Abstract
Background
The aim of this study was to investigate the reorganization in ipsilesional and contralesional thalamic radiation fibers after unilateral focal thalamic stroke in sensory disturbance patients.
Material/Methods
We recruited 12 patients with acute unilateral thalamic infarction and sensory disturbance and 12 healthy age- and sex-matched controls. All patients underwent diffusion tensor imaging (DTI) and were assessed with National Institutes of Health stroke scale (NIHSS), Barthel index (BI), and paragraph 8 of NIHSS (NIHSS8) at 1 week (W1), 4 weeks (W4), 3 months (M3), and 6 months (M6) after thalamic infraction. The relationship between FA changes and the clinical scores changes were then examined.
Results
NIHSS and NIHSS8 scores decreased while BI scores increased gradually from W1 to M6 in patients, but not in controls. FA values of the patients gradually increased in ipsilesional and contralesional thalamic radiation fibers from W1 to M6. In addition, the FA values in patients were significantly higher at M3 and M6 compared to W1. No significant changes were observed in the controls. Regarding the relationship between FA changes and the clinical scores changes, the FA increases were negatively correlated with NIHSS and NIHSS8 decrease while FA increases were positively correlated with BI increases.
Conclusions
Our results indicate that reorganization occurred after unilateral focal thalamic infarct not only in ipsilesional, but also in contralesional thalamic radiation fibers in patients with sensory disturbance. In addition, the results suggested that the reorganization can support and promote stroke restoration.
MeSH Keywords: Cognitive Reserve, Neurology, Thalamic Diseases
Background
Stroke is a major cause of death and long-term disability, with motor disability and sensory disturbance as the most common sequela in survivors of stroke. Although current therapies for the brain injury caused by stroke are limited, most survivors achieve some degree of functional recovery [1–3]. The recovery mechanism of motor disability and sensory disturbance from stroke has been studied previously. Regeneration of the corticospinal tract (CST) in motor restoration was demonstrated in animal stroke models [4–6]. Studies investigating the mechanism of motor function recovery in stroke patients with diffusion tensor imaging (DTI) showed that the fractional anisotropy (FA) value of ipsilesional CST decreased in the early stage (<~1 month), but increased in the later stage (>~6 months), accompanied with motor function recovery. These studies showing an increase of FA value in the ipsilesional CST suggested that the nerve fiber regeneration in ipsilesional CST contributes to motor recovery [2,7–9]. Moreover, other DTI studies showed that the increase in FA value was also found in the contralesional CST after stroke, accompanied with motor function recovery, which also indicated that the nerve fibers regeneration in the contralesional CST contributed to motor recovery [10–12]. Moreover, it has been shown that sensory disturbance can profoundly affect motor function restoration, and that sensory training can promote the restoration of sensory function and motor function [13–16].
However, there are few studies investigating the mechanism of sensory disturbance recovery after stroke. Sensory disturbance is one of the major and unique symptoms in thalamic stroke patients, and was observed to improve in some patients with acute thalamic stroke [17,18]. However, the mechanism of sensory function recovery after stroke remains largely unknown. Based on the nerve fibers regeneration in both ipsilesional and contralesional CST after stroke contributing to the motor recovery, we hypothesize that the nerve fibers regeneration in both ipsilesional and contralesional thalamic radiation fibers contribute to sensory function recovery after a unilateral focal thalamic stroke. In this study, patients with acute unilateral focal thalamic infarction underwent longitudinal diffusion tensor imaging examination along the time course of sensory restoration.
Material and Methods
Subjects
Patients (ages 18–65 years) with acute unilateral focal thalamic infarction were recruited at the Department of Neurology of the First Affiliated Hospital of Guangxi Medical University. All patients first underwent a brain computed tomography (CT) scan, and then a brain magnetic resonance imaging (MRI) scan within 24 h from symptom onset. Cerebral hemorrhage was excluded in all of the patients, and the acute unilateral focal thalamic infarcts in those patients were demonstrated as a hypointensity lesion constricted in the thalamus on CT images, and a hypointensity lesion on T1 images, as well as a hyperintensity lesion on T2, FLAIR, and diffusion-weighted imaging (DWI) images constricted in the thalamus. All the patients recruited were conscious and had some degree of sensory disturbance in one side of the body and limbs. Patients with severe systemic diseases such as heart, liver, and kidney diseases, as well as with a history of central nervous system diseases such as stroke and multiple sclerosis, were excluded from the study. Age- and sex-matched healthy controls were recruited. All the patients recruited received similar conservative therapy, including antiplatelet, blood pressure, and blood glucose level control treatment. This study was approved by the Medical Ethics Committee of the institution. All participants provided written informed consent.
