Abstract
Purpose
To determine the normal range of intraocular pressure (IOP) in the young and its association with certain corneal parameters using a non-contact device.
Methods
Subjects were selected from students of Mashhad University of Medical Sciences through stratified sampling. All participants had visual acuity testing, corneal imaging, a comprehensive slit-lamp examination by an ophthalmologist, and IOP measurement using a non-contact air-puff tonometer.
Results
Of the 1280 invitees, 1073 (83.8%) participated, and 1027 were eligible. Mean IOP was 16.38 mmHg [95% confidence interval (CI): 16.22–16.53] in the total sample, 16.14 mmHg (95% CI: 15.84–16.45) in men, and 16.48 mmHg (95% CI: 16.31–16.66) in women. There was a significant IOP difference between myopes and emmetropes (P = 0.031). Based on the multiple linear regression model, IOP associated directly with age and central corneal thickness (CCT), and inversely with corneal diameter, spherical equivalent (SE), and keratoconus. Based on standardized coefficients of the regression model, CCT and SE had the strongest association with IOP.
Conclusions
In the present study, we demonstrated the IOP distribution in a young population using a non-contact method. CCT and SE were strongly associated with IOP.
Keywords: Intraocular pressure, Cross-sectional study, Distribution, Air-puff tonometer
Introduction
Intraocular pressure (IOP) is an ocular index, and high IOP is a major risk factor of glaucoma.1, 2 Associations between increased IOP and older age,3, 4 higher blood pressure,5, 6, 7 myopia,8, 9 and central corneal thickness (CCT)10, 11, 12 have been reported in previous studies. However, results in different races are inconsistent about the association between IOP and age, and some studies have demonstrated a reverse relationship.13, 14, 15 In terms of the relationship between CCT and IOP, although many studies confirm this hypothesis, there are conflicting results that suggest the association may be due to age-related changes.5, 16
As a quantitative continuous variable, it is not easy to set a clear-cut value between normal and abnormal IOP. Knowledge of the normal range and average IOP in different populations is necessary for diagnostic and therapeutic purposes, and several studies have demonstrated the IOP distribution in various races and populations.17, 18, 19
Most previous studies on IOP distribution have shown its normal range in the middle-aged and the elderly, and few studies have reported IOP in younger populations.17, 18, 20 Also, the majority have used Goldmann applanation tonometry (GAT), while non-contact air-puff tonometers are becoming common in clinical practice.21 The latter method tends to overestimate the IOP compared to GAT; nonetheless, their results have shown high agreement. In light of its non-contact approach, it provides a more practical method for population-based studies22 compared to GAT.
In this study, we intend to describe the normal range of IOP in a young population using a non-contact air-puff tonometer. Results of this study can serve as a reference for the Middle East population as well as young populations on the global level. Also, due to the paucity of literature on normal population IOP with non-contact air-puff tonometers, results of this study can be considered for developing nomograms as well.
Methods
The target population of this study was the actively enrolled students of Mashhad University of Medical Sciences in 2013. Subjects were selected through stratified cluster sampling by considering all students in each academic department as a stratum. Stratification was then done based on the students' entrance year. Finally, we used student ID numbers to perform random sampling proportionate to the number of students in each stratum. The Ethics Committee of Mashhad University of Medical Sciences reviewed and approved the study proposal. Before enrollment, all participants signed an informed consent after the study purpose, and its methodology was explained to them.
Selected students were invited to participate in the study, and they were enrolled after signing informed consents. All interviews and examinations were performed at one site. In the interview, which was done first, we collected demographics as well as information regarding history of keratoconus and other ocular pathologies, history of allergies and eye rubbing habits, history of glaucoma and medical or surgical treatment for glaucoma, previous refractive and other ocular surgeries as well as contact lens use. After completing the interview, participants proceeded to the examination stage.
First, uncorrected visual acuity (UCVA) was tested with a Snellen E chart at 6 m. Then objective refraction was done using auto-refraction (Topcon RM8800, Topcon Corporation, Japan), and results were refined through retinoscopy (Heine Beta 200 retinoscope, HEINE Optotechnik, Germany). Participants were then tested subjectively for best distance optical correction, and their best distance visual acuity (BCVA) was determined. All vision and refraction tests were conducted by a skilled optometrist.
