Abstract
Background
Increased lipid accumulation in renal tubular epithelial cells (TECs) contributes to their injury and dysfunction and progression of tubulointerstitial fibrosis. Berberine (BBR), a natural plant alkaloid isolated from traditional medicine herbs, is effective in lowing serum lipid, and has a protective effect on chronic kidney disease (CKD) with dyslipidemia, including diabetic nephropathy. The aim of this study was to investigate the effect of BBR on palmitate (PA)-induced lipid accumulation and apoptosis in TECs.
Material/Methods
Human kidney proximal tubular epithelial cell line (HK-2) cells were treated with PA, BBR, and/or palmitoyltransferase 1A (CPT1A) inhibitor Etomoxir. Intracellular lipid content was assessed by Oil Red O and Nile Red staining. Cell apoptosis rate was evaluated by flow cytometry assay. The expression of apoptosis-related protein cleaved-caspase3 and fatty acid oxidation (FAO)-regulating proteins, including CPT1A, peroxisome proliferator-activated receptor α (PPARα), and PPARγ co-activator-1α (PGC1α), was measured by Western blot analysis and immunofluorescence.
Results
In the present study, PA treatment increased intracellular lipid deposition accompanied by elevated apoptosis in TECs compared with control group, whereas the protein expression of CPT1A, PPARα, and PGC1α, did not correspondingly increase in TECs. BBR significantly up-regulated the protein expression of CPT1A, PPARα, and PGC1α in TECs treated with or without PA, and reversed PA-induced intracellular lipid accumulation and apoptosis. Moreover, the CPT1A inhibitor Etomoxir counteracted the protective effect of BBR in TECs.
Conclusions
These in vitro findings suggest that PA can induce intracellular lipid accumulation and apoptosis in TECs, and the mechanism may be associated with inducing defective FAO, whereas BBR can protect TECs against PA-induced intracellular lipid accumulation and apoptosis by promoting FAO.
MeSH Keywords: Apoptosis, Berberine, Fatty Acid Oxidation, Palmitic Acid, Tubular Epithelial Cells
Background
Chronic kidney disease (CKD) is usually accompanied by lipid abnormalities [1–3]. Since the “lipid nephrotoxicity hypothesis” was first proposed by Moorhead in 1982 [4], increasing evidence has supported the hypothesis that filtered non-esterified fatty acid or lipoprotein causes renal epithelial cell injury and promotes the progression of renal disease [5].
Intracellular lipid accumulation has been found in human and mouse kidneys with tubulointerstitial fibrosis [6]. Moreover, lipid accumulation in the kidney leads to structural impairment of tubular epithelial cells (TECs) and associated inflammation and fibrosis in high-fat diet (HFD)-fed mice [7]. These findings demonstrate that lipid accumulation in TECs plays a critical role in tubulointerstitial fibrosis. TECs depend primarily on fatty acid oxidation (FAO) as their energy source. Intracellular lipid accumulation is associated with an imbalance between uptake and oxidation of fatty acids [8]. Moreover, it has been shown that improving FAO protects mice from tubulointerstitial fibrosis [6]. These findings suggest that defective FAO can lead to lipid accumulation in TECs and thus contribute to TEC injury and tubulointerstitial fibrosis.
Berberine (BBR), a natural plant alkaloid originally extracted from Coptis chinensis (“Huanglian” in Chinese), has been commonly used to treat diarrhea for at least a century in China, and its safety has been confirmed [9–11]. In recent years, it has been found that BBR is effective in lowering serum lipid (including serum cholesterol, triglyceride, and LDL-cholesterol), has a significant effect on the treatment of CKD with dyslipidemia (including diabetic nephropathy), and has been widely used in clinical therapy [12,13]. However, the mechanism responsible for the renal-protective effect of BBR has not been completely understood. Some researchers reported that the renal-protective effect of BBR may be associated with anti-oxidative stress and inhibiting aldose reductase in diabetic nephropathy [14]. Recently, to further explore how BBR protects against cell and organ injury caused by dyslipidemia, studies on the effect of BBR on intracellular lipid were performed. It has been shown that BBR can reduce lipid accumulation and promote FAO in liver in db/db mice [15], and can ameliorate PA-induced apoptosis in pancreatic β cells in vitro [16]. However, the specific mechanism has not been well defined, and it remains unclear whether BBR has a similar effect in TECs. In the present study we investigated the effect of BBR on PA-induced lipid accumulation and apoptosis in TECs using an in vitro model and sought to determine the underlying mechanism.