Data acquisition
Demographic information, including age, sex, and risk factors for stroke such as hypertension, hyperlipidemia, diabetes, smoking, alcohol consumption, and atrial fibrillation were obtained. National Institutes of Health Stroke Scale (NIHSS) and Barthel index (BI) were used to assess the general neurological function and functional independence, respectively. Paragraph 8 of the NIHSS (NIHSS8) was used to assess the degree of sensory disturbance. All patients underwent the neurological function assessment detailed above at 2 h before MRI scans at 1 week (W1), 4 weeks (W4), 3 months (M3), and 6 months (M6) after thalamic infarction.
MRI protocols
All MRI scans were performed using an Achieva 3.0 T MR Scanner (Intera Achieva; Philips, The Netherlands). The baseline scan was in the anteroposterior plane, using a standard head coil. Consecutive slices were acquired in an identical location for all sequences, with a 2-mm slice thickness without a gap. T1-FLAIR with TR/TE=2250/7.7 ms, fast spin-echo T2 with TR/TE=3800/120 ms, and traditional FLAIR with TR/TE/TI=9000/120/2200 ms were used. For DTI, a spin-echo echo planar imaging sequence with TR/TE=10000/115 ms, NEX=2, matrix=128×128, and field of view=24×24 cm2 was used. Diffusion-weighted images were obtained with b=1000 s/mm2 and diffusion-sensitive gradients were applied along 64 gradient directions. In addition, a reference image without diffusion weighting (b=0 s/mm2) was acquired. Acquisitions were repeated 20 times and the results were averaged.
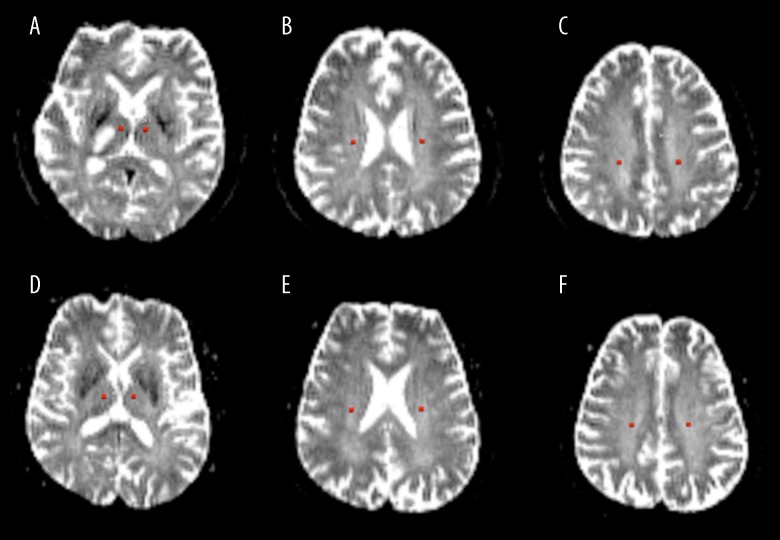
The DTI data were further analyzed with Philips PRIDE software. Regions of interest (ROIs) were defined as a 4-voxel circle, and were placed along the thalamic radiata fiber pathway in both left and right side at 3 positions: the thalamus, corona radiata, and semiovale. The ROIs were carefully placed to avoid the ischemic lesion and thalamic fibers connecting to the lesion (Figure 1). The quantitative MD value derived from the trace of the diffusion tensor (MD=trace[D]/3) and FA value derived from directionality of intravoxel diffusivity were extracted at these regions. The average regional FA and MD values were obtained by averaging in the ROIs.
Figure 1.
Definition of regions of interest (ROIs) were defined a 4-voxel circle on ADC images (b=0). For the patients’ contralesional side (left side of images A–C) and control group (images D–F), ROIs were placed along the thalamic radiata fiber pathway in both left and right side at 3 positions: thalamus, corona radiata, and semiovale. For the patients, the ROIs in the ipsilesional side (right side of images A–C) were carefully placed to avoid the ischemic lesion and the thalamic fibers connecting to the lesion. There was no abnormal signal outside the ischemic lesion in patient images or in control subject images.
