males.
Keywords: Parental Bonding, oxytocin receptor gene, social abilities, Gene * Environment, physiological responses to social distress
Introduction
Social bonds constitute a foundation of human development. Phylogenetically, social interactions enhance human survival (Guilley et al., 2005; Holt-Lunstad et al., 2010; Perry, 2002; Rodriguez-Laso et al., 2007); ontogenetically, social interactions assure human survival in the first years of life and trigger the development of cognitive, social, and emotional abilities (Winston & Chicot, 2016). Phylogenesis and ontogenesis intersect insofar as social abilities, in adulthood, foster cooperation, reproduction, and nursing offspring and have developmental roots in initial social bonds forged between caregivers and infants. That said, each individual develops unique social sensitivities because of the combined effect of individual characteristics and environmental factors (Pluess, 2015). Therefore, understanding how social sensitivities develop requires a multilevel approach that takes into account individual-level genetic factors and environmental-level experiential factors that foster social abilities. Here, we attempted such an effort.
Genes and Social Sensitivity
The genetics literature points to associations between different allelic structures and the expression of distinct social sensitivities (Pluess, 2015; Windhorst et al., 2014).
Theoretically, researchers have proposed that sensitivity to the environment is not positive or negative in itself, but rather depends on the experienced context (Boyce & Ellis, 2005). Individuals less sensitive to the environment will be protected from adversities, but also benefit less from favorable experiences. By contrast, individuals biologically predisposed to be more sensitive to their environment, when experiencing greater adversities and stress, will show poorer developmental outcomes compared to less sensitive individuals, whereas individuals more sensitive to their environment will benefit more from favorable environments showing better developmental outcomes compared with less-sensitive individuals (Belsky et al., 2007).
Within this general perspective, notable are genes that encode for the receptors of oxytocin (OXTR), and specifically in the oxytocin receptor gene one region correlated with social behaviors is rs2254298 (for a review see Ebstein et al., 2012). Two allelic forms have been identified for this region, A and G. However findings are not unequivocal in the role these two allelic forms play in social development. Some research points to the G allele as the genotype linked to better social development (qua protective factor) and the A allele as the genotype linked to poorer social development (qua risk factor). Adolescent girls homozygous for the G allele (GG genotype) are less sensitive to early adversities in the social environment, such as having depressed and anxious mothers, compared with A carriers, and individuals with the G allele in this region (compared with the A allele) show more empathy (Thompson et al., 2011; Wu et al., 2012). From an anatomical point of view, A carriers also show greater amygdala volume which is purportedly a cerebral correlate of greater susceptibility to life events (Furman et al., 2011). Also, Marusak and colleagues (2015) found greater amygdala volume, a heightened amygdala activation in response to social stimuli, and reduced behavioral performance in A carriers (A/A or A/G genotypes), compared to G/G homozygous, only when exposed to stressful life events. Other findings, however, show that the G allele appears in patients with depression and in adults with higher levels of separation anxiety (Costa et al., 2009), and that there could be distinct effects of the OXTR gene genotype in different ethnic populations. Such inconsistency concerning associations of G and A alleles with individual development may be attributable to environmental factors not heretofore considered in this research. G and A alleles may not be directly linked to different levels of social development; rather, G and A alleles might affect development by differently influencing individuals’ sensitivity to the environment (Boyce & Ellis, 2005). For instance, the G allele renders individuals less vulnerable to the early environment, so later development would not be expected to differ according to early experience, whereas the A allele could enhance individual vulnerability to the environment suggesting that people with positive early experiences develop more adaptively than G carriers, and people with poor experiences develop more maladaptive behaviors than G carriers (Brüne, 2012). It is therefore important to understand the effects of genes on environmental sensitivity and to assess early experiences together with genetic composition.
Environment and Social Sensitivity
Although genes help to structure development, individuals’ responses to stressful events are greatly influenced by early experiences normally embedded in caregiver-infant interactions (Sable, 2008). Children learn about and then develop social sensitivities beginning with social experiences in the first years of life (Bornstein, 2015). Parents’ behaviors help to shape children’s mature social sensitivities. Parental bonding is deeply involved in this process (Huppert et al., 2010; Patton et al., 2001; Winston & Chicot, 2016). Parents who nurture their infants and who willingly fulfil their infants’ physiological and psychological needs for security and emotional closeness will foster a better and more stable emotional development in their offspring, who will be able to cope with stressful events in an adaptive way. By contrast, children who experience poor parenting tend to develop poorer emotional pathways and will react to distress with maladaptive responses (Francis & Meaney, 1999; Picardi et al., 2013).
