Abstract
Purpose:
The purpose of this study was to estimate the prevalence of blood-borne viral infections (triple H: HBV-hepatitis B virus, HCV-hepatitis C virus, and HIV-human immunodeficiency virus) among cataract patients, sought possible risk associations and discuss feasibility of universal preoperative screening.
Methods:
This prospective, cross-sectional study enrolled consecutive patients of senile cataract. They were screened by immunoassay-based rapid diagnostic card tests for blood-borne viral infections. Positive cases were confirmed with confirmatory ELISA tests. Seropositive patients were enquired about the exposure to possible risk associations for acquiring these infections. Cost of card test per patient was calculated.
Results:
The prevalence of seropositivity for triple H viral infections (HBV, HCV, and HIV) among patients of senile cataract was 5.9% (95% confidence interval [CI]: 5.3–6.6), and HCV was most common viral infection. The dental extraction was most common (54%; 95% CI:48-60) possible risk association. The total cost of primary screening per patient for triple H infections(HBV, HCV, and HIV) was $0.93.
Conclusion:
The prevalence of blood-borne viral infection among cataract patients is high in this area. Awareness of the prevalence of blood-borne viral infections in service area, along with knowledge of rate of accidental exposure and risk of transmission would help to understand cost-effectiveness of universal preoperative screening before cataract surgery.
Keywords: Cataract, cost-analysis, hepatitis b virus, hepatitis c virus, human immunodeficiency virus, prevalence, screening
Cataract surgery is one of the most commonly performed surgical procedures. In India more than 6 million cataract surgeries were done during 2015-16.[1] In the past two decades, cataract surgery rate has gone up to 6.6/1000 population.[2] With the advent of surgical techniques, though topical anesthesia is getting popular, peribulbur anesthesia is most often used.[3,4] The technique of peribulbar anesthesia involves giving mixture of anesthetic drugs in peribulbar space of orbit using syringe. There is risk of accidental needleprick injury during this step. In addition, all techniques of cataract surgery require use of sharp instruments for performing different steps of surgery, thus, a potential risk of sustaining accidental injuries and contracting blood-borne infections.
The blood-borne pathogens that are most commonly involved in occupational transmission are hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV).[5,6] The prevalence of HBV among health-care workers is two to four times higher than of the general population.[7] A general surgeon is estimated to sustain 0.8 injuries/100 h of operating time, resulting in a 6.9% lifetime risk of contracting hepatitis C and a 0.15% lifetime risk of HIV infection.[8] The reported incidence of needlestick injury in eye care in India is 0.07/1000 surgeries.[9] The prevalence of these blood-borne viral infections is on the rise, and the World Health Organization (WHO) reported that 252 million people are infected with HBV and 71 million with HCV.[10] The WHO estimates that HIV has infected 36.7 million people globally.[11] Majority of carriers of these viral diseases are asymptomatic.[6] The presence of viral particles for HBV, HCV, and HIV in aqueous humour has been reported.[12,13,14] Experimental studies have demonstrated risk of passing viral infection(s) during sequential phacoemulsification surgery.[15] However, viability of virion in nonbiological systems, dose to establish infection, and chances of infectivity vary among viruses.[16,17,18] Studies done among patients presenting for ocular surgeries, prevalence varies between 1% and 4% for HBV, 0.5%–6% for HCV, and 0.3 for HIV with wide regional variations.[19,20,21] There is lack of guidelines on the preoperative screening of patients before cataract surgery.
We designed this study with objective to determine the hospital-based prevalence of HBV, HCV, and HIV infections in patients coming for elective cataract surgery in this area; look for possible risk associations for these infection(s) and discuss need and feasibility of universal preoperative screening for viral seropositivity among patients of cataract surgery.