Statistical analysis
All statistical analyses were performed using SPSS16.0 (Abacus Concepts, Inc, Chicago, IL), and all data are shown as means ± standard deviations. As the thalamic infarction appeared in either the left or right side in patients, we used the mean FA values and MD from both sides of the control subjects to compare with patients’ DTI quantitative measurements from either the ipsilesional or contralesional side to avoid any possible bias. A 2-sample t test was used first to compare the FA value and MD quantitative data in the regions studied between the patients and the controls. To reveal the progressive effects over time, the DTI parameters of subjects and the clinical scores of patients at each time points were analyzed using 2-way repeated-measures analysis of variance (RMANOVA) with post hoc testing [19,20]. Then, the data between 2 time points were analyzed using the least squared difference (LSD) method with Bonferroni correction. Spearman correlation analysis was used to investigate the association between the absolute value of percent change {[(M6–W1)/W1]×100%} of FA values in the ipsilesional and contralesional thalamic radiatation fibers and the absolute value of percent change of clinical scores, including NIHSS, BI, and NIHSS8. Values of P<0.05 were considered statistically significant.
Results
Subjects
There were 12 patients (3 females and 9 males) and 12 age- and sex-matched controls (3 females and 9 males). The ages of patients and controls (patients: 50.33±7.62 vs. control 51.47±8.43, P>0.05) were not significantly different. Each of the patients had 1 or more vascular factors and sensory disturbance in limbs of one side, and 6 of them had limb weakness. A focal ischemic lesion confined to the thalamus was confirmed in all patients, and there were no lesions elsewhere (Table 1). There were no lesions in the healthy controls.
Table 1.
General patient information.
| No. | Gender | Age (years) | Risk Factors | Lesion site | Major symptoms |
|---|---|---|---|---|---|
| 1 | M | 53 | Diabetes, smoking | Left thalamus | Right limbs sensory disturbance without weakness |
| 2 | M | 57 | Hyperlipidemia | Left thalamus | Right face and limbs sensory disturbance with limbs minor weakness |
| 3 | F | 50 | Hypertension | Right thalamus | Left limbs sensory disturbance with minor weakness |
| 4 | M | 48 | Hypertension, Smoking | Right thalamus | Right face and limbs sensory disturbance with right limbs minor weakness |
| 5 | M | 38 | Hypertension, Drinking | Left thalamus | Right face and limbs sensory disturbance without weakness |
| 6 | M | 43 | Hypertension, Diabetes | Left thalamus | Right limbs sensory loss without weakness |
| 7 | F | 45 | Hyperlipidemia | Left thalamus | Right face and limbs sensory disturbance without weakness |
| 8 | M | 44 | Hyperlipidemia,Smoking | Right thalamus | Left limbs sensory disturbance without weakness |
| 9 | M | 60 | Hyperlipidemia,High homocysteine | Left thalamus | Right face and limbs sensory disturbance with right limbs minor weakness |
| 10 | M | 46 | Hyperlipidemia, Smoking | Right thalamus | Left and limbs sensory disturbance without weakness |
| 11 | M | 62 | High homocysteine | Left thalamus | Right face and limbs sensory loss with right limbs minor weakness |
| 12 | F | 58 | Hypertension, Diabetes | Left thalamus | Right face and limbs sensory loss with right limbs minor weakness |
M6 – six months; M – Male; F – female.
DTI
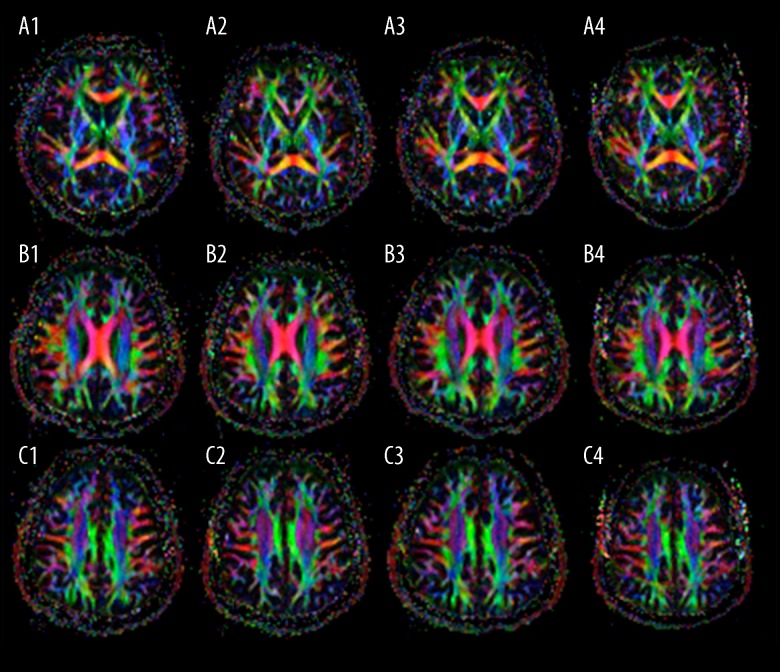
Within the first week from onset, T2-weighted, FLAIR, and DTI images showed a focal hyperintensity signal confined in one side of the thalamus without abnormal signal intensities in other regions. The ischemic lesion had lower FA values and higher MD values. No significant changes were observed in the thalamic radiatation fibers pathway besides the one connecting to the ischemic lesion from W1 to M6 (Figure 2). No significant FA nor MD changes were observed in controls.