Iris color was determined by viewing the participant's non-dilated eye using a penlight. The color was compared to a standard color chart developed based on the standards used in the Beaver Dam Eye Study.23 At the conclusion of these tests, imaging was done using Orbscan II (Bausch & Lomb Inc., Rochester, New York, USA) by a second skilled optometrist following the manufacturer's instructions. Images with acceptable quality were used to extract mean keratometry, corneal diameter (white to white), pupil diameter, anterior chamber depth (ACD), and CCT. In the next stage, all participants had a full slit-lamp examination of the anterior and posterior segments by an ophthalmologist. In examining the posterior segment, a +90 diopter (D) lens was used to inspect the disc size, color, and vascularity, as well as the cup-to-disc ratio.
Finally, IOP was measured without local anesthesia using the Topcon CT-80 (Topcon Corporation, Tokyo, Japan) noncontact tonometer set on automatic alignment. For this purpose, the skilled technician made 3 consecutive measurements, and the average of the 3 was recorded. The process was explained to the participants beforehand to prepare them for the puff of air that would touch their eye. They were instructed to remain relaxed, breathe normally, and undo the collar button if it was tight. The maximum acceptable variation among the 3 readings was 2.0 mmHg, otherwise, measurements were repeated after 10 min.
Exclusion criteria included history of self-reported glaucoma, history of medical or surgical treatment for glaucoma, suspicion of glaucoma based on optic nerve head exam (cup/disc >0.5, cup/disc asymmetry >0.2, neuroretinal rim loss), any ocular pathology affecting IOP and its measurement such as anterior segment inflammation or infections, conditions related to secondary glaucoma such as pigment dispersion syndrome and chronic uveitis, any history of refractive or intraocular surgery, history of ocular trauma, and use of contact lenses.
Statistical analysis performed using the Statistical Package for Social Sciences (SPSS) Version 20.0 (Chicago, IL,USA). IOP data were summarized as mean and 95% confidence intervals (CI). We used histograms and skewness and kurtosis parameters to demonstrate the distribution of IOP in this population. We also determined 50th, 95th, and 99.5th IOP percentiles. Simple and multiple linear regression models were used to examine associations of IOP with other studied parameters. Refractive error was defined based on spherical equivalent (SE); an SE of −1.0D or less was defined as myopia, and SE ≥+1.00D was defined as hyperopia.
Results
Of a total of 6745 students, 1330 individuals were selected, and 1211 (91.5%) of them participated in the study. After applying the exclusion criteria, the study was done using data from 1044 people. The mean age of the participants was 26.1 ± 2.3 years (range, 20–34 years), and 57.1% (n = 596) were female. Due to the high correlation between fellow eyes (IOP Pearson Correlation = 0.852), here we present results from the right eyes only.
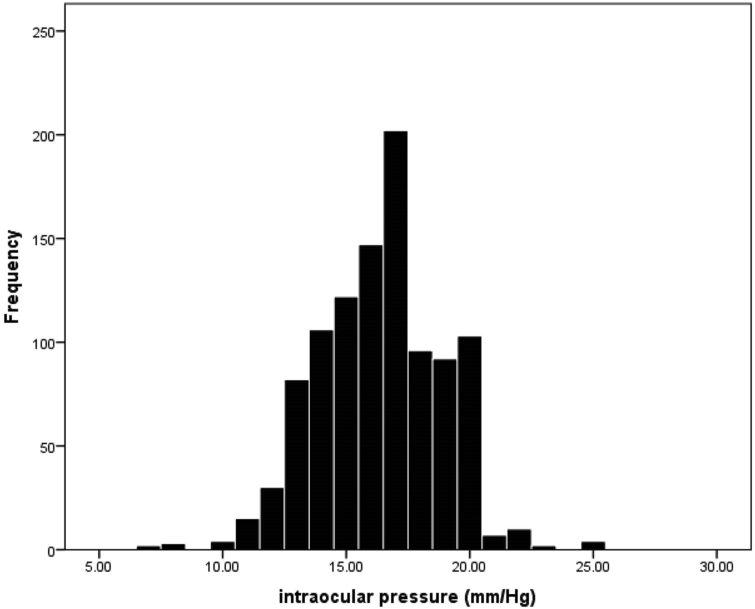
Fig. 1 shows the IOP distribution in the studied population, which was skewed to the left (negative) with a skewness value of −0.095 and a kurtosis index of 0.322. The 25th, 50th, 95th, and 99.5th IOP percentiles were 15.0, 17.0, 20.0, and 23.0 mmHg, respectively. Mean IOP was 16.38 mmHg (95% CI: 16.22–16.53) in the total sample, 16.14 mmHg (95% CI: 15.84–16.45) in men, and 16.48 mmHg (95% CI: 16.31–16.66) in women. IOP was significantly higher in women (P = 0.043).