Material and Methods
Cell culture
Human kidney proximal tubular epithelial cell line (HK-2) cells were obtained from the American Type Culture Collection (Manassas, VA, USA). HK-2 cells were cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 100 U/ml penicillin, and 100 mg/ml streptomycin in a humidified 5% CO2 incubator at 37°C. For treatment of HK-2 cells, sodium palmitate (Sigma, USA, P9767) was prepared as a 2.5 mmol/L stock solution, as described previously [17]. For treatment of HK-2 cells, BBR (Sigma, USA) was prepared as a 25 mmol/L stock solution by dissolving it in DMSO, and Etomoxir (Selleck, USA) was prepared as a 50 mmol/L stock solution by dissolving it in DMSO. Cells were treated with sodium palmitate after pretreatment with BBR and/or Etomoxir, and then intracellular lipid accumulation, FAO-related protein expression, and cell apoptosis were assessed.
Flow cytometric assay
Apoptosis was detected using the Annexin V-FITC/propidium iodide (PI) apoptosis assay kit (Sungene Biotech, China). Briefly, treated cells were collected by centrifugation, washed twice with ice-cold PBS, and then resuspended in 500 μl of 1× Annexin V binding buffer containing 5 μl of Annexin V-FITC and 3 μl of PI. After incubation for 10 min at room temperature, the percentage of apoptotic cells was analyzed using a flow cytometer (BD FACSCalibur, Becton-Dickinson, USA). The apoptosis rate of HK-2 cells was calculated as early apoptotic (annexin V+/PI−) rate plus late apoptotic (annexin V+/PI+) rate.
Western blot analysis
Cells were lysed in RIPA lysis buffer (Beyotime, China), sonicated for 15 s, and then centrifuged at 12 000 g for 15 min at 4°C. Then, the protein samples were mixed with loading buffer, heated at 100°C for 5 min, separated by electrophoresis in 10% or 12% tris-glycine polyacrylamide gradient gels, transferred onto PVDF membrane (Millipore, USA), blocked with 5% non-fat milk for 1 h at room temperature, and then incubated overnight at 4°C with different primary antibodies: mouse anti-carnitine palmitoyltransferase 1A (CPT1A, 1: 1000, Abcam, UK), rabbit anti-cleaved-caspase3 (1: 1000, CST, USA), rabbit anti-peroxisome proliferator-activated receptor α (PPARα, 1: 500, Santa Cruz, USA), rabbit anti-PPARγ co-activator-1α (PGC1α, 1: 1000, Novus, USA), or mouse anti-β-actin (1: 5000, Sungene Biotech, China). After washing with TBS-T, the membrane was incubated with goat anti-mouse IgG(H+L) horse-radish peroxidase (1: 8000, MultiSciences, China) or goat anti-rabbit IgG(H+L) horse-radish peroxidase (1: 8000, MultiSciences, China) for 1 h and then incubated with ECL reagent (Advansta, USA). Protein blot images were captured by an imaging densitometer (Fusion FX5, Vilber Lourmat, France), band densitometry was scanned by the Fusion analysis software, and targeted protein expression levels were quantitated relative to β-actin and expressed as fold change (normalized to control group).
Immunofluorescence
Cells were fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X-100 for 10 min, blocked with 5% BSA (Sigma, USA) for 1 h at room temperature, incubated overnight at 4°C with mouse anti-CPT1A antibody (1: 400, Abcam, UK), washed with PBS 3 times, and then incubated with green-fluorescent Alexa Fluor 488 goat anti-mouse IgG antibody (1: 400, Invitrogen, USA) for 1 h. After washing with PBS 3 times, cells were stained with 4, 6-diamidino-2-phenylindole (DAPI; Invitrogen, USA) for 3 min and visualized with a fluorescence microscope (DM4000 B LED, Leica, Germany) at wavelengths of 488 nm for excitation and 525 nm for emission.
Measurement of intracellular lipid content
Intracellular lipid content was measured with Oil Red O and Nile Red staining. Cells were first fixed with 4% paraformaldehyde for 30 min. For Oil Red O staining, cells were stained with Oil Red O (0.18g/ml, Sigma, USA) for 15 min at 37°C, and then counterstained with hematoxylin for 3 min. For Nile Red staining, cells were incubated with Nile Red (0.1 ug/ml, Sigma, USA) for 10 min, and then stained with DAPI for 3 min. Then, cells were visualized under a microscope (DM4000 B LED, Leica, Germany). All fluorescence intensity was quantified using Image J software.
Statistical analysis
Data are presented as mean ±SEM from 3 independent experiments. Analyses were carried out using GraphPad Prism Software version 7.00 (San Diego, CA). Differences among different groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for analysis of differences between groups. The level of statistical significance was defined as p<0.05.