Methods
This prospective, cross-sectional, hospital-based study included consecutive patients of senile cataract (aged 50 years and above) who were tested seropositive for one or more viral infections (HBV, HCV, and/or HIV) during preoperative screening. This study was conducted between June 2015 and May 2017 at department of ophthalmology of medical college situated in Western Haryana, in North India. It was approved by the institutional ethical committee and adhered to the Declaration of Helsinki. Preoperatively cataract patients were subjected to detailed ocular examination and laboratory investigations. All patients signed informed common consent for serological evaluation for HBV, HCV, and HIV. Other laboratory investigations included complete blood count, random blood sugar, and urine examination. The tests were carried out in serology laboratory of department of microbiology of the institute. Tests were carried out by qualified, trained technicians under supervision of microbiologist. Results were interpreted as per the WHO and National AIDS Control Organization (NACO) guidelines for interpretation of rapid diagnostic card tests.[22,23,24,25] Test reports were signed by microbiologists. The serological screening was done by one step immunoassay-based rapid diagnostic card tests for HBV (Hepacard; Diagnostic Enterprises, Parwanoo, India), anti-HCV antibodies (HCV TRI-DOT, Diagnostic Enterprises, Parwanoo, India), and HIV (HIV TRI-DOT, Diagnostic Enterprises, Parwanoo, India). The HBV test card is based on antigen capture or “sandwich technique”, with manufacturers claim to detect 11 subtypes of HBV with 100% sensitivity and 99.4% specificity. HCV test card is based on “flow-through” technology, and have HCV antigens for core, NS3, NS4, and NS5. It has 100% sensitivity and 98.9% specificity. The HIV test cards has 100% sensitivity and 100% specificity and have separate dots for HIV-1 and HIV-2; however, the results were reported as a whole. Patients were provided with pretest information individually before sample collection at Integrated Counselling and test centre. Patients who were seropositive for HIV on primary screening were subjected to re-testing twice, as per the strategy 2A of NACO guidelines.[25] Posttest counseling was offered to both HIV positive and negative patients, and HIV-positive patients were referred to anti-retroviral therapy center for registration and baseline investigations. In case of HBV and HCV positivity confirmation was done by ELISA-based serological tests. Cases positive on confirmatory tests were included in study. An ophthalmologist presented questionnaire about exposure to risk factors to find probable source of acquiring infection. The elicited risk factors included, history of blood transfusion, unprotected sex with multiple partners, receiving injections from unqualified local medical practitioner, history of previous surgery, history of dental extraction, and in case of men shaving from barber. An unlisted response was recorded under heading “others.” The information was recorded in separate pro forma and kept confidential. The case file was stamped with “nursing barrier” remark on the cover to ensure safety of paramedical and medical personnel as well to maintain personal secrecy of patient. All seropositive patients received consultation with physician. The type-specific (HBV, HCV, or HIV) seropositive patients were scheduled for cataract surgery on specific assigned day to prevent “theoretical possibility” of cross-transmission to nonseropositive patients during sequential phacoemulsification. In operation theater, protocols were followed for giving peribulbar anesthesia and for phacoemulsification surgery which included double gloving, use of impervious gown, and eye protection. During surgery, operating surgeon and assistant used special protective kit meant for operating on viral seropositive cases. Disposal of needles and sharp instruments was done as per established institutional policy, which in brief included disposal in puncture-proof containers. We maintain separate tips for phacoemulsification for operating on each seropositive type cases. The tubing and tips are autoclaved twice, once on day after surgery and again on day before next assigned surgery day for seropositive cases.
We obtained price value of each test card from purchase department of institute. The screening cost was calculated in US dollars at conversion rate of 1$=Rs 68.
The demographic details such as age, gender, and address were extracted from the case records. Patients who underwent bilateral cataract surgery during this period were considered as single case for demographic and statistical calculations. The data were entered into Excel sheet (MS Office; Microsoft Corp., USA) as categorical data. The prevalence of the viral infections was reported in percentage. Chi-square test of independence was used for calculating gender-based difference in prevalence. The level of significance was set at <0.05. The statistical calculation was done using OpenEpi (Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version 3.01. www.OpenEpi.com).
Results
A total of 4529 patients, 1848 men (41%) and 2681 (59%) women, underwent cataract surgery between June 2015 and May 2017. Of these, 267 patients were seropositive for one or more blood-borne viral infections. The mean age of seropositive patients was 62 ± 9 years (Range 50–85 years). Seropositivity was significantly higher (P < 0.0001) among men (9.7%; 180 of 1848), compared to women (3.2%; 87 of 2681). The residential address of these patients spread to several neighboring districts [Fig. 1].
Figure 1.