Fig. 1.
Distribution of intraocular pressure in 20- to 34-year-old individuals.
As demonstrated in Table 1, mean IOP had an age-related ascending trend starting from 16.22 mmHg in the 20–22 year age group to 16.57 mmHg in the over 28 year age group. Linear regression indicated that the age-related increase in IOP was statistically significant (P = 0.046), even after adjusting for gender (P = 0.044). Table 1 also shows the mean IOP by iris color; there was no significant association between iris color and IOP (P = 0.823).
Table 1.
Mean intraocular pressure (IOP) and 95% confidence interval (CI) by age, gender, and iris color.
| n | Mean IOP | SD | 95% CI of the mean |
|||
|---|---|---|---|---|---|---|
| Lower band | Higher band | |||||
| Total | 1044 | 16.38 | 2.52 | 16.22 | 16.53 | |
| Gender | Male | 448 | 16.14 | 2.79 | 15.84 | 16.45 |
| Female | 596 | 16.48 | 2.38 | 16.31 | 16.66 | |
| Age | 20–22 | 170 | 16.22 | 2.59 | 15.84 | 16.61 |
| 23–25 | 292 | 16.08 | 2.51 | 15.78 | 16.37 | |
| 26–28 | 325 | 16.55 | 2.43 | 16.24 | 16.86 | |
| >28 | 257 | 16.57 | 2.53 | 16.30 | 16.85 | |
| Iris color | Blue | 3 | 15.33 | 2.08 | 10.16 | 20.50 |
| Light brown | 194 | 16.22 | 2.61 | 15.85 | 16.59 | |
| Medium brown | 438 | 16.40 | 2.49 | 16.16 | 16.63 | |
| Dark brown | 389 | 16.43 | 2.53 | 16.18 | 16.68 | |
| Green | 19 | 16.53 | 2.20 | 15.47 | 17.58 | |
IOP: Intraocular pressure.
SD: Standard deviation.
Mean IOP was 15.51 mmHg (95% CI: 15.36–16.27) in emmetropic participants, 16.46 mmHg (95% CI: 16.30–16.63) in myopes (range of SE: −1.0D to −14.5D), and 15.36 mmHg (95% CI: 14.15–16.58) in cases with hyperopia (range of SE: +1.0D to +13.25D). Analysis of variance using Scheffe's test revealed significant IOP differences between myopic and emmetropic groups (P = 0.031).
There were 26 cases of keratoconus among the study participants. Mean IOP was 13.11 mmHg (95% CI: 11.63–14.60) in keratoconic eyes and significantly lower than the 16.64 mmHg (95% CI: 16.3–16.6) in non-keratoconic eyes (P < 0.001).
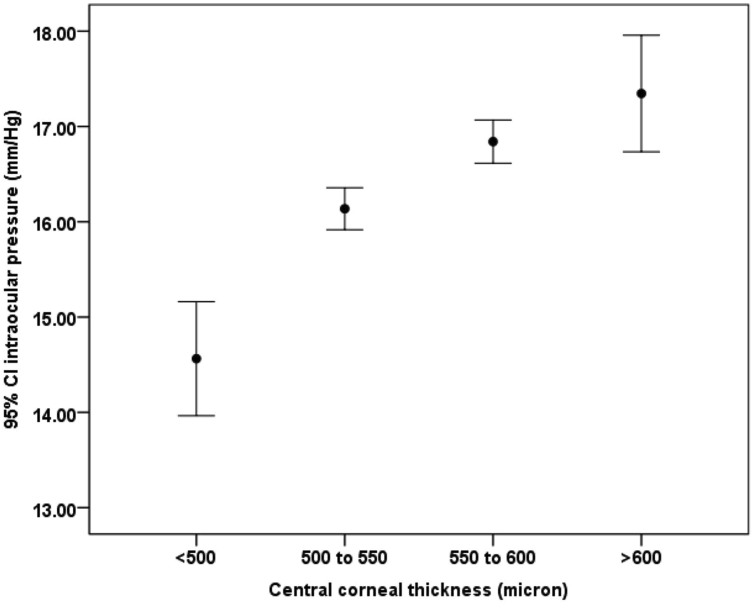
Fig. 2 illustrates the mean IOP in CCT categories. IOP significantly increased at higher CCT; IOP was 14.56 mmHg in the under 500 μm thickness group and increased to 17.35 mmHg in those with a CCT more than 600 μm.
Fig. 2.
Association between intraocular pressure and central corneal thickness.