Figure 2.
Representative FA pseudo-color pictures from a patient at W1, W4, M3, and M6 time points. Images A–C show the thalamus, corona radiata, and semiovale axial level, respectively. Images A1–A4, B1–B4, and C1–C4 were derived from W1 to M6. There was no abnormal signal outside the ischemic lesion in patient images or in control subject images.
Compared to the controls, the FA values in thalamic radiation fibers, including thalamus, corona radiata, and semiovale for both ipsilesional and contralesional sides, were significantly increased at M3 to M6 time points. The FA values in thalamic radiation fibers, including the thalamus, corona radiata and semiovale, for both ipsilesional and contralesional sides increased gradually from W4 to M6 (Table 2). However, there were no significant MD differences in the thalamic radiata pathway, including the thalamus, corona radiata, and semiovale, for both ipsilesional and contralesional sides when compared to the controls at each time point. In addition, the MD values in the thalamic radiation pathway for both ipsilesional and contralesional sides remained similar from W1 to M6 (Table 3). Both FA and MD in the thalamic radiata pathway in the control group also remained similar over time.
Table 2.
FA values of thalamic radiation fibers at each time point (n=12).
| Regions located in corona radiata pathway | W1 | W4 | M3 | M6 |
|---|---|---|---|---|
| Patients ipsilesinal side | ||||
| Thalamus | 0.42±0.02 | 0.43±0.08 | 0.46±0.071)2)3) | 0.49±0.061)2)3)4) |
| Corona radiata | 0.49±0.05 | 0.50±0.12 | 0.54±0.091)2)3) | 0.58±0.071)2)3)4) |
| Semiovale | 0.36±0.07 | 0.36±0.09 | 0.39±0.101)2)3) | 0.0.42±0.081)2)3)4) |
| Patients contralesional side | ||||
| Thalamus | 0.42±0.03 | 0.43±0.05 | 0.45±0.061)2)3 | 0.48±0.051)2)3)4) |
| Corona radiata | 0.50±0.06 | 0.51±0.11 | 0.53±0.081)2)3 | 0.57±0.051)2)3)4) |
| Semiovale | 0.35±0.06 | 0.36±0.06 | 0.38±0.071)2)3 | 0.41±0.061)2)3)4) |
| Controls | ||||
| Thalamus | 0.42±0.04 | 0.42±0.07 | 0.42±0.09 | 0.43±0.10 |
| Corona radiata | 0.49±0.03 | 0.50±0.09 | 0.50±0.06 | 0.49±0.05 |
| Semiovale | 0.35±0.04 | 0.35±0.06 | 0.36±0.09 | 0.35±0.07 |
W1 – one week; W4 – four weeks; M3 – three months; M6 – six months. The control subjects data of the values MD and FA derived from the mean values from both left and right sides at each location. Two sample t test:
compared to the same time points, controls, P<0.05; MRANOVA:
compared to patients, W1,
compared to patients, W4,
compared to patients, M3, P<0.05 respectively.
Table 3.
MD values of thalamic fiber pathway at each time point (n=12).
| Locations in corona radiata pathway | W1 | W4 | M3 | M6 |
|---|---|---|---|---|
| Patients ipsilesinal side | ||||
| Thalamus | 0.64±0.03 | 0.65±0.02 | 0.65±0.03 | 0.64±0.03 |
| Corona radiata | 0.67±0.02 | 0.68±0.02 | 0.68±0.01 | 0.68±0.01 |
| Semiovale | 0.70±0.02 | 0.73±0.03 | 0.72±0.07 | 0.72±0.03 |
| Patients contralesional side | ||||
| Thalamus | 0.64±0.03 | 0.65±0.03 | 0.63±0.03 | 0.64±0.03 |
| Corona radiata | 0.66±0.04 | 0.67±0.04 | 0.65±0.03 | 0.66±0.02 |
| Semiovale | 0.72±0.03 | 0.73±0.05 | 0.74±0.02 | 0.73±0.03 |
| Controls | ||||
| Thalamus | 0.65±0.02 | 0.66±0.06 | 0.65±0.05 | 0.66±0.04 |
| Corona radiata | 0.66±0.01 | 0.65±0.04 | 0.66±0.03 | 0.66±0.05 |
| Semiovale | 0.71±0.02 | 0.70±0.07 | 0.71±0.04 | 0.71±0.05 |
W1 – one week; W4 – four weeks; M3 – three months; M6 – six months. For controls, The MD and FA values were derived from the mean values from both left and right sides at each location.