Role of Physiological Activations
Genetic factors do not directly and univocally determine behavioral development, but moderate behavioral outcomes in conjunction with environmental factors. Therefore, genetic predispositions and environmental factors together shape individual behaviors. Specifically, effects of gene*environment interaction likely take place at the physiological level (Kanthak et al., 2016). Here we focus on Autonomic Nervous System (ANS) activation to investigate how gene*environment interaction moderates automatic physiological development. We evaluate heart rate changes and peripheral skin temperature (on the nose) to better assess the activation of both the parasympathetic and sympathetic branches of the ANS (Ioannou et al., 2013; Kothgassner et al., 2016; Manini et al., 2013; Quas et al., 2000).
Responses to Cry to Model Responses to Social Distress
Cry is one of the earliest and most prominent social communicative signals (Stewart et al., 2013). Evolutionarily, cries index the presence of a problematic, possible threatening situation and, especially baby cries, arouse in individuals a specific set of physiological and emotional patterns which underlie a sense of alertness and promptness to address the cause (Venuti et al., 2012: Messina et al., 2016). Ontogenetically, cries are essential for infants’ survival because is the cry is one of the few signals through which babies can communicate their needs to parents (Laurent & Ablow, 2012). Given the social importance of cry and its automatic elicitation of arousal and distress in human beings, we chose responses to cry stimuli to model responses to social distress.
Aims of the Study
In brief, genetics and experience interact in shaping individuals’ lasting social sensitivities. This study aimed to test how adults’ genotype for oxytocin receptors interact with their reported social history in parental bonding in childhood to shape their physiological responses to distressing social stimuli. We hypothesized a genotype*environment interaction (OXTR rs2254298 SNP * parental bonding in childhood) on physiological responses to distress. Specifically, we expected only A carriers (G/A or A/A genotype), the genotype more sensitive to the environment, to show differential physiological activation in response to distressing stimuli according to participants’ early experience with parents. Specifically, we expected (i) individuals’ with A alleles who experienced better parental behaviors to show better coping abilities in reaction to stress, as represented by a heart rate decrease and by a temperature increase, (ii) A carriers who experienced poorer parental behaviors to show poorer coping abilities in response to prolonged stress, represented by a heart rate increase and a temperature decrease, and (iii) G/G homozygotes to show relatively similar physiological responses independent of their experienced parental behavior.
Methods
Participants
Forty-two adult males (M = 24.7 years, SD = 5.05) were recruited through a database of volunteers available through the University of Trento web site. Informed consent was obtained from all participants, and the study was conducted in accord with ethical principles stated in the Helsinki declaration.
Stimuli
The stimuli were 30 15-s audio clips of distressed vocalizations, ten clips for each of 3 categories: infant cries, adult female cries, and bonobo cries. Cries were chosen because of their evolutionary significance and because they have been found to elicit distress and specific physiological responses in adults (De Pisapia et al., 2013; Esposito et al., 2012; Messina et al., 2016). This research aimed to assess physiological responses elicited by social distress, and infant and female cries could have a specific evolutionary salience to male adults, so both human infant and adult female cries were included as stimuli. Also, to test whether the investigated physiological activations were specific to human distress vocalizations or a generalized response to social distress, bonobo cries were included in the stimulus set. All cry stimuli were normalized for intensity, and the in volume was kept constant for all the presentations for all participants. Each audio clip was presented following 10 s of silence. Audio clips were organized into three different randomized sequences, and presentation order of the three sequences was counterbalanced across participants. Stimulus sequences were created using open source software Audacity.
Procedure
The study was conducted in three parts. First, participants completed an on- line self-report questionnaire to assess their parental bonding status in childhood. Second, participants’ heart rate and peripheral temperature were recorded throughout the presentation of the 30 audio clips. Before the beginning of the audio sequence, 30 s of heart rate and nose temperature were recorded to assess participants’ physiological baseline. Finally, a buccal mucosa sample was collected from each participant. The experimenters that ran the physiological experiment and collected the buccal mucosa sample were always blind to participants’ PBI responses.
Parental Bonding
The Parental Bonding Instrument (PBI; Parker et al., 1979) is a 50-item self-report questionnaire developed to measure the principal parental dimensions of care and overprotection, indicative of parental bonding during childhood. Participants filled in two forms, one for maternal behaviors and one for paternal behaviors. Both dimensions, care and overprotection, are measured on continuous scales, where values range from 0 to 3. In our sample, the PBI Cronbach’s alphas were medium-high for maternal PBI (care α =73; overprotection α = 73) and for paternal PBI (care α = 65; overprotection α = 83). Care measures parental attention to needs, and overprotection measures parental protectiveness (“Spoke to me in a warm and friendly voice”; “Tried to control everything I did”, respectively). High scores on the care dimension are interpreted as high parental warmth, affection, emotional closeness, and empathy (Arrindell et al., 1998, Dalsant et al., 2015). High scores on the overprotection dimension usually represent too great parental control over children, intrusion, and prevention of independent behavior (Arrindell et al., 1998; Rigby et al., 2007; Rikhye et al., 2008).