We used a multiple linear regression model to examine IOP relationships with parameters extracted from Orbscan II (Table 2). IOP showed direct relationships with age and CCT, and inverse associations with corneal diameter, SE, and mean keratometry reading. According to the standard coefficients of this model, IOP related most strongly with CCT followed by SE. In the next model, we added keratoconus as a variable (Table 2); all variables showed a relation similar to the previous model except mean keratometry reading. This model showed a lower IOP in keratoconic cases compared to non-keratoconic eyes after adjusting for age, gender, and other variables.
Table 2.
Association between intraocular pressure and studied variables based on two multiple linear regression models.
| Model 1 |
Model 2 (model 1 + Keratoconus) |
|||||
|---|---|---|---|---|---|---|
| Coefficients (95% CI) | SC | P-value | Coefficients (95% CI) | SC | P-value | |
| WTW (mm) | −0.649 (−1.106–0.192) | −0.102 | 0.005 | −0.48 (−0.941–0.019) | −0.076 | 0.041 |
| PD (mm) | 0.061 (−0.1–0.221) | 0.022 | 0.458 | 0.056 (−0.103–0.215) | 0.021 | 0.492 |
| ACD (mm) | 0.216 (−0.391–0.824) | 0.024 | 0.485 | 0.100 (−0.505–0.706) | 0.011 | 0.745 |
| CCT (micron) | 0.017 (0.013–0.022) | 0.256 | <0.001 | 0.015 (0.011–0.02) | 0.227 | <0.001 |
| Age (year) | 0.052 (0.007–0.097) | 0.068 | 0.023 | 0.046 (0.002–0.091) | 0.060 | 0.042 |
| Gender (male/female) | 0.151 (−0.181–0.482) | 0.028 | 0.372 | 0.106 (−0.224–0.436) | 0.020 | 0.527 |
| SE (diopter) | −0.15 (−0.208–0.093) | −0.155 | <0.001 | −0.152 (−0.209–0.095) | −0.157 | <0.001 |
| Mean-keratometry (diopter) | −0.107 (−0.205–0.01) | −0.075 | 0.031 | −0.036 (−0.139–0.066) | −0.026 | 0.487 |
| Keratoconus (yes/no) | −2.115 (−3.147–1.083) | −0.132 | <0.001 | |||
ACD: Anterior chamber depth.
CCT: Central corneal thickness.
CI: Confidence interval.
PD: Pupil diameter.
SC: Standardized coefficients.
SE: Spherical equivalent.
WTW: White to white.
Discussion
The present study is one of the few studies describing IOP distribution in a young population, as measured with a non-contact device. Non-contact devices are more convenient for the physician and the patient because they require no corneal anesthesia and there is no risk of corneal surface abrasion. Their ease of use also makes them more suitable for epidemiologic studies. Nonetheless, there is a limited number of studies using this method in normal populations.24, 25 While non-contact IOP readings in our young sample can be used as a baseline reference for other studies, lack of similar studies limits our ability to compare our results with those in other populations. In general, non-contact methods tend to overestimate IOP compared to GAT.26, 27, 28, 29 Inter-device differences range between 0.7 mmHg and 3.0 mmHg, depending on the age of the sample population. However, results with these two methods show very high agreement.26, 27, 28
The IOP distribution in this study was skewed to the left while studies in older populations mostly exhibited right skewness.3, 30, 31 Considering the young age range of our sample population, high IOP readings are not expected, and thus, distribution skewness was not unexpected. The 99.5th percentile of IOP was 23.0 mmHg in our study; since previous studies have used GAT, it is not easy to draw valid comparisons.