Clinical scores
At the onset of the disease, all patients had sensory disturbance in one side of the limbs and/or body. In addition, 6 patients had limb weakness. All patients received standard treatment options for ischemic stroke, consisting of antiplatelet aggregation and antilipemic agent. All patients exhibited good functional recovery. Patients’ NIHSS and NIHSS8 scores decreased while BI scores increased gradually from W1 to M6 (Table 4).
Table 4.
Clinical scores at four time points (n=12).
| W1 | W4 | M3 | M6 | |
|---|---|---|---|---|
| NIHSS | 5.92±3.70 | 3.25±2.491) | 2.00±1.911)2) | 0.83±1.271)2)3) |
| NIHSS8 | 1.42±0.51 | 1.25±0.45 | 0.75±0.451)2) | 0.25±0.451)2)3) |
| BI | 57.08±14.84 | 73.75±16.111) | 86.22±9.951)2) | 95.97±8.541)2)3 |
W1 – one week; W4 – four weeks; M3 – three months; M6 – six months; NIHSS – National Institutes of Health Stroke Scale; NIHSS8 – Paragraph 8 of National Institutes of Health Stroke Scale score; BI – Barthel Index. MRANOVA:
compared to W1,
compared to W4,
compared to M3, P<0.05 respectively.
According to MRANOVA, patients’ NIHSS and NIHSS8 scores decreased, but BI scores increased gradually from W1 to M6. These results indicated that neurological function recovered gradually from W1 to M6 in the patients.
Correlations between clinical scores and DTI parameters
Spearman correlation analysis revealed that the increased percentage of FA values in both ipsilesional and contralesional thalamic radiation fibers were negatively correlated with the decreased percentage of NIHSS and NIHSS8, while they were positively correlated with the increased percentage of BI (Table 5).
Table 5.
Correlations between the percentage changes in the FA value and clinical score (n=12).
| Region of FA value | NIHSS r (P) | NIHSS8 r (P) | BI r (P) |
|---|---|---|---|
| Patients ipsilesinal side | |||
| Thalamus | −0.345 (0.000)1) | −0.209 (0.000)1) | 0.373 (0.001)2) |
| Corona radiata | −0.404 (0.000)1) | −0.369 (0.001)1) | 0.368 (0.011)2) |
| Semiovale | −0.319 (0.000)1) | −0.267 (0.000)1) | 0.424 (0.027)2) |
| Patients contralesional side | |||
| Thalamus | −0.274 (0.062) | −0.136 (0.362) | 0.244 (0.098) |
| Corona radiata | −0.187 (0.000)1) | −0.232 (0.02)1) | 0.267 (0.011)2) |
| Semiovale | −0.294 (0.000)1) | −0.341 (0.000)1) | 0.319 (0.003)2) |
NIHSS – National Institutes of Health Stroke Scale; NIHSS8 – Paragraph 8 of National Institutes of Health Stroke Scale score; BI – Barthel Index. Spearman correlation analysis:
P<0.01;
P<0.05.
Discussion
In this study, the NIHSS and NIHSS8 scores decreased while BI scores increased gradually from W1 to M6 after unilateral focal thalamic stroke in sensory disturbance patients, indicating that neurological sensory and motor functions recovered gradually over time. Further, we explored the recovery mechanism by applying DTI longitudinally. Previous animal studies have shown that neural stem cells in the subventricular zone of the lateral ventricle and the dentate gyrus of the hippocampus can be activated after stroke. These stem cells can proliferate and produce neuroblasts that migrate to the infarcted area [21–23], triggering synaptic plasticity and axon growth [24–26]. These observations have been reported for neurogenesis and nerve fiber generation, and contribute to repair of the infarcted brain as well as neurological recovery. Furthermore, an autopsy study demonstrated neurogenesis in the regions surrounding the affected area within 10 days of acute lethal cerebral infarction [27], indicating that neurogenesis is an important part of human neurological restoration after stroke. However, the detection and monitoring of such neurogenesis and nerve fiber generation in stroke survivors with sensory disturbance have remained largely unexplored.