Heart Rate
HR was measured to assess participants’ parasympathetic arousal and stressful/calming state. An increase in heart rate indexes an increase in attention and promptness to action, whereas a decrease reflects the activation of a calming response in response to external stimulation (Bernston et al., 1997; Bradley, 2009). Specifically, a HR decrease in response to prolonged stress-eliciting stimuli is interpreted as a coping reaction which reflects an adaptive deactivation of the stress axis (Pavlov et al., 2015). Pavlov and colleagues (2015) showed participants images eliciting negative emotions, asking them either to look at them or to reduce their negative feeling towards them. When asked to reduce their negative attitude, participants rated images as less negative and had a lower cardiac blood output and lower total peripheral resistance, which are indexes of more efficient down-regulation of negative affect. A HR increase in response to prolonged stress-eliciting stimuli reflects a failure in deactivating prolonged sympathetic activation which is thought to lead to maladaptive consequences due to prolonged stress.
Temperature
To index sympathetic activity, we measured participants’ peripheral surface body temperature at the tip of the nose using a thermistor (Applent at4524 multi-channel temperature meter) for the duration of the experiment. Temperature fluctuations also indicate participants’ promptness to action. Previous studies have shown significant decreases in nose skin temperature in response to stress-eliciting stimuli in adults, infants, and non-human primates (Kuraoka & Nakamura, 2011; Ioannou et al., 2013; Manini et al., 2013). Therefore, we expected to find only in A carriers exposed to poorer parental behaviors a greater decrease in nose temperature in response to prolonged stress-eliciting stimuli, underlying poorer coping abilities, but we expected a greater increase in nose temperature in response to the same stimuli in A carriers exposed to better parental behaviors.
Genetic Assessment
DNA extraction and genotyping were conducted by ACGT, Inc. (Wheeling, IL). DNA was extracted from each kit using the Oragene DNA purification reagent. DNA concentrations were evaluated using spectroscopy (NanoDrop Technologies, USA). Each DNA sample was polymerase chain reaction (PCR) amplified for the rs2254298 region target with the primers 5′-TGA AAG CAG AGG TTG TGT GGA CAG G-3′ and 5′-AAC GCC CAC CCC AGT TTC TTC-3′. A PCR reaction of 20 ll consisting of 1.5 ll of genomic DNA from the test sample, PCR buffer, 1 mMeach of forward and reverse primers, 10 mM deoxyribonucleotides, KapaTaq polymerase, and 50 mM MgCl2 was performed. Cycling conditions included an initial 15 min denaturation at 95_C, and 35 cycles of 94 _C (30 s), 60 _C (60 s), 72 _C (60 s), and a final extension of 72_C for 10 min. PCR reactions were genotyped with an ABI 3730xl Genetic Analyzer (Applied Biosystems Inc.) and normalized with GeneScan 600 LIZ (Applied Biosystems, Inc.) size standards run on each sample. Genotype data were analyzed using GeneMapper ID (Applied Biosystems, Inc.). Participants possessing at least one A allele (A/A or G/A) were classified into a single A carriers group (Marusak et al., 2015). In the general population, the distribution of different genotypes for this DNA region is 70-80% of G/G homozygous and 20-30% for A carriers. The distribution in our sample was 71% for G/G homozygous and 29% for A carriers.
Analysis
For each physiological measure, the average levels of heart rate and nose temperature were calculated for each stimulus presentation, then physiological values were calculated as difference scores from baseline. The baseline for each participant was assessed by recording HR and nose temperature for 30 s before the beginning of the audio sequence. The different distress vocalization sounds (human infant, human woman, bonobo) were considered separately to test participants’ reactions to distinct distress types. For each participant a mean value of HR and temperature in response to each different type of sound was calculated. Prior to data analysis, univariate and multivariate distributions of heart rate values, temperature values, and behavioral judgment scores were examined for normality, homogeneity of variance, outliers, and influential cases (Fox, 1997). Furthermore, the distance of each case to the centroid was evaluated to screen for multidimensional outliers (Fox, 1997) defined as a value 2 SDs above/below the mean. A total of 3 values (out of 126 observations) were considered outliers and excluded from the analysis. For each physiological measure one multivariate ANOVA was performed with the physiological values as the dependent variable, the distress cry type (infant, woman, bonobo) as a within-subject factor, the OXTR gene genotype (G/G, A carriers) as a between-subject factor, and the PBI dimensions (maternal care, maternal overprotection, paternal care, and paternal overprotection) as continuous covariates. Therefore, overall for each physiological dependent variable six main effects, nine 2-way interaction effects, and four 3-way interaction effects were considered. Because our primary interest was in how genetics moderate responses to distressing social stimuli, we only report main effects of genotype and any significant interactions with genotype. Main effects and lower-order interactions of other variables (e.g., cry type and parental bonding) were included in the model only to aid interpretation of higher-order interactions with genotype.