Mean IOP was statistically significantly higher in women, but the mean inter-gender difference was less than 0.5 mmHg, which seems to be clinically insignificant. On the other hand, the significance was not observed in the final model where other variables were fit. Therefore, our findings showed no significant relation between gender and IOP. Previous studies on this topic are inconclusive as well, and those reporting a significant relation could be biased by confounding factors. In the study by Hashemi et al.,3 mean IOP was higher in women in the univariate analysis, but this was no longer significant after controlling for hypertension and diabetes, and thus, the higher IOP in women was attributed to the higher prevalence of these two conditions in women. Similarly, most studies that used multivariate models to control for confounding factors found that IOP was not gender related.3
Although our sample population was young (range: 20–34 years), age was found to be a risk factor for higher IOP. Most European studies (e.g. the study in Italy4) as well as the Beaver Dam Eye Study,32 the Barbados Eye Study,33 and the Framingham Eye Study showed that IOP increased with age.32 On the contrary, studies in Eastern Asian countries have suggested that IOP decreases with aging.13, 15 Some studies have shown that IOP increases with age up to the 6th decade of life and decreases thereafter. Krzyzanowska-Berkowska et al.34 used a non-contact tonometer in a sample of children and found no significant IOP change between the ages of 5 and 15 years. In the Tehran Eye Study, mean IOP was 0.2 mmHg higher in the 20–30 year age group compared to the 10–20 year age group.3
Refractive errors demonstrated a significant relation with IOP, such that myopes had the highest readings, and this relation was confirmed in the multivariate model. Although the relation between myopia and open angle glaucoma is well known,35 there are contradicting findings regarding the relation of IOP with myopia in normal cases. Some studies found no such relation36, 37 while others, including our study, found them to be related.32, 38, 39 Different theories have been suggested. Some authors attribute the IOP-myopia relation to the longer axial length which causes increased stress on the global wall and reduced ocular rigidity.40 Another hypothesis suggests that since the ciliary body of the myopic eye is in a posterior position relative to the Schlemm's canal, there is less mechanical advantage for opening trabecular meshwork spaces which can lead to raised IOP.41 The third hypothesis is based on the genetic relation between myopia and raised IOP. According to this hypothesis, steroid responsiveness is significantly higher in myopes, especially high myopes, compared to the general population, and genes related to raised IOP, in particular the GLC1A locus on chromosome 1q21-q31, could be overrepresented in myopic cases.42 These are all hypotheses, and each (or a combination of several) can be responsible for the higher IOP among myopes. Other mechanisms may also be involved, and this calls for further research. It should also be noted that the risk of raised IOP increases at higher degrees of myopia.43 Therefore, failure to show a relationship between IOP and myopia in certain studies can be due to their small sample size and the limited number of cases with moderate and high myopia.
In agreement with previous studies,44, 45, 46 CCT directly associated with IOP, and as demonstrated in the results, this relationship was observed after adjusting for variables such as age and keratometry. Even in the multiple model, IOP and CCT had the strongest correlation. The relationship between IOP and CCT has been demonstrated in studies using non-contact methods as well. Some studies attribute the relationship between IOP and age to the CCT; they argue that IOP increases with age in populations that exhibit an age-related increase in corneal thickness, and IOP is lower in populations whose elderly have thinner corneas.5, 16
Our study demonstrated lower IOP readings in cases with smaller corneal diameters. Since this relationship was observed in the multiple model after adjusting for CCT, we cannot attribute it to the association between corneal diameter and CCT. Further studies are needed in this regard.
Based on our findings, keratoconus patients had lower IOP compared to non-keratoconus individuals. Read and Collins rejected any association between IOP and keratoconus47, 48 and the best explanation for the relationship seems to be low corneal biomechanical properties such as the corneal resistance factor and corneal hysteresis in keratoconic eyes.48 This finding should be considered with caution on account of the limited number of keratoconus patients in this study.
The strength of this study is the large sample size and the age range of the participants which makes it one of the few studies addressing IOP in younger populations. In light of the limited information available regarding normal IOP in this age range, our study results can be used for a more accurate interpretations in the young. Also, examining the effect of different demographic and ocular parameters can provide valuable information about the determinants of IOP. Of the limitations of this study, we should first mention the study sample. Since they were all students of a particular university, generalizing results to the general population should be done with caution. Another limitation is lack of GAT IOP readings which is the gold standard method for IOP measurement.
In conclusion, we demonstrated the IOP distribution in a normal young population using a non-contact method. Results of this study can be used as a reference for other studies. CCT and spherical equivalent strongly associated with IOP. In clinical settings, the IOP range in keratoconus patients should be taken into consideration.
Acknowledgement
This research was supported by the Deputy of Research of Mashhad University of Medical Sciences (grant code: 910521).