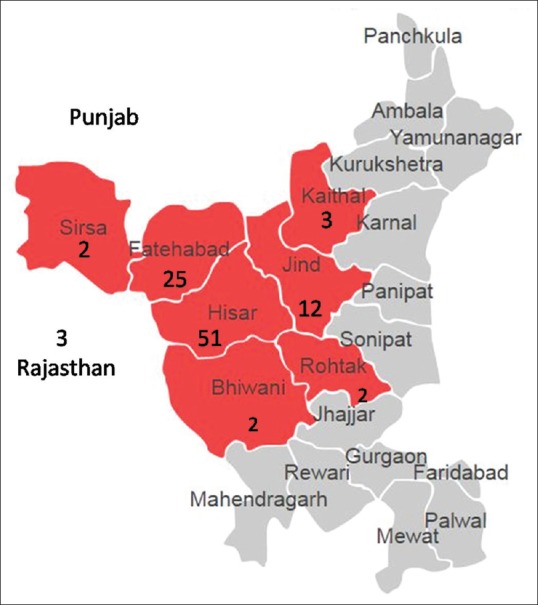
District or residence area of seropositive patients. (The number below district name shows percentage of seropositive patients. These numbers are relative, as proportions of patients coming for any area for the cataract surgery in this institute vary)
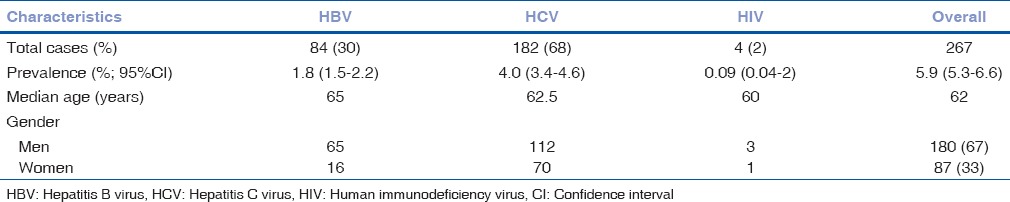
In this study, we found hospital based overall prevalence of seropositivity for triple H viral infections among patients of senile cataract was 5.9% (95% confidence interval [CI]: 5.3–6.6), and HCV was most common viral infection [Table 1]. Only 26 cases (10%; 95% CI: 7–14) were aware about their seropositive status. All the four cases of HIV were aware about their seropositive status, but only two of them revealed it to the treating surgeon before preoperative screening. Two patients (0.7%) had dual infection of HBV and HCV.
Table 1.
Demographic characteristic of seropositive senile cataract patients
Risk association history was reported by 151 (56%; 95% CI: 50–62) patients. History of dental extraction was noted in 145 patients (54%; 95% CI: 48–60). Among males, practice of shaving at saloons was most common risk factor [Table 2].
Table 2.
Frequency of probable risk associations for acquiring viral infection*

The screening cost per card to institute was $ 0.37 for HIV, $ for 0.41 for HCV, and $ 0.15 for HBV. This resulted in $ 0.93 spent per patient for primary screening for viral seropositivity in a patient for cataract surgery. Thus, cost of screening 4529 patients comes to $4212 (4529 × 0.93); and $2106 being spent each year for screening cataract patients for viral markers. The running cost for carrying out the tests, which would be higher, required many considerations in institute beyond scope of this study. The reported incidence of needlestick injury in eye care in India is 0.07/1000 surgeries.[9] This means one needlestick injury would result after 14,286 ocular surgeries. The transmission (seroconversion) risk after percutaneous exposure averages 0.3% for HIV, 1.8% for HCV, and 6%–30% for HBV.[6] This means in case of HIV one seroconversion would occur after 333 exposures. Similarly, one seroconversion would happen after 50 exposures in case of HCV and after 3.33 exposures in HBV. Taking into consideration, the incidence of needlestick injuries among ophthalmologists in India (0.007%) and transmission rate of 0.3% for HIV, one seroconversion would happen when operating on 47,57,238 cases. The cost of screening each patient for HIV in our study was $0.37; this means the cost of screening to foreknow one accidental seroconversion in case of HIV is $17,60,178. In a similar way at prevailing rates of injuries and transmission and current cost of test, the approximate expenditure to foreknow one seroconversion in case of HCV would be $2,92,863 and between $7143 and $35,715 in case of HBV depending on the prevalence. The accumulative cost to foreknow one accidental seroconversion comes to $17,10,939. We could not estimate cost involved to foreknow transmission in sequential phacoemulsification as still data on the rate of transmission is not available in literature.
