Abstract
A young male presented with diminution of vision left eye, attributable to full-thickness macular hole, and submacular hemorrhage, following closed globe injury 2 weeks ago. The patient was managed successfully with 25-gauge vitrectomy, subretinal injection of tissue plasminogen activator and aspiration of liquefied blood through the macular hole, internal limiting membrane peeling, short-acting gas tamponade, and prone positioning. This resulted in good visual improvement, type 1 macular hole closure, and restoration of foveal architecture. The outcome and rationale of treatment in this unique scenario is discussed.
Keywords: Closed globe injury, full-thickness macular hole, pars plana vitrectomy, subretinal hemorrhage, subretinal tissue plasminogen activator
Closed globe injury is a common cause of full-thickness macular hole (FTMH).[1] Surgical management of posttraumatic FTMHs with vitrectomy results in good anatomical and visual outcomes,[2] especially in the presence of intact optic nerve and retinal pigment epithelium (RPE), which can be affected concurrently due to trauma.[2] The presence of submacular hemorrhage is associated with poor visual outcome.[3] This poor outcome may be attributed to damage to photoreceptors and RPE due to prolonged exposure to blood and its components.[4,5] An underlying choroidal rupture is a known association and may also affect the visual outcome. We describe surgical management of a patient with posttraumatic FTMH associated with submacular hemorrhage of 2 weeks duration. To the best of our knowledge, there are no reports of FTMH with submacular hemorrhage managed with vitrectomy and transmacular hole aspiration of hemorrhage.
Case Report
A 25-year-old male presented with visual decline following closed globe injury left eye, sustained 2 weeks ago, due to a firecracker-related accident. His best-corrected visual acuity (BCVA) was 20/20 and 20/120 in the right and the left eye, respectively. Examination of anterior segment of both eyes and fundus right eye was unremarkable. Fundus examination of the left eye revealed an FTMH and horizontally oval area of subretinal hemorrhage underlying the macular hole and extending temporally [Fig. 1a]. A temporal circumferential choroidal rupture was also noted 1-disc diameters (DD) away from macular hole. Retinal periphery was normal. Swept source optical coherence tomography (SS-OCT) (DRI, Triton Topcon Inc.,) revealed least hole diameter of 656 μ and submacular hemorrhage [Fig. 1b]. Dispersed vitreous hemorrhage was noted inferiorly.
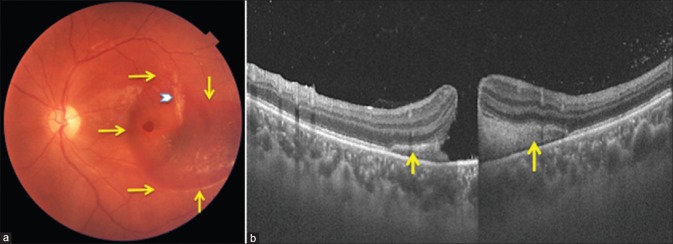
Figure 1.
(a) Color fundus photograph at presentation shows full-thickness macular hole, subretinal hemorrhage extending across the fovea (extent marked by yellow arrows) and temporal choroidal rupture (white chevron). (b) Vertical swept source optical coherence tomography scan through the hole showing macular hole and subretinal bleed (yellow arrows)
After informed consent, the patient underwent standard 3-port 25-gauge pars plana vitrectomy under peribulbar anesthesia. Following core vitrectomy, posterior vitreous detachment was induced using Triamcinolone Acetonide crystals (Aurocort™, Aurolab, Madurai, India). Fluid air exchange was done, and 50 microgram (in 0.05 ml) tissue plasminogen activator (tPA) (Alteplase, Actilyse™, Zydus Cadila, German Remedies, India) was injected into the subretinal space, through the FTMH, using a soft tip cannula. This resulted in shallow retinal detachment at the macula and few clots of hemorrhage evacuated into the vitreous cavity [Fig. 2a]. After 5 min, air was replaced with fluid, and internal limiting membrane (ILM) was peeled after staining with 0.05% brilliant blue G dye (Ocublue Plus™, Aurolab, Madurai, India), using pinch and peel technique with Eckardt type disposable ILM forceps [Fig. 2b]. A large peel of around 2 DDs was performed. After completion of ILM peeling, subretinal hemorrhage was aspirated through the macular hole using soft-tip canula in passive suction mode [Fig. 2c and d]. Any touch with RPE was avoided, and no attempts were made to aspirate blood outside the macula. A repeat fluid air exchange was done, and air was replaced with 25% sulfur hexafluoride. The patient was advised routine postoperative medication and prone position for 72 h.