Footnotes
Funding: This project was supported by Mashhad University of Medical Sciences.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Carbonaro F., Hysi P.G., Fahy S.J., Nag A., Hammond C.J. Optic disc planimetry, corneal hysteresis, central corneal thickness, and intraocular pressure as risk factors for glaucoma. Am J Ophthalmol. 2014;157(2):441–446. doi: 10.1016/j.ajo.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Chauhan B.C., Mikelberg F.S., Artes P.H. Canadian Glaucoma Study: 3. Impact of risk factors and intraocular pressure reduction on the rates of visual field change. Arch Ophthalmol. 2010;128(10):1249. doi: 10.1001/archophthalmol.2010.196. [DOI] [PubMed] [Google Scholar]
- 3.Hashemi H., Kashi A., Fotouhi A., Mohammad K. Distribution of intraocular pressure in healthy Iranian individuals: the Tehran Eye study. Br J Ophthalmol. 2005;89(6):652–657. doi: 10.1136/bjo.2004.058057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonomi L., Marchini G., Marraffa M. Prevalence of glaucoma and intraocular pressure distribution in a defined population: the Egna-Neumarkt Study. Ophthalmology. 1998;105(2):209–215. doi: 10.1016/s0161-6420(98)92665-3. [DOI] [PubMed] [Google Scholar]
- 5.Wong T.T., Wong T.Y., Foster P.J., Crowston J.G., Fong C.-W., Aung T. The relationship of intraocular pressure with age, systolic blood pressure, and central corneal thickness in an Asian population. Investig Ophthalmol Vis Sci. 2009;50(9):4097–4102. doi: 10.1167/iovs.08-2822. [DOI] [PubMed] [Google Scholar]
- 6.Tomoyose E., Higa A., Sakai H. Intraocular pressure and related systemic and ocular biometric factors in a population-based study in Japan: the Kumejima study. Am J Ophthalmol. 2010;150(2):279–286. doi: 10.1016/j.ajo.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Memarzadeh F., Ying-Lai M., Azen S.P., Varma R., Group L.A.L.E.S. Associations with intraocular pressure in Latinos: the Los Angeles Latino eye study. Am J Ophthalmol. 2008;146(1):69–76. doi: 10.1016/j.ajo.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L., Li J., Zheng Y. Intraocular pressure in Northern China in an urban and rural population: the Beijing eye study. Am J Ophthalmol. 2005;140(5):913–915. doi: 10.1016/j.ajo.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 9.Nomura H., Ando F., Niino N., Shimokata H., Miyake Y. The relationship between intraocular pressure and refractive error adjusting for age and central corneal thickness. Ophthalmic Physiol Opt. 2004;24(1):41–45. doi: 10.1046/j.1475-1313.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- 10.Thapa S.S., Paudyal I., Khanal S., Paudel N., Mansberger S.L., van Rens G.H. Central corneal thickness and intraocular pressure in a Nepalese population: the Bhaktapur Glaucoma Study. J Glaucoma. 2012;21(7):481–485. doi: 10.1097/IJG.0b013e3182182c0f. [DOI] [PubMed] [Google Scholar]
- 11.Rao A., Kumar M., Prakash B., Varshney G. Relationship of central corneal thickness and intraocular pressure by iCare rebound tonometer. J Glaucoma. 2014;23(6):380–384. doi: 10.1097/IJG.0b013e318279b819. [DOI] [PubMed] [Google Scholar]
- 12.Wang D., Huang W., Li Y. Intraocular pressure, central corneal thickness, and glaucoma in Chinese adults: the Liwan Eye Study. Am J Ophthalmol. 2011;152(3):454–462. doi: 10.1016/j.ajo.2011.03.005. e451. [DOI] [PubMed] [Google Scholar]
- 13.Nomura H., Ando F., Niino N., Shimokata H., Miyake Y. The relationship between age and intraocular pressure in a Japanese population: the influence of central corneal thickness. Curr eye Res. 2002;24(2):81–85. doi: 10.1076/ceyr.24.2.81.8161. [DOI] [PubMed] [Google Scholar]
- 14.Shiose Y. The aging effect on intraocular pressure in an apparently normal population. Arch Ophthalmol. 1984;102(6):883–887. doi: 10.1001/archopht.1984.01040030703023. [DOI] [PubMed] [Google Scholar]