Diffusion tensor imaging (DTI) uses water molecules as a ubiquitous marker to provide an unprecedented and quantitative capability to probe tissue microstructures noninvasively [28]. It has been extensively used to evaluate microstructural changes in the white matter of the brain in vivo [29,30]. Previous studies using animal models have shown that synaptic plasticity and axonal growth are associated with increases in FA in recovered brain regions after stroke [31,32]. In addition, other DTI studies have shown that a decreased FA accompanied with an unchanged MD in CST can indicate degeneration of white matter at early stages of stroke [33,34], and the degree of FA decrease in CST can be used to predict motor outcome of patients [35–37]. Previous studies also showed that FA was increased in ipsilesional CST after stroke, indicating nerve fiber regeneration in CST [2,7–9]. These FA increases were also associated with neurogenesis, synaptic plasticity and axon growth, and motor function recovery after stroke [2,7–9]. Several other studies showed that FA increased gradually in contralesional CST after a focal ischemic stroke, indicating that regeneration in contralesional CST fibers was positively correlated with motor function recovery after stroke [10,11]. These results show that the mechanism of motor function recovery after stroke involves regeneration in ipsilesional CST and in contralesional CST fibers.
However, the mechanism of sensory function recovery after stroke is still largely unknown. Since the sensory stimulation from peripheral nerves to the sensory cortex is disrupted, sensory disturbance is one of the major and unique symptoms of patients with thalamic stroke [38,39]. In the present study, our results show that compared to the controls, FA values in both ipsilesional and contralesional thalamic radiation fibers increased between M3 and M6 time points. Moreover, the FA values in both ipsilesional and contralesional thalamic radiation fibers increased gradually from W4 to M6. Meanwhile, the MD values in both ipsilesional and contralesional thalamic radiation fibers were unchanged. Thus, our results indicate that after a focal ischemic thalamus stroke, the nerve fiber generation existed not only in ipsilesional but also in contralesional thalamic radiation fibers. Furthermore, the increased percentage of FA values in both ipsilesional and contralesional thalamic radiation fibers was negatively correlated with the decreased percentage of NIHSS and NIHSS8, and positively correlated with the increased percentage of BI. These results indicate that the nerve fiber generation in both ipsilesional and contralesional thalamic radiation fibers support and promote neurological function recovery. Since the function of thalamic radiation fibers is to deliver sensory stimulation from the thalamus to the sensory cortex, and sensory disturbance was the major symptom of the patients in present study, our findings suggest that the nerve fiber generation in both ipsilesional and contralesional thalamic radiation fibers detected with DTI are responsible for the sensory function recovery after thalamic stroke.
Conclusions
This study investigated brain structural changes using DTI and neurological function recovery using functional evaluation. We demonstrated neurological function recovery in patients with significantly increased FA values in both ipsilesional and contralesional thalamic radiation fibers. Furthermore, the percentages of increase of FA values over time in both ipsilesional and contralesional thalamic radiation fibers were correlated with changes in neurological function over time. These results suggest that unilateral focal thalamic stroke induces nerve fiber regeneration not only in ipsilesional but also in contralesional thalamic radiation fibers, and the nerve fiber regeneration supports and promotes neurological function recovery. Since sensory disturbance was the major symptom of our patients, and the function of thalamic radiation fibers is to deliver sensory stimulation from the thalamus to the sensory cortex, our findings strongly suggest that the regenerating thalamic radiation fibers are the main feature of sensory recovery after thalamic stroke, which may benefit clinicians in designing a better treatment scheme for patients with cerebral infarction.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by National Natural Science Foundation of China (NSFC No. 30860088 and 81260186 ), Guangxi Natural Science Foundation (GNFC No. 0832134, 0991149, 2015GXNSFAA139228 and 2016GXNSFAA380281) and National Key R&D Program of China (2017YFC1307500)