Discussion
In our study, the overall prevalence of triple H viral infections was 5.9% among cataract patients. In similar studies from India and Pakistan, viral seroprevalence ranged between 4% and 16% among cataract patients.[19,20,26,27] Average estimated prevalence of HBV, HCV, and HIV in the general population in India is 3%–4%, 0.094%–15%, and 0.3%, respectively, with regional variations.[28,29] The overall prevalence of seropositivity in our study is comparable to national prevalence [Fig. 2]. In our study population, the prevalence of HCV was higher than the general population in India. In community-based study done in this region, the prevalence of HCV was 22%.[30] Authors attributed high HCV prevalence rate to practice of using unsterilized needles, syringes, and equipment by local private practitioners. In a study by Sood et al., HCV (5.2%) was most prevalent while in another Indian study on 560 patients for ocular surgeries HBV (3.92%) was predominant infection.[20,31] Awareness and knowledge of specific pockets of high prevalence of a virus, such as HCV in this area in our study, is relevant to ophthalmologist (or health-care professional) to allow selective screening.
Figure 2.
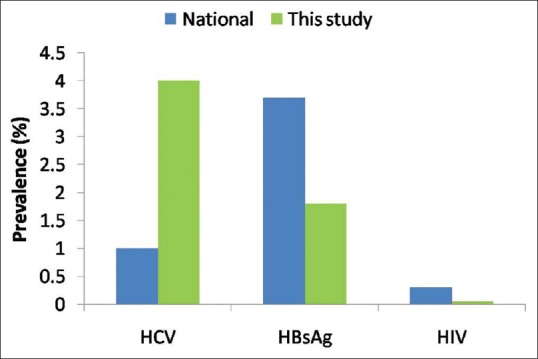
Comparison of the prevalence of viral infections among cataract patients in this study with population-based national data
In many studies, higher HCV positivity is noticed in the age group of 41–60 years, perhaps attributable to indulgence of this age group in more risky behavior and long latency of HCV.[31,32] The same age group (40–60 years) was most commonly affected in studies involving patients for ocular surgeries, including the present study.[20] The reason could be that this age group patients constitute most patients attending ophthalmology outpatient's department.
We used rapid diagnostic cards for primary screening in our study because they provide an easy, quick, and affordable mean for screening with reasonably good sensitivity and specificity – HBV (sensitivity 99%, specificity 95.5%–99.4%), HCV (sensitivity 95.5%–99.4%, specificity-97.1%–100%), and HIV (sensitivity 100%, specificity 99.4%–100%).[22,23,24]
Seropositive patients’ poses risk of transmission to persons involved in medical care by accident prick injuries or through contact of body fluids. The odds of prick injuries among ophthalmologists were third highest among surgical procedures in the study by Mele et al.[33] The chances of viral transmission in sequential cataract surgery in humans are not known, though viral transmission has been demonstrated during sequential phacoemulsification in experimental studies.[15] There exists no clinical study or published case report (s) on disease transmission through ocular fluids or phacoemulsification. However, in some cases, the presence of HBV and HCV has been reported in aqueous humour.[12,13,14] Further, viral viability outside body, dose to establish infection, and chances of infectivity vary among viruses.[16,17,18]
Universal testing for all preoperative patients of cataract would add to cost of surgery. The cost-benefit ratio of screening 4529 patients in our study could not be calculated because rate of transmission of these viral infections during cataract surgery is not known. Some of the studies do not justify universal preoperative screening in elective surgical procedures.[34,35,36] Ahmed and Bhattacharya reviewed feasibility of universal preoperative screening for these viral infections in India and did not consider it cost-effective.[36] Universal screening is advocated for the prevalence >1 in 1000 in general population and selective screening for the prevalence <1 in 1000.[36] This way universal screening would be recommendable for HCV and HBV, and selective screening for HIV, if take national average prevalence in consideration. The cost to foreknow one seroconversion in our study was $17,10,939 per accidental exposure. Universal screening of all patients coming for cataract surgery may not be viable economically in all setups. Further, we do not know yet, does preoperative knowledge of seropositive status of patient decreases the incidence of needlestick injuries. In our study, 90% of patients denied any awareness about seropositive status. In our study, 2 out of 4 cases did not reveal their HIV status before preoperative screening test though they were aware about their seropositive status. Since carrier state is asymptomatic in these cases, does this poses a risk for propagation in the absence of screening?