- 15.Han Y.S., Lee J.W., Lee J.S. Intraocular pressure and influencing systemic health parameters in a Korean population. Indian J Ophthalmol. 2014;62(3):305. doi: 10.4103/0301-4738.116453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonnu P., Ho T., Newson T. The influence of central corneal thickness and age on intraocular pressure measured by pneumotonometry, non-contact tonometry, the Tono-Pen XL, and Goldmann applanation tonometry. Br J Ophthalmol. 2005;89(7):851–854. doi: 10.1136/bjo.2004.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoehn R., Mirshahi A., Hoffmann E.M. Distribution of intraocular pressure and its association with ocular features and cardiovascular risk factors: the Gutenberg Health Study. Ophthalmology. 2013;120(5):961–968. doi: 10.1016/j.ophtha.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 18.Sakalar Y.B., Keklikci U., Unlu K., Alakus M.F., Yildirim M., Dag U. Distribution of central corneal thickness and intraocular pressure in a large population of Turkish school children. Ophthalmic Epidemiol. 2012;19(2):83–88. doi: 10.3109/09286586.2011.649227. [DOI] [PubMed] [Google Scholar]
- 19.Park S.-S., Lee E.-H., Jargal G., Paek D., Cho S.-I. The distribution of intraocular pressure and its association with metabolic syndrome in a community. J Prev Med Public Health. 2010;43(2):125–130. doi: 10.3961/jpmph.2010.43.2.125. [DOI] [PubMed] [Google Scholar]
- 20.Landers J., Henderson T., Craig J. Distribution and associations of intraocular pressure in indigenous Australians within central Australia: the Central Australian Ocular health study. Clin Exp Ophthalmol. 2011;39(7):607–613. doi: 10.1111/j.1442-9071.2011.02507.x. [DOI] [PubMed] [Google Scholar]
- 21.Farhood Q.K. Comparative evaluation of intraocular pressure with an air-puff tonometer versus a Goldmann applanation tonometer. Clin Ophthalmol (Auckland, NZ) 2013;7:23–27. doi: 10.2147/OPTH.S38418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiose Y., Kitazawa Y., Tsukahara S. Epidemiology of glaucoma in Japan–a nationwide glaucoma survey. Jpn J Ophthalmol. 1990;35(2):133–155. [PubMed] [Google Scholar]
- 23.Klein R., Klein B.E., Jensen S.C., Cruickshanks K.J. The relationship of ocular factors to the incidence and progression of age-related maculopathy. Arch Ophthalmol. 1998;116(4):506–513. doi: 10.1001/archopht.116.4.506. [DOI] [PubMed] [Google Scholar]
- 24.Yaoeda K., Shirakashi M., Fukushima A. Measurement of intraocular pressure using the NT-4000: a new non-contact tonometer equipped with pulse synchronous measurement function. J Glaucoma. 2005;14(3):201–205. doi: 10.1097/01.ijg.0000159120.03747.48. [DOI] [PubMed] [Google Scholar]
- 25.Vernon S., Jones S. Intraocular pressure asymmetry in a population tested with the Pulsair non-contact tonometer. Eye. 1991;5(Pt 6):674–677. doi: 10.1038/eye.1991.124. [DOI] [PubMed] [Google Scholar]
- 26.Hubanova R., Aptel F., Zhou T., Arnol N., Romanet J.-P., Chiquet C. Comparison of intraocular pressure measurements with the Reichert Pt100, the Keeler Pulsair intellipuff portable noncontact tonometers, and Goldmann applanation tonometry. J Glaucoma. 2015;24(5):356–363. doi: 10.1097/01.ijg.0000435776.99193.41. [DOI] [PubMed] [Google Scholar]
- 27.Vincent S.J., Vincent R.A., Shields D., Lee G.A. Comparison of intraocular pressure measurement between rebound, non-contact and Goldmann applanation tonometry in treated glaucoma patients. Clin Exp Ophthalmol. 2012;40(4):e163–e170. doi: 10.1111/j.1442-9071.2011.02670.x. [DOI] [PubMed] [Google Scholar]
- 28.Salim S., Linn D.J., Echols J.R., Netland P. Comparison of intraocular pressure measurements with the portable PT100 noncontact tonometer and Goldmann applanation tonometry. Clin Ophthalmol. 2009;3:341–344. doi: 10.2147/opth.s5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu S., Sheu M., Hsu A. Comparisons of intraocular pressure measurements: Goldmann applanation tonometry, noncontact tonometry, Tono-Pen tonometry, and dynamic contour tonometry. Eye. 2009;23(7):1582–1588. doi: 10.1038/eye.2009.77. [DOI] [PubMed] [Google Scholar]
- 30.Rotchford A.P., Johnson G.J. Glaucoma in Zulus: a population-based cross-sectional survey in a rural district in South Africa. Arch Ophthalmol. 2002;120(4):471–478. doi: 10.1001/archopht.120.4.471. [DOI] [PubMed] [Google Scholar]
- 31.Rahman M.L., Bunce C., Healey P.R. Commingling analyses of central corneal thickness and adjusted intraocular pressure in an older Australian population. Investig Ophthalmol Vis Sci. 2010;51(5):2512–2518. doi: 10.1167/iovs.09-4270. [DOI] [PubMed] [Google Scholar]