Two comparisons out of 20 proved significant. Regression coefficients were used to analyze the effect of the covariate on the dependent variable, and Cohen’s d was used to evaluate the magnitude of significant effects. Finally, if an interaction between genotype and PBI was found, participants were divided in two groups, high and low PBI dimensions using the median split procedure, and post-hoc Student’s t tests were carried out within those groups to investigate whether the physiological activations between G/G and A carriers were significantly different.
Ready activation of the sympathetic nervous system in response to a single cry is considered an adaptive response because it underlies heightened arousal in anticipation of threat and promptness to play out adaptive comforting, for example moving to pick the infant up or cradling the baby. However, because we were interested in physiological responses to social stress and the cry was used as a model for it, activations to cries of the same type were collapsed, and for each participant three mean responses were calculated, one for each cry type. Considering a single response to a series of cries allowed us to investigate adults’ responses to prolonged social stress, rather than adults’ responses to single cries. Therefore, in this experiment we considered parasympathetic activation (lower HR, higher temperature) to be a coping and calming reaction in response to social stress, whereas a heightened sympathetic arousal (higher HR, lower temperature) was considered to be a physiologically stressful response.
Results
Means and standard deviations of the measured variables are provided in Table 1.
Table 1.
Means(SD) of dependent variables (Heart Rate and Nose Temperature) for G/G homozygotes and A carriers divided in two groups (high vs low score) for each PBI dimension.
| G/G | A Carriers | |||
|---|---|---|---|---|
| Hearth Rate (bpm) | High | Low | High | Low |
| Maternal Care | 0.19 (2.97) | 0.29 (2.41) | −0.49 (1.26) | 2.13 (2.68) |
| Paternal Care | 0.21 (2.94) | 0.26 (2.46) | 1.77 (3.28) | 0.89 (2.02) |
| Maternal Overprotection | 0.46 (2.76) | −0.05 (2.66) | 2.14 (2.87) | 0.82 (2.41) |
| Paternal Overprotection | −0.57 (3.03) | 0.94 (2.19) | 2.32 (2.07) | 0.19 (2.71) |
| Nose Temperature (°C) | ||||
| Maternal Care | 0.37 (1.72) | −1.15 (2.17) | −0.27 (2.55) | −0.15 (1.36) |
| Paternal Care | 0.06 (1.85) | −0.80 (1.24) | 0.71 (1.35) | −0.84 (1.85) |
| Maternal Overprotection | −0.60 (2.00) | −0.01 (2.16) | 0.04 (1.17) | −0.31 (2.07) |
| Paternal Overprotection | −0.07 (1.90) | −0.58 (2.24) | −0.90 (2.02) | 0.52 (1.25) |
Heart Rate
Paternal Overprotection
A significant interaction between paternal overprotection and genotype emerged for HR (F(1,41) = 8.54, p< .01, d = .99). No main effect of genotype or other interactions with genotype were significant. Paternal overprotection was positively associated with heart rate, r = .38 for A carriers; it was negatively associated with heart rate for G/G homozygous, r = −.35 [Fig. 1a]. Single Pearson’s rs were not significant. However, the difference between the slopes for G/G and A carriers, tested with Fisher’s z, was statistically significant (z = −2.02, p < .05). Furthermore, post-hoc Student’s t tests on the G/G vs. A carriers in low- and high-overprotection groups (using median split) revealed that HR activations were significantly different (t(18) = −2.13, p< .05) between G/G homozygotes and A carriers only when they had experience of high paternal overprotection [Fig. 1b].
Figure 1.