References
- 1.Zhang J, Meng L, Qin W, et al. Structural damage and functional reorganization in ipsilesional m1 in well-recovered patients with subcortical stroke. Stroke. 2014;45:788–93. doi: 10.1161/STROKEAHA.113.003425. [DOI] [PubMed] [Google Scholar]
- 2.George E, Heier L, Kovanlikaya I, Greenfield J. Diffusion tensor imaging of pyramidal tract reorganization after pediatric stroke. Childs Nerv Syst. 2014;30:1135–39. doi: 10.1007/s00381-013-2351-x. [DOI] [PubMed] [Google Scholar]
- 3.Chelette KC, Carrico C, Nichols L, Sawaki L. Long-term cortical reorganization following stroke in a single subject with severe motor impairment. Neurorehabilitation. 2013;33:385–89. doi: 10.3233/NRE-130968. [DOI] [PubMed] [Google Scholar]
- 4.Wahl AS, Omlor W, Rubio JC, et al. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344:1250–55. doi: 10.1126/science.1253050. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Chopp M, Ding X, et al. Axonal remodeling of the corticospinal tract in the spinal cord contributes to voluntary motor recovery after stroke in adult mice. Stroke. 2013;44:1951–56. doi: 10.1161/STROKEAHA.113.001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Zhang RL, Li Y, et al. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40:2546–51. doi: 10.1161/STROKEAHA.109.547265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang SH, Byun WM, Han BS, et al. Recovery of a partially damaged corticospinal tract in a patient with intracerebral hemorrhage: A diffusion tensor image study. Restor Neurol Neurosci. 2006;24:25–29. [PubMed] [Google Scholar]
- 8.Jang SH, Kim SH, Cho SH, et al. Demonstration of motor recovery process in a patient with intracerebral hemorrhage. Neurorehabilitation. 2007;22:141–45. [PubMed] [Google Scholar]
- 9.Rong D, Zhang M, Ma Q, et al. Corticospinal tract change during motor recovery in patients with medulla infarct: A diffusion tensor imaging study. Biomed Res Int. 2014:524096. doi: 10.1155/2014/524096. 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaechter JD, Fricker ZP, Perdue KL, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp. 2009;30:3461–74. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang SH, Yi JH, Choi BY, et al. Changes of the corticospinal tract in the unaffected hemisphere in stroke patients: A diffusion tensor imaging study. Somatosens Mot Res. 2016;33:1–7. doi: 10.3109/08990220.2016.1142435. [DOI] [PubMed] [Google Scholar]
- 12.Kwak SY, Yeo SS, Choi BY, et al. Corticospinal tract change in the unaffected hemisphere at the early stage of intracerebral hemorrhage: A diffusion tensor tractography study. Eur Neurol. 2010;63:149–53. doi: 10.1159/000281108. [DOI] [PubMed] [Google Scholar]
- 13.Bolognini N, Russo C, Edwards DJ. The sensory side of post-stroke motor rehabilitation. Restor Neurol Neurosci. 2016;34:571–86. doi: 10.3233/RNN-150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staines WR, Black SE, Graham SJ, McIlroy WE. Somatosensory gating and recovery from stroke involving the thalamus. Stroke. 2002;33:2642–51. doi: 10.1161/01.str.0000032552.40405.40. [DOI] [PubMed] [Google Scholar]
- 15.Lima NM, Menegatti KC, Yu E, et al. Motor and sensory effects of ipsilesional upper extremity hypothermia and contralesional sensory training for chronic stroke patients. Top Stroke Rehabil. 2015;22:44–55. doi: 10.1179/1074935714Z.0000000023. [DOI] [PubMed] [Google Scholar]
- 16.Seo NJ, Kosmopoulos ML, Enders LR, Hur P. Effect of remote sensory noise on hand function post stroke. Front Hum Neurosci. 2014;8:934. doi: 10.3389/fnhum.2014.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohara S, Lenz FA. Reorganization of somatic sensory function in the human thalamus after stroke. Ann Neurol. 2001;50(6):800–3. doi: 10.1002/ana.10041. [DOI] [PubMed] [Google Scholar]
- 18.Ohye C, Shibazaki T, Hirai T, et al. Plastic change of thalamic organization in patients with tremor after stoke. Appl Neurophysiol. 1985;48(1–6):288–92. doi: 10.1159/000101145. [DOI] [PubMed] [Google Scholar]
- 19.Costantino C, Galuppo L, Romiti D. Short-term effect of local muscle vibration treatment versus sham therapy on upper limb in chronic post-stroke patients: A randomized controlled trial. Eur J Phys Rehabil Med. 2017;53(1):32–40. doi: 10.23736/S1973-9087.16.04211-8. [DOI] [PubMed] [Google Scholar]