- 32.Klein B., Klein R., Linton K. Intraocular pressure in an American community. The Beaver Dam eye study. Investig Ophthalmol Vis Sci. 1992;33(7):2224–2228. [PubMed] [Google Scholar]
- 33.Leske M.C., Connell A.M., Wu S.-Y., Hyman L., Schachat A.P. Distribution of intraocular pressure: the Barbados eye study. Arch Ophthalmol. 1997;115(8):1051–1057. doi: 10.1001/archopht.1997.01100160221012. [DOI] [PubMed] [Google Scholar]
- 34.Krzyżanowska-Berkowska P., Asejczyk-Widlicka M., Pierscionek B. Intraocular pressure in a cohort of healthy eastern European schoolchildren: variations in method and corneal thickness. BMC Ophthalmol. 2012;12(1):61. doi: 10.1186/1471-2415-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S.-J., Lu P., Zhang W.-F., Lu J.-H. High myopia as a risk factor in primary open angle glaucoma. Int J Ophthalmol. 2012;5(6):750. doi: 10.3980/j.issn.2222-3959.2012.06.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee A., Saw S., Gazzard G., Cheng A., Tan D. Intraocular pressure associations with refractive error and axial length in children. Br J Ophthalmol. 2004;88(1):5–7. doi: 10.1136/bjo.88.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yassin S.A., Al-Tamimi E.R. Age, gender and refractive error association with intraocular pressure in healthy Saudi participants: a cross-sectional study. Saudi J Ophthalmol. 2016;30(1):44–48. doi: 10.1016/j.sjopt.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weih L.M., Mukesh B.N., McCarty C.A., Taylor H.R. Association of demographic, familial, medical, and ocular factors with intraocular pressure. Arch Ophthalmol. 2001;119(6):875–880. doi: 10.1001/archopht.119.6.875. [DOI] [PubMed] [Google Scholar]
- 39.Choi J.A., Han K., Park Y.-M., Park C.K. Age-related association of refractive error with intraocular pressure in the Korea National health and Nutrition examination survey. PloS One. 2014;9(11):e111879. doi: 10.1371/journal.pone.0111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid K.L., Li R.W., Edwards M.H., Lew J.K. The expandability of the eye in childhood myopia. Curr eye Res. 2003;26(2):65–71. doi: 10.1076/ceyr.26.2.65.14513. [DOI] [PubMed] [Google Scholar]
- 41.Moses R.A., Grodzki W., Etheridge E.L., Wilson C.D. Schlemm's canal: the effect of intraocular pressure. Investig Ophthalmol Vis Sci. 1981;20(1):61–68. [PubMed] [Google Scholar]
- 42.Mitchell P., Hourihan F., Sandbach J., Wang J.J. The relationship between glaucoma and myopia: the Blue Mountains eye study. Ophthalmology. 1999;106(10):2010–2015. doi: 10.1016/s0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 43.McMonnies C.W. An examination of the relation between intraocular pressure, fundal stretching and myopic pathology. Clin Exp Optometry. 2016;99(2):113–119. doi: 10.1111/cxo.12302. [DOI] [PubMed] [Google Scholar]
- 44.Marjanović I., Kontić D., Hentova-Senćanić P., Marković V., Božić M. Correlation between central corneal thickness and intraocular pressure in various age groups. Srp Arh Celok Lek. 2010;138(5-6):279–286. doi: 10.2298/sarh1006279m. [DOI] [PubMed] [Google Scholar]
- 45.Kniestedt C., Lin S., Choe J. Correlation between intraocular pressure, central corneal thickness, stage of glaucoma, and demographic patient data: prospective analysis of biophysical parameters in tertiary glaucoma practice populations. J Glaucoma. 2006;15(2):91–97. doi: 10.1097/00061198-200604000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Lekskul M., Aimpun P., Nawanopparatskul B. The correlations between Central Corneal Thickness and age, gender, intraocular pressure and refractive error of aged 12-60 years old in rural Thai community. J Med Assoc Thail Chotmaihet thangphaet. 2005;88:S175–S179. [PubMed] [Google Scholar]
- 47.Read S.A., Collins M.J. Intraocular pressure in keratoconus. Acta Ophthalmol. 2011;89(4):358–364. doi: 10.1111/j.1755-3768.2009.01690.x. [DOI] [PubMed] [Google Scholar]
- 48.Gkika M., Labiris G., Giarmoukakis A., Koutsogianni A., Kozobolis V. Evaluation of corneal hysteresis and corneal resistance factor after corneal cross-linking for keratoconus. Graefe's Archive Clin Exp Ophthalmol. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2012;250(4):565–573. doi: 10.1007/s00417-011-1897-0. [DOI] [PubMed] [Google Scholar]