Results
BBR ameliorated PA-induced apoptosis in TECs
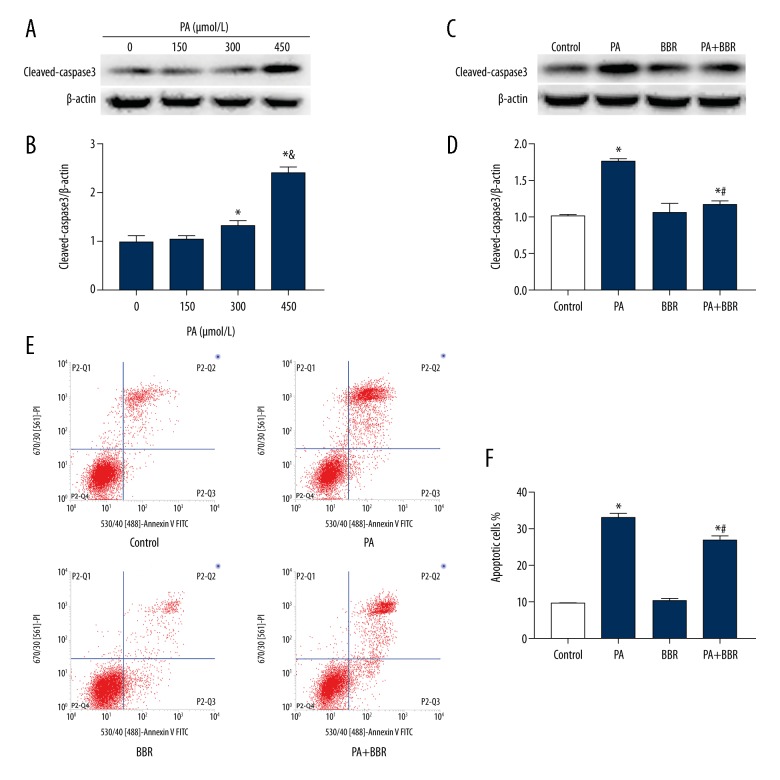
HK-2 cells were treated with different concentration of PA for 24 h, and cleaved-caspase3 protein expression was measured by Western blotting. There was a significant increase in cleaved-caspase3 protein expression in the 300 μmol/L PA group and 450 μmol/L PA group compared with the control group (Figure 1A, 1B). Moreover, cleaved-caspase3 protein expression in the 450 μmol/L PA group was dramatically higher than that in the 300 μmol/L PA group (Figure 1A, 1D). Thus, we chose PA at a concentration of 450 μmol/L to treat HK-2 cells in the following experiments. Using annexin V-FITC/PI staining and flow cytometric analysis, we also confirmed that the exposure of HK-2 cells to 450 μmol/L PA induced obvious apoptosis in TECs (Figure 1E, 1F). In addition, HK-2 cells were incubated with 450 μmol/L PA in the presence or absence of 12.5 μmol/L BBR for 24 h, and BBR treatment ameliorated PA-induced apoptosis (Figure 1C–1F).
Figure 1.
Berberine ameliorated palmitate-induced apoptosis in HK-2 cells. (A) Representative Western blot analyses of cleaved-caspase3 expression in HK-2 cells treated with different concentrations of palmitate for 24 h. (B) Densitometric analysis of cleaved-caspase3 expression in Figure A. (C) Representative Western blot analyses of cleaved-caspase3 protein expression in HK-2 cells treated with or without 450 μmol/L palmitate in the presence or absence of 12.5 μmol/L berberine. (D) Densitometric analysis of cleaved-caspase3 expression in Figure C. (E) Representative cytograms of apoptosis. (F) Quantification of cell apoptosis. All the statistical data are expressed as the mean ±SEM; n=3. * p<0.05 vs. 0 μmol/L or control group, & p<0.05 vs. 300 μmol/L, # p<0.05 vs. PA group.