- 20.Gulde P, Hughes CM, Hermsdörfer J. Effects of stroke on ipsilesional end-effector kinematics in a multi-step activity of daily living. Front Hum Neurosci. 2017;11:42. doi: 10.3389/fnhum.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105(1):33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 22.Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98(8):4710–15. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parent JM, Vexler ZS, Gong C, et al. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52(6):802–13. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 24.Cui X, Chopp M, Shehadah A, et al. Therapeutic benefit of treatment of stroke with simvastatin and human umbilical cord blood cells: neurogenesis, synaptic plasticity, and axon growth. Cell Transplant. 2012;21(5):845–56. doi: 10.3727/096368911X627417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui X, Chopp M, Zacharek A, et al. Niacin treatment of stroke increases synaptic plasticity and axon growth in rats. Stroke. 2010;41(9):2044–49. doi: 10.1161/STROKEAHA.110.589333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Zacharek A, Cui X, et al. Treatment of stroke with a synthetic liver X receptor agonist, TO901317, promotes synaptic plasticity and axonal regeneration in mice. J Cereb Blood Flow Metab. 2010;30(1):102–9. doi: 10.1038/jcbfm.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marti-Fabregas J, Romaguera-Ros M, Gomez-Pinedo U, et al. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology. 2010;74:357–365. doi: 10.5214/ans.0972-7531.1017308. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ann Neurosci. 2010;17:134–35. [Google Scholar]
- 28.Mukherjee P, Berman JI, Chung SW, et al. LDiffusion tensor MR imaging and fiber tractography: Theoretic underpinnings. Am J Neuroradiol. 2008;29(4):632–41. doi: 10.3174/ajnr.A1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu FM, Dai J, Couto TA, et al. Diffusion tensor imaging tractography reveals disrupted white matter structural connectivity network in healthy adults with insomnia symptoms. Front Hum Neurosci. 2017;11:583. doi: 10.3389/fnhum.2017.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makola M, Douglas Ris M, Mahone EM, et al. Long-term effects of radiation therapy on white matter of the corpus callosum: A diffusion tensor imaging study in children. Pediatr Radiol. 2017;47(13):1809–16. doi: 10.1007/s00247-017-3955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Q, Zhang ZG, Ding GL, et al. MRI detects white matter reorganization after eural progenitor cell treatment of stroke. NeuroImage. 2006;32:1080–89. doi: 10.1016/j.neuroimage.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 32.van der Zijden JP, van der Toorn A, van der Marel K, Dijkhuizen RM. Longitudinal in vivo MRI of aleration in perilesional tissue after transient ischemic stroke in rats. Exp Neurol. 2008;212(1):207–12. doi: 10.1016/j.expneurol.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 33.Palacios EM, Martin AJ, Boss MA, et al. Toward precision and reproducibility of diffusion tensor imaging: A multicenter diffusion phantom and traveling volunteer study. Am J Neuroradiol. 2017;38:537–45. doi: 10.3174/ajnr.A5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imura T, Nagasawa Y, Inagawa T, et al. Prediction of motor outcomes and activities of daily living function using diffusion tensor tractography in acute hemiparetic stroke patients. J Phys Ther Sci. 2015;27:1383–86. doi: 10.1589/jpts.27.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nael K, Trouard TP, Lafleur SR, et al. White matter ischemic changes in hyperacute ischemic stroke: voxel-based analysis using diffusion tensor imaging and MR perfusion. Stroke. 2015;46:413–18. doi: 10.1161/STROKEAHA.114.007000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Zhang Y, Xing S, et al. Secondary neurodegeneration in remote regions after focal cerebral infarction: A new target for stroke management? Stroke. 2012;43:1700–5. doi: 10.1161/STROKEAHA.111.632448. [DOI] [PubMed] [Google Scholar]
- 37.Liang Z, Zeng J, Zhang C, et al. Progression of pathological changes in the middle cerebellar peduncle by diffusion tensor imaging correlates with lesser motor gains after pontine infarction. Neurorehabil Neural Repair. 2009;23:692–98. doi: 10.1177/1545968308331142. [DOI] [PubMed] [Google Scholar]
- 38.Barbaud A, Hadjout K, Blard JM, Pagès M. Improvement in essential tremor after pure sensory stroke due to thalamic infarction. Eur Neurol. 2001;46(1):57–59. doi: 10.1159/000050762. [DOI] [PubMed] [Google Scholar]
- 39.Chen CC, Chen JT, Wu ZA, et al. Long latency responses in pure sensory stroke due to thalamic infarction. Acta Neurol Scand. 1998;98(1):41–48. doi: 10.1111/j.1600-0404.1998.tb07376.x. [DOI] [PubMed] [Google Scholar]