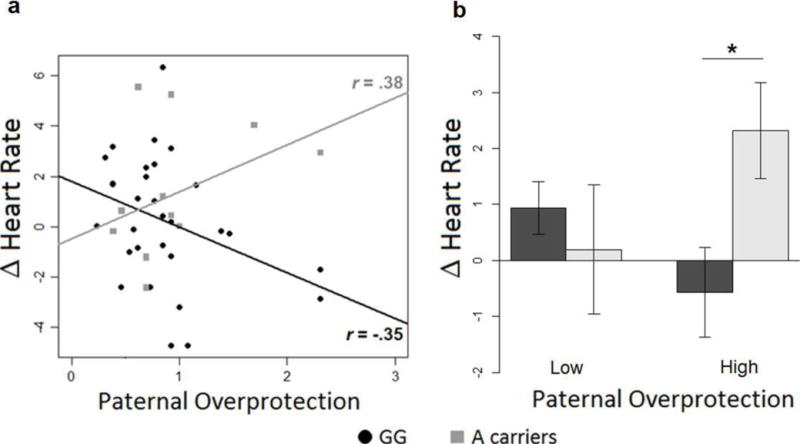
a–b. Effect of the interaction between paternal overprotection and genotype on HR changes. a) Correlations between Heart Rate responses to distress vocalizations (calculated as difference from baseline) and experienced paternal overprotection. Black circles = G/G homozygous; grey squares = A carriers. Lines represent the linear models for G/G homozygous (black) and A carriers (grey). In the figure the r-values represent Pearson’s r correlations. b) Comparison between HR mean changes from baseline in G/G homozygotes (black) and A carriers (gray) divided in high and low paternal overprotection.
Maternal Care, Maternal Overprotection, and Paternal Care
No significant main effect of genotype or interactions between maternal care, maternal overprotection or paternal care and genotype were found.
Temperature
Paternal Care
A significant interaction between paternal care and genotype was found (F(1,41) = 4.25, p < .05, d = .70). Paternal care was positively associated with temperature both for A carriers (r =.28) and G/G homozygous (r =.21) [Fig. 2a]. Pearson’s rs were not significantly different. Furthermore, post-hoc Student’s t tests on the G/G vs. A carriers in high and low paternal care groups (using median split) revealed no significant differences [Fig. 2b].
Figure 2.
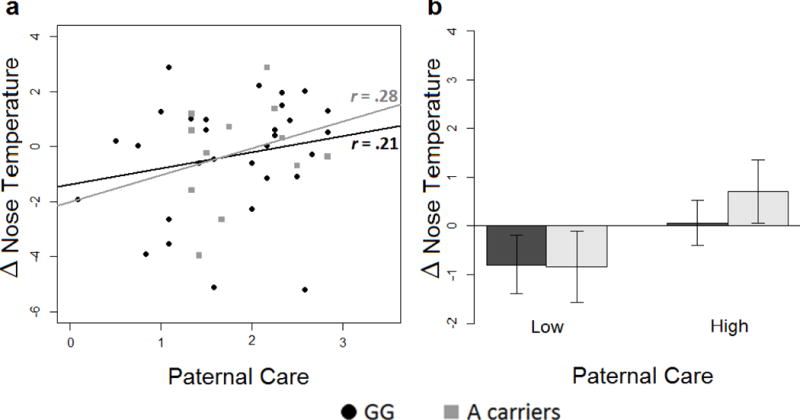
a–b. Effect of the interaction between paternal care and genotype on nose temperature changes. a) Correlations between nose temperature responses (calculated as difference from baseline) and experienced parental care, independently from stimuli type. Black circles = G/G homozygous; grey squares = A carriers. Lines represent the linear models for G/G homozygous (black) and A carriers (grey). In the figure the r-values represent Pearson’s r correlation. b) Comparison between nose temperature mean changes in G/G homozygotes (black) and A carriers (gray) divided in high and low paternal care.
Maternal Care, Maternal and Paternal Overprotection
No significant main effect of genotype or interaction between maternal care, maternal overprotection or paternal overprotection and genotype was found for temperature.
Discussion
Social sensitivities arise from the combined effects of individual characteristics, such as genes, and environmental factors, such as early social experiences. Among environmental factors, early caregiver interactions play a special role, possibly because early social experiences can influence individuals’ life-long social relationships (Ainsworth, 1970; Bowlby, 1969). Children who experience more optimal interactional patterns with parents characterized by security and emotional closeness, tend to develop healthier social relationships, emotional regulation, and ability to cope with distress in maturity (Sable, 2008). At the molecular level, social behavior is influenced by hormones, including notably oxytocin (Meinlschmidt & Heim, 2007; Pedersen & Boccia, 2002). Previous research shows how different alleles on the OXTR gene, that code for the expression of oxytocin receptors, relate to distinct levels of social sensitivity and susceptibility to distress. OXTR alleles may differ for several single nucleotide polymorphisms (SNP), and one SNP related to the development of social sensitivity appears in region rs2254298 of the OXTR gene (Ebstein et al., 2012).