Figure 2.
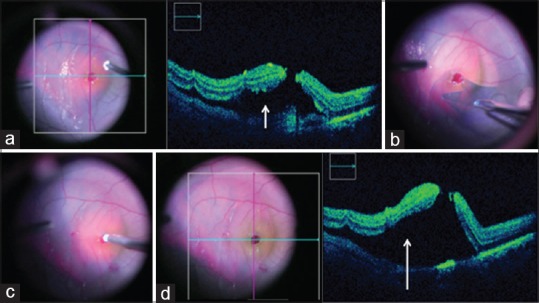
(a) Intraoperative optical coherence tomography after injection of subretinal tissue plasminogen activator through the hole shows induction of shallow retinal detachment (white arrow). (b) Intraoperative color photographs show internal limiting membrane peeling in progress. (c) Aspiration of subretinal blood through the macular hole. (d) Intraoperative optical coherence tomography after aspiration of blood shows absence of subretinal bleed and persisting neurosensory detachment (white arrow)
At 1-week follow-up, BCVA in the left eye improved to 20/80 with Type I closure of the macular hole [Fig. 3a and b]. Ellipsoid zone (EZ) and external limiting membrane (ELM) were discontinuous as seen on SS-OCT. At three months, BCVA improved to 20/30, and EZ and ELM were restored on SS-OCT [Fig. 3c and d]. The patient was advised regular follow-up.
Figure 3.
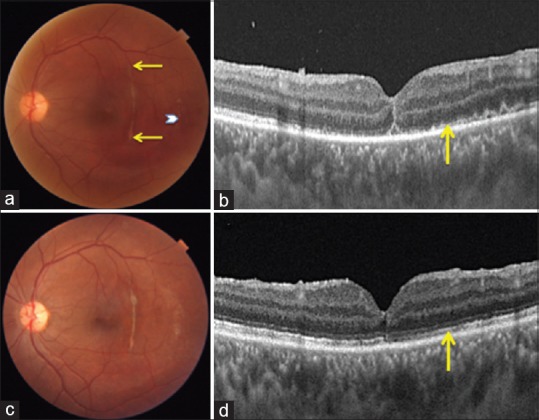
(a) Color photograph at 1 week postoperatively shows closure of macular hole, residual subretinal bleed temporally (white chevron) and full extent of choroidal rupture (ends marked by yellow arrows). (b) Vertical swept source optical coherence tomography scan at 1-week visit confirms Type 1 closure of macular hole and discontinuous ellipsoid zone and external limiting membrane (yellow arrow). (c) Three months postoperative color photograph shows complete resolution of subretinal bleed. (d) Vertical swept source optical coherence tomography scan, congruous to 3b, shows restoration of ellipsoid zone and external limiting membrane (yellow arrow)
Discussion
Continued advancements in techniques and equipment have improved the outcomes in macular hole surgery immensely over the last decade.[6] Similar improvements have occurred in outcomes of traumatic FTMH after surgical repair.[2] However, the presence of traumatic optic neuropathy, RPE changes, and subretinal hemorrhage, frequent accompaniments in posttraumatic holes, continue to be the limiting factors for visual outcome in posttraumatic macular holes.[7,8]
Removal of submacular hemorrhage was the main challenge in the present case. In view of 2 weeks old injury, tPA was used to liquefy the hemorrhage. Performing fluid air exchange before the injection of submacular tPA, allowed gentle injection without damaging the RPE, allowed diffusion of tPA into the subretinal space, and avoided dilution of tPA on admixture with vitreous fluid. Subretinal tPA injection has been found to be safe in the previous studies.[9] Once the ILM peeling was complete, liquefied subretinal hemorrhage could be easily aspirated using soft tip cannula through the macular hole. The use of tPA was further justified as aspiration of clotted blood through the hole could have led to the enlargement of the macular hole. Hemorrhage in the macular area was removed, and no attempts were made to clear the blood away from the macula, so as to avoid any damage to the RPE underlying macular hole.