Most of the patients were not only unaware about their seropositive status but also possible source of infection. Although studying actual cause of infection in these patients was beyond the scope of this study, we tried to highlight possible risk associations. Blood transfusion and use of re-useable glass syringes are among the risk factors for HCV epidemiology in India.[28] In our study, we observed that blood transfusion was not a common risk factor. This could be due to improved transfusion practices and awareness among donors. Seroprevalence of HBV, HCV, and HIV was 1.7%, 1.0%, and 0.3%, respectively, among blood donors.[37,38] History of dental extraction, shaving at saloon, and history of taking injections from local medical practitioners were important risk associations. In a study by Verma et al., history of injection from the local practitioner and dental treatment were two most common risk factors for HCV infection.[30] Approximately 60%–90% of total injections administered in India are estimated to be unsafe.[28] Although no exact data is available, it is estimated that 70% of health-care providers in rural India receive no structured or formal training for practicing medicine.[39] There is no data available on their practice pattern on safe use of syringes, asepsis, and awareness about the fact that infections can transmit through reuse of syringes. Similarly, shaving at saloon has been recognized as potential, possible source of transmission of viral infection.[40,41] Among barbers, either level of awareness is low, or practice pattern is poor, posing risk of transmission.[42,43]
Several limitations of this study must be considered. This is a single-center study and included patients from limited geographical area; hence, seroprevalence and its pattern may not be representative. Larger multicenter study would be needed to know distribution of types of seropositive cases in different regions. The calculation of cost-benefit analysis of universal screening would have been useful but could not be done due to lack of data on viral transmission in ophthalmic practice.
Conclusion
Our study found the prevalence of blood-borne viral infections, mainly HCV, among cataract patients is high in this area. Sensitization of ophthalmologist to be aware about pattern of seroprevalence of viral infections in their practice area could help in observing safeguards against accidental injury and transmission. Choosing between practices of observing universal precautions versus universal preoperative screening of patients undergoing cataract surgery require further studies. Currently, there is lack of data on how ophthalmologists screen for viral seropositivity in their patients preoperatively before cataract surgery as well studies on safeguards being observed during sequential cataract surgeries to prevent accidental exposure and transmission among patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.National Program for control of blindness. State wise targets and achievements for various eye diseases during 2015-16. Available at http://npcb.nic.in/writereaddata/mainlinkfile/File320.pdf .
- 2.Murthy G, Gupta SK, John N, Vashist P. Current status of cataract blindness and vision 2020: The right to sight initiative in India. Indian J Ophthalmol. 2008;56:489–94. doi: 10.4103/0301-4738.42774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik A. Efficacy and performance of various local anesthesia modalities for cataract surgery. J Clin Exp Ophthalmol. 2013;S1:007. [Google Scholar]
- 4.Adekoya BJ, Onakoya AO, Balogun BG, Oworu O. Current practice of ophthalmic anesthesia in Nigeria. Middle East Afr J Ophthalmol. 2013;20:341–4. doi: 10.4103/0974-9233.120022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayanniyi AA, Olatunji FO, Majengbasan T, Ayanniyi RO, Danfulani M. Ophthalmic practice health hazards among ophthalmologists in a resource-limited setting. Asian Pac J Trop Dis. 2011;1:17–20. [Google Scholar]
- 6.Beltrami EM, Williams IT, Shapiro CN, Chamberland ME. Risk and management of blood-borne infections in health care workers. Clin Microbiol Rev. 2000;13:385–407. doi: 10.1128/cmr.13.3.385-407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rele M, Mathur M, Turbadkar D. Risk of needle stick injuries in health care workers-a report. Indian J Med Microbiol. 2002;20:206–7. [PubMed] [Google Scholar]
- 8.Scardino PT. A hazard surgeons need to address. Nat Clin Pract Urol. 2007;4:347. doi: 10.1038/ncpuro0854. [DOI] [PubMed] [Google Scholar]