Results for this SNP from previous research are not determinative in stating which allele, G or A, is protective or which a risk factor correlates with the development of maladaptive social behaviors and responses to stress (Brüne, 2012; Costa et al., 2009; Thompson et al., 2011). Here we aimed to investigate how different alleles in the OXTR gene interact with early parental bonding status to shape adults’ physiological responses to distressing social stimuli. We measured participants’ heart rate and nose skin temperature while exposing them to different cries. Individuals carrying the A allele (A/G or A/A) in this region of the OXTR gene showed differential physiological responses to stressful stimuli depending on their history of interactions with their fathers. Specifically, A carriers with a history of paternal overprotection showed increased heart rate compared to baseline in response to social distress, and G/G homozygotes with a history of paternal overprotection decreased heart rate in response to the same stimuli. A carriers with a history of good paternal care showed a trend in increasing nose temperature, whereas those with poor paternal care showed a trend in decreasing nose temperature. Overall, A carriers with better early interactional patterns with their caregiver in childhood, represented by lower paternal overprotection and higher paternal care, reacted to social distress with calming physiological responses (Bradley, 2009; Ioannou et al., 2013; Manini et al., 2013).
These findings accord with existing literature in that they show an effect of the interaction between early social experiences and genetic factors on individuals’ later development. Specifically, the moderation of genetic predispositions on environmental effects over physiological activations support the sensitivity hypothesis (Boyce & Ellis, 2005; Pluess, 2015; Windhorst et al., 2014): genes affect individual development through an indirect pathway, shaping sensitivity to environmental experiences. This pattern obtained for heart rate responses. Indeed, individuals with the so-called risk genetic factor, the A allele, may develop either worse or better than G/G individuals, according to their experienced social environment (Belsky et al., 2007). For example, when exposed to a less nurturing social environment, individuals carrying the A allele display a diminished ability to cope with stressful events and show greater physiological reactivity to distressing stimuli (as indexed by increased HR). However, when exposed to a more caring social environment, individuals carrying the A allele display an enhanced ability to cope with stressful events (as indexed by increased temperature). This double possibility for A carriers – to develop in either a better or poorer way – compared to G homozygotes, may underlie the presence in literature of multivocal results about the influence of OXTR rs2254298 genotype on adult social behavior. For temperature changes, a significant effect of paternal care and genotype was found. However, both G/G homozygotes and A carriers showed a positive correlation between paternal care scores and temperature increase, even if the pattern of activation differed between the two groups. The findings concerning temperature seem to reflect an effect of experienced paternal behavior (the higher paternal care, the higher the nose temperature), rather than an interaction between environment and genes. Previous studies showed a decrease in nose skin temperature in response to stress-eliciting stimuli (Kuraoka & Nakamura, 2011; Ioannou et al., 2013; Manini et al., 2013). We argue that an increase in nose temperature in response to stress-eliciting stimuli is likely a more adaptive reaction to better cope with a distressing situation. For neither heart rate nor temperature changes an effect of the interaction between genotype and cry type was found. This result underlies a generalized response to social stressors of different types. We argue that the lack of cry type effect may be due to the role that OXTR gene play in social behavior. Specifically, oxytocin is involved in affiliative and social behaviors in a broad sense, which may implicate that different OXTR gene genotypes underlie distinct patterns of physiological activations in reaction to a wide range of social-related stimuli. It is worth noting that in this experiment males’ physiological responses to distressing situations, for both HR and nose temperature, were more affected by their reported paternal bonding, rather than maternal bonding. Likely, the model of paternal behavior in handling stressful situations is more influential than the maternal model for male responding to distress. However, this difference may also have its basis in distinct internal familial dynamics which were not assessed here. This point calls for additional study. Therefore, genes combined with early social experiences to shape individual development of sensitiveness to distressing stimuli from childhood to adulthood. Our findings reveal a physiological biomarker of the interaction between genetic and experiential contributions to one kind of social sensitivity.
Limitations and future directions
This study had some limitations which point to future directions. First, in subsequent research the sample size should be increased to better account for the unequal allele distribution in the population, and strengthen the power of the interaction effects. Second, individuals’ early interactional patterns with parents were assessed using the PBI questionnaire, which is a retrospective report. Individuals’ retrospective memories are not a direct measure, and they may potentially be biased memories or people’s personal interpretations of events. A viable (but very expensive) way to overcome the issue of the retrospective nature of the PBI would be to undertake a longitudinal study directly assessing both the early interactional patterns with parents in childhood and individuals’ social development in adulthood. Furthermore, we tested males only. In future studies, female responses to distress should also be assessed to investigate the possible differential role of mothers and fathers in boys’ and girls’ social development.
Acknowledgments
All participants in this study are gratefully acknowledged. This research was supported by grants from the FP7 PEOPLE-Marie Curie Career Integration Grants (GA-2013-630166), NTU-SUG Grant (2014–2017), and the Intramural Research Program of the NIH, NICHD.