The procedure resulted in excellent anatomic and visual outcome. Outer retinal layers (EZ and ELM) were seen to be discontinuous at 1-week follow-up. However, at 3 months postoperative, these layers became continuous with concomitant improvement in visual acuity. This has been seen in the previous studies and signifies that retinal architecture and visual improvement may occur till late after macular hole surgery.[10]
Conclusion
This case describes the successful management of posttraumatic FTMH associated with submacular bleed and highlights that subtle change in the surgical plan can provide excellent outcomes in complex surgical situations.
Informed consent
Informed consent was obtained from the participant included in the study for publication of clinical data and photographs.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Ankita Srivastava, M. Optom., Shilky Singh, M. Optom. Dr. Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi - 110 029, India.
References
- 1.Miller JB, Yonekawa Y, Eliott D, Kim IK, Kim LA, Loewenstein JI, et al. Long-term follow-up and outcomes in traumatic macular holes. Am J Ophthalmol. 2015;160:1255–80. doi: 10.1016/j.ajo.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Liu W, Grzybowski A. Current management of traumatic macular holes. J Ophthalmol. 2017;2017:1748135. doi: 10.1155/2017/1748135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrocal MH, Lewis ML, Flynn HW., Jr Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol. 1996;122:486–93. doi: 10.1016/s0002-9394(14)72107-5. [DOI] [PubMed] [Google Scholar]
- 4.Hochman MA, Seery CM, Zarbin MA. Pathophysiology and management of subretinal hemorrhage. Surv Ophthalmol. 1997;42:195–213. doi: 10.1016/s0039-6257(97)00089-1. [DOI] [PubMed] [Google Scholar]
- 5.Benner JD, Hay A, Landers MB, 3rd, Hjelmeland LM, Morse LS. Fibrinolytic-assisted removal of experimental subretinal hemorrhage within seven days reduces outer retinal degeneration. Ophthalmology. 1994;101:672–81. doi: 10.1016/s0161-6420(94)31279-6. [DOI] [PubMed] [Google Scholar]
- 6.Spiteri Cornish K, Lois N, Scott NW, Burr J, Cook J, Boachie C, et al. Vitrectomy with internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole. Ophthalmology. 2014;121:649–55. doi: 10.1016/j.ophtha.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Youssri AI, Young LH. Closed-globe contusion injuries of the posterior segment. Int Ophthalmol Clin. 2002;42:79–86. doi: 10.1097/00004397-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Erdurman FC, Sobaci G, Acikel CH, Ceylan MO, Durukan AH, Hurmeric V, et al. Anatomical and functional outcomes in contusion injuries of posterior segment. Eye (Lond) 2011;25:1050–6. doi: 10.1038/eye.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang W, Garg SJ, Maturi R, Hsu J, Sivalingam A, Gupta SA, et al. Management of thick submacular hemorrhage with subretinal tissue plasminogen activator and pneumatic displacement for age-related macular degeneration. Am J Ophthalmol. 2014;157:1250–7. doi: 10.1016/j.ajo.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Morawski K, Jędrychowska-Jamborska J, Kubicka-Trząska A, Romanowska-Dixon B. The analysis of spontaneous closure mechanisms and regeneration of retinal layers of a full-thickness macular hole: Relationship with visual acuity improvement. Retina. 2016;36:2132–9. doi: 10.1097/IAE.0000000000001074. [DOI] [PubMed] [Google Scholar]