- 9.Rishi E, Shantha B, Dhami A, Rishi P, Rajapriya HC. Needle stick injuries in a tertiary eye-care hospital: Incidence, management, outcomes, and recommendations. Indian J Ophthalmol. 2017;65:999–1003. doi: 10.4103/ijo.IJO_147_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO Global Hepatitis Report. 2017. Available from: http://www.who.int/mediacentre/news/releases/2017/global-hepatitis-report/en/
- 11.World Health Organization. Global Health Observatory (GHO) Data. [Last accessed on 2017 Oct 18]. Available from: http://www.who.int/gho/hiv/epidemic_status/cases_all/en/
- 12.Temel A, Seber E, Gunay M. Detection of hepatitis B surface antigen in aqueous humor. Acta Ophthalmol (Copenh) 1990;68:205–8. doi: 10.1111/j.1755-3768.1990.tb01905.x. [DOI] [PubMed] [Google Scholar]
- 13.Atas M, Karatepe Hashas AS, Demircan S, Sarıguzel FM, Baskan B, Yuvacı I, et al. The investigation of HCV RNA in tear fluid and aqueous humor in patients with anti-HCV antibody positive who underwent cataract surgery. Ocul Immunol Inflamm. 2016;24:297–301. doi: 10.3109/09273948.2014.985386. [DOI] [PubMed] [Google Scholar]
- 14.Kashiwagi K, Gohdo T, Sato S, Iijima H, Tsukahara S. Detection of HIV-RNA in aqueous humor and subretinal fluid in an HIV carrier with rhegmatogenous retinal detachment. Jpn J Ophthalmol. 2000;44:687–9. doi: 10.1016/s0021-5155(00)00278-1. [DOI] [PubMed] [Google Scholar]
- 15.Coelho RP, Garcia TV, Paula JS, Cruz AA, Rocha EM, Figueiredo LT, et al. Viral contamination during sequential phacoemulsification surgeries in an experimental model. Arq Bras Oftalmol. 2012;75:174–7. doi: 10.1590/s0004-27492012000300005. [DOI] [PubMed] [Google Scholar]
- 16.Ciesek S, Friesland M, Steinmann J, Becker B, Wedemeyer H, Manns MP, et al. How stable is the hepatitis C virus (HCV). Environmental stability of HCV and its susceptibility to chemical biocides? J Infect Dis. 2010;201:1859–66. doi: 10.1086/652803. [DOI] [PubMed] [Google Scholar]
- 17.Abdala N, Stephens PC, Griffith BP, Heimer R. Survival of HIV-1 in syringes. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:73–80. doi: 10.1097/00042560-199901010-00011. [DOI] [PubMed] [Google Scholar]
- 18.Thompson SC, Boughton CR, Dore GJ. Blood-borne viruses and their survival in the environment: Is public concern about community needlestick exposures justified? Aust N Z J Public Health. 2003;27:602–7. doi: 10.1111/j.1467-842x.2003.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 19.Tahir MA, Cheema A, Tareen S. Frequency of hepatitis-B and C in patients undergoing cataract surgery in a tertiary care centre. Pak J Med Sci. 2015;31:895–8. doi: 10.12669/pjms.314.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambastha A, Kusumesh R, Bhasker G. Why should viral markers be mandatory in ocular surgeries: A Hospital based retrospective study. J Clin Diagn Res. 2016;10:LC09–11. doi: 10.7860/JCDR/2016/21386.8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alhassan MB, Unung P, Adejor G. HIV and HBsAg seropositivity amongst patients presenting for ocular surgery at a tertiary eye care hospital in Nigeria. Open Ophthalmol J. 2013;7:18–9. doi: 10.2174/1874364101307010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Last accessed on 2017 Nov 05]. Available from: http://www.who.int/diagnostics_laboratory/evaluations/en/hep_B_rep1.pdf .
- 23. [Last accessed on 2017 Nov 05]. Available from: http://www.apps.who.int/iris/bitstream/10665/66829/1/WHO_BCT_BTS_01.2.pdf .
- 24. [Last accessed on 2017 Nov 05]. Available from: http://www.who.int/diagnostics_laboratory/publications/15032_hiv_assay_report18.pdf?ua=1 .
- 25.National Guidelines for HIV Testing. Published by National AIDS Control Organization, Ministry of Health and Family Welfare, Government of India. 2015:40. [Google Scholar]
- 26.Naeem SS, Siddiqui EU, Kazi AN, Khan S, Abdullah FE, Adhi I, et al. Prevalence of hepatitis ‘B’ and hepatitis ‘C’ among preoperative cataract patients in Karachi. BMC Res Notes. 2012;5:492. doi: 10.1186/1756-0500-5-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohano MK, Su L, Narsani AK, Jawed M, Naveed H. Frequency of hepatitis B surface antigen (HBsAg) and hepatitis C antibody (HCVAb) seropositivity among preoperative eye surgery patients. BJMP. 2016;9:a918. [Google Scholar]
- 28. [Last accessed on 2017 Oct 23]. Available from: http://www.searo.who.int/india/publication/technical_consultation_world_hepatitis-day2014.pdf .