References
- Ainsworth MDS, Bell SM. Attachment, exploration and separation: illustrated by the behavior of one-year-olds in a strange situation. Child Development. 1970;41(1):49–67. [PubMed] [Google Scholar]
- Arrindell WA, Gerlsma C, Vandereycken W, Hageman WJJM, Daeseleire T. Convergent validity of the dimensions underlying the Parental Bonding Instrument (PBI) and the EMBU. Personality and Individual Differences. 1998;24(3):341–350. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For Better and For Worse. Differential Susceptibility to Environmental Influences. Current Directions In Psychological Science. 2007;16(6):300–304. [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Van Der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bornstein MH. Children’s parents. In: Bornstein MH, Leventhal T, editors. Ecological settings and processes in developmental systems. Volume 4 of the Handbook of child psychology and developmental science. Hoboken, NJ: Wiley; 2015. pp. 55–132. 7e. Editor-in-chief: R M Lerner. [Google Scholar]
- Bowlby J. Attachment and Loss. 2nd. Vol. 1. New York: Basic Books; 1999 [1969]. Attachment. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Natural selective attention: orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne M. Does oxytocin receptor (OXTR) polymorphism (rs2254298) confer “vulnerability” for psychopathology or “differential susceptibility”? Insights from evolution. BMC Medicine. 2012;10(1):38. doi: 10.1186/1741-7015-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Pini S, Gabelloni P, Abelli M, Lari L, Martini C. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34:1506–1514. doi: 10.1016/j.psyneuen.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Dalsant A, Truzzi A, Setoh P, Esposito G. Maternal bonding in childhood moderates autonomic responses to distress stimuli in adult males. Behavioral Brain Research. 2015;292:428–431. doi: 10.1016/j.bbr.2015.06.026. [DOI] [PubMed] [Google Scholar]
- De Pisapia N, Bornstein MH, Rigo P, Esposito G, De Falco S, Venuti P. Gender differences in directional brain responses to infant hunger cries. Neuroreport. 2013;24(3):142–146. doi: 10.1097/WNR.0b013e32835df4fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Hormones and Behavior. 2012;61:359–379. doi: 10.1016/j.yhbeh.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Esposito G, Nakazawa J, Venuti P, Bornstein MH. Perceptions of distress in young children with autism compared to typically developing children: a cultural comparison between Japan and Italy. Research in Developmental Disabilities. 2012;33(4):1059–1067. doi: 10.1016/j.ridd.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. Applied regression analysis, linear models, and related methods. Thousand Oaks, CA, US: Sage Publications; 1997. [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Current Opinion in Neurobiology. 1999;9:128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Furman DJ, Chen MC, Gotlib IH. Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology. 2011;36(6):891–897. doi: 10.1016/j.psyneuen.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley E, Pin S, Spini D, d’Epinay CL, Herrmann F, Michel JP. Association between social relationships and survival of Swiss octogenarians. A five-year prospective, population-based study. Aging Clin Exp Res. 2005;17(5):419–425. doi: 10.1007/BF03324632. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social Relationships and Mortality Risk: A Meta-analytic Review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert FA, Abbott RA, Ploubidis GB, Richards M, Kuh D. Parental practices predict psychological well-being in midlife: life-course associations among women in the 1946 British birth cohort. Psychological Medicine. 2010;40:1507–1518. doi: 10.1017/S0033291709991978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou S, Ebisch S, Aureli T, Bafunno D, Ioannides HA, Merla A. The Autonomic Signature of Guilt in Children: A Thermal Infrared Imaging Study. PLoS ONE. 2013;8(11):e79440. doi: 10.1371/journal.pone.0079440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neuroscience Letter. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthak MK, Chen FS, Kumsta R, Hill LK, Thayer JF, Heinrichs M. Oxytocin receptor gene polymorphism modulates the effects of social support on heart rate variability. Biological Psychology. 2016;117:43–49. doi: 10.1016/j.biopsycho.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothgassner OD, Felnhofer A, Hlavacs H, Beutl L, Palme R, Kryspin-Exner I, Glenk LM. Salivary cortisol and cardiovascular reactivity to a public speaking task in a virtual and real-life environment. Computers in Human Behavior. 2016;62:124–135. [Google Scholar]
- Kuraoka K, Nakamura K. The use of nasal skin temperature measurements in studying emotion in macaque monkeys. Physiology & Behavior. 2011;102:347–355. doi: 10.1016/j.physbeh.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. The missing link: Mothers’ neural response to infant cry related to infant attachment behaviors. Infant Behavior & Development. 2012;35:761–772. doi: 10.1016/j.infbeh.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Molecular Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Manini B, Cardone D, Ebisch SJH, Bafunno D, Aureli T, Merla A. Mom feels what her child feels: thermal signatures of vicarious autonomic response while watching children in a stressful situation. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Furman DJ, Kuruvadi N, Shattuk DV, Joshi SH, Thomason ME. Amygdala responses to salient social cues vary with oxytocin receptor genotype in youth. Neuropsychologia. 2015;79:1–9. doi: 10.1016/j.neuropsychologia.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biological Psychiatry. 2007;61:1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Messina I, Cattaneo L, Venuti P, De Pisapia N, Serra M, Esposito G, Bornstein MH. Frontiers in Psychology. 2015;6:1909. doi: 10.3389/fpsyg.2015.01909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G, Tupling H, Brown LB. A parental bonding instrument. British Journal of Medical Psychology. 1979;152:1–10. [Google Scholar]
- Patton GC, Coffey C, Posterino M, Carlin JB, Wolfe R. Parental “affectionless control” in adolescent depressive disorder. Soc Psychiatry Psychiatr Epidemiol. 2001;36:475–480. doi: 10.1007/s001270170011. [DOI] [PubMed] [Google Scholar]
- Pavlov SV, Reva NV, Loktev KV, Korenyok VV, Aftanas LI. Impact of long-term meditation practice on cardiovascular reactivity during perception and reappraisal of affective images. International Journal of Psychophysiology. 2015;95:363–371. doi: 10.1016/j.ijpsycho.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress. 2002;5(4):259–267. doi: 10.1080/1025389021000037586. [DOI] [PubMed] [Google Scholar]
- Perry BD. Childhood Experience and the Expression of Genetic Potential: What Childhood Neglect Tells Us About Nature and Nurture. Brain and Mind. 2002;3:79–100. [Google Scholar]
- Picardi A, Caroppo E, Fabi E, Proietti S, Di Gennaro G, Martinotti G. Attachment and Parenting in Adult Patients with Anxiety Disorders. Clinical Practice & Epidemiology in Mental Health. 2013;9:157–163. doi: 10.2174/1745017901309010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M. Vantage Sensitivity: Environmental Sensitivity to Positive Experiences as a Function of Genetic Differences. Journal of Personality. 2015 doi: 10.1111/jopy.12218. [DOI] [PubMed] [Google Scholar]
- Quas JA, Hong M, Alkon A, Boyce WT. Dissociations between psychobiologic reactivity and emotional expression in children. Developmental Psychobiology. 2000;37(3):153–175. doi: 10.1002/1098-2302(200011)37:3<153::aid-dev4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Rigby K, Slee PT, Martin G. Implications of inadeguate parental bonding and peer victimization for adolescent mental health. Journal of Adolescence. 2007;30:801–812. doi: 10.1016/j.adolescence.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Rikhye K, Tyrka AR, Kelly MM, Gagne GG, Carpenter LL. Interplay between childhood maltreatment, parental bonding, and gender effects: impact on quality of life. Child Abuse and Neglect. 2008;32:19–34. doi: 10.1016/j.chiabu.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Laso A, Zunzunegui MV, Otero A. The effect of social relationships on survival in elderly residents of anSouthern European community: a cohort study. BMC Geriatrics. 2007;7(19) doi: 10.1186/1471-2318-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable P. What is Adult Attachment? Clinical Social Work Journal. 2008;36(1):21–30. [Google Scholar]
- Stewart AM, Lewis GF, Heilman KJ, Davila MI, Coleman DD, Porges SW. The covariation of acoustic features of infant cries and autonomic state. Physiology & Behavior. 2013;120:203–210. doi: 10.1016/j.physbeh.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescence girls. Psychoneuroendocrinology. 2011;36(1):144–147. doi: 10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuti P, Caria A, Esposito G, de Pisapia N, Bornstein MH, de Falco S. Differential brain responses to cries of infants with autistic disorder and typical development: an fMRI Study. Research in Developmental Disabilities. 2012;33(6):2255–2264. doi: 10.1016/j.ridd.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windhorst DA, Mileva-Seitz VR, Linting MI, Hofman A, Jaddoe VWV, Bakermans-Kranenburg MJ. Differential Susceptibility in a Developmental perspective: DRD4 and Maternal Sensitivity Predicting Externalizing Behavior. Developmental psychobiology. 2014;57(1):35–49. doi: 10.1002/dev.21257. [DOI] [PubMed] [Google Scholar]
- Winston R, Chicot R. The importance of early bonding on the long-term mental health and resilience of children. London Journal of Primary Care. 2016;8(1):12–14. doi: 10.1080/17571472.2015.1133012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Zhi L, Su Y. The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. Journal of Affective Disorders. 2012;138:468–472. doi: 10.1016/j.jad.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biological Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]