- 29.National HIV Counselling and Testing Services (Hcts) Guidelines. National AIDS Control Organization. [Last accessed on 2017 Oct 24]. Available from: http://www.naco.gov.in/sites/default/files/National%20HIV%20Counselling%20%26%20Testing%20Services%20Guideline,%20Dec%202016.pdf .
- 30.Verma R, Behera BK, Jain RB, Arora V, Chayal V, Gill PS, et al. Hepatitis C, a silent threat to the community of Haryana, India: A community-based study. Australas Med J. 2014;7:11–6. doi: 10.4066/AMJ.2014.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sood A, Sarin SK, Midha V, Hissar S, Sood N, Bansal P, et al. Prevalence of hepatitis C virus in a selected geographical area of Northern India: A population based survey. Indian J Gastroenterol. 2012;31:232–6. doi: 10.1007/s12664-012-0251-8. [DOI] [PubMed] [Google Scholar]
- 32.Singh P, Kaur R, Kaur A. Frequency distribution of hepatitis C virus in different geographical regions of Punjab: Retrospective study from a tertiary care centre in North India. J Nat Sci Biol Med. 2014;5:56–8. doi: 10.4103/0976-9668.127288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mele A, Spada E, Sagliocca L, Ragni P, Tosti ME, Gallo G, et al. Risk of parenterally transmitted hepatitis following exposure to surgery or other invasive procedures: Results from the hepatitis surveillance system in Italy. J Hepatol. 2001;35:284–9. doi: 10.1016/s0168-8278(01)00111-8. [DOI] [PubMed] [Google Scholar]
- 34.Weber P, Eberle J, Bogner JR, Schrimpf F, Jansson V, Huber-Wagner S, et al. Is there a benefit to a routine preoperative screening of infectivity for HIV, hepatitis B and C virus before elective orthopaedic operations? Infection. 2013;41:479–83. doi: 10.1007/s15010-012-0373-z. [DOI] [PubMed] [Google Scholar]
- 35.Winkelmann M, Sorrentino JN, Klein M, Macke C, Mommsen P, Brand S, et al. Is there a benefit for health care workers in testing HIV, HCV and HBV in routine before elective arthroplasty? Orthop Traumatol Surg Res. 2016;102:513–6. doi: 10.1016/j.otsr.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed R, Bhattacharya S. Universal screening versus universal precautions in the context of preoperative screening for HIV, HBV, HCV in India. Indian J Med Microbiol. 2013;31:219–25. doi: 10.4103/0255-0857.115623. [DOI] [PubMed] [Google Scholar]
- 37.Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, et al. Asystematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31(Suppl 2):61–80. doi: 10.1111/j.1478-3231.2011.02540.x. [DOI] [PubMed] [Google Scholar]
- 38.Arora D, Arora B, Khetarpal A. Seroprevalence of HIV, HBV, HCV and syphilis in blood donors in Southern Haryana. Indian J Pathol Microbiol. 2010;53:308–9. doi: 10.4103/0377-4929.64295. [DOI] [PubMed] [Google Scholar]
- 39.Pulla P. Are India's quacks the answer to its shortage of doctors? BMJ. 2016;352:i291. doi: 10.1136/bmj.i291. [DOI] [PubMed] [Google Scholar]
- 40.Khan G, Rizvi TA, Blair I, Adrian TE. Risk of blood-borne infections in barber shops. J Infect Public Health. 2010;3:88–9. doi: 10.1016/j.jiph.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Eroglu C, Zivalioglu M, Esen S, Sunbul M, Leblebicioglu H. Detection of hepatitis B virus in used razor blades by PCR. Hepat Mon. 2010;10:22–5. [PMC free article] [PubMed] [Google Scholar]
- 42.Eltayeb NH, Mudawi HY. Knowledge and practice of barbers regarding transmission of blood-borne viruses in Khartoum state. Ann Trop Med Public Health. 2013;6:80–3. [Google Scholar]
- 43.Jokhio AH, Bhatti TA, Memon S. Knowledge, attitudes and practices of barbers about hepatitis B and C transmission in Hyderabad, Pakistan. East Mediterr Health J. 2010;16:1079–84. [PubMed] [Google Scholar]