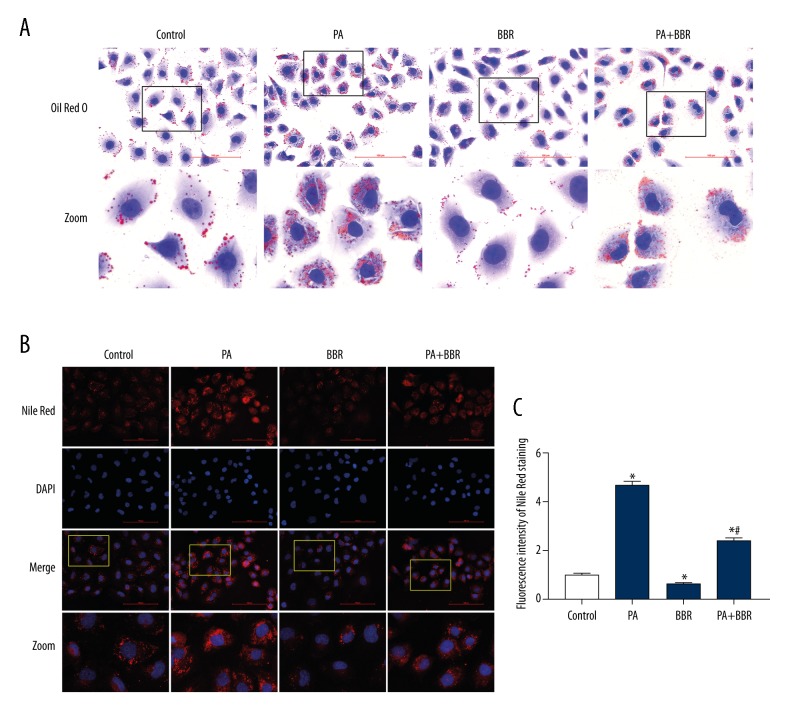
BBR decreased PA-induced lipid accumulation in TECs
Intracellular lipid content was detected with the use of Oil Red O and Nile Red staining. PA induced a significant increase of lipid content in HK-2 cells, whereas BBR significantly reduced PA-induced intracellular lipid accumulation (Figure 2A–2C). Oil Red O can stain neutral triglycerides, and Nile Red can stain varies of neutral lipids including neutral triglycerides and cholesteryl esters. Therefore, increased intracellular lipid induced by PA contained neutral triglycerides.
Figure 2.
Berberine decreased palmitate-induced lipid accumulation in HK-2 cells. Intracellular lipid content was detected by Oil Red O staining (400×) (A) and Nile Red staining (400×) (B). The lower panels show enlarged views of the boxed areas in the upper panels in Figure A. The lowest panels show enlarged views of the boxed areas in the upper panels in Figure B. (C) Fluorescent intensity from 5 randomly selected microscopic fields per group in Figure b was captured and analyzed. The statistical data are expressed as the mean ±SEM; n=3. * p<0.05 vs. control group. # p<0.05 vs. PA group.
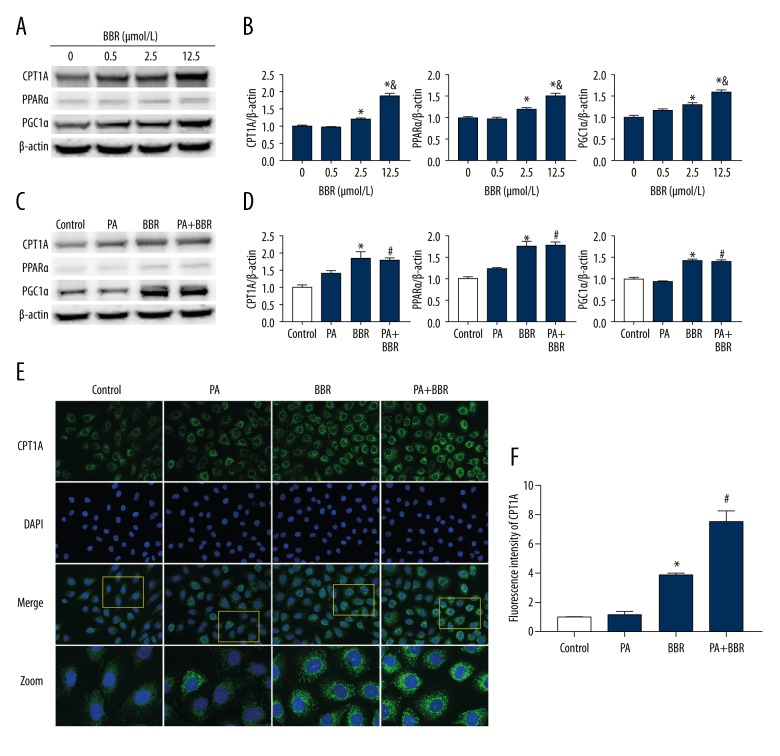
BBR promoted FAO of TECs
To investigate how BBR decreases PA-induced lipid accumulation in TECs, we assessed the expression of FAO-related proteins, including CPT1A, PPARα, and PGC1α, by Western blotting. After treatment with 2.5 μmol/L or 12.5 μmol/L BBR, the expression of each of these 3 proteins was significantly up-regulated compared with the control group, whereas 12.5 μmol/L BBR caused a higher level than that of 2.5 μmol/L BBR (Figure 3A, 3B). We chose BBR at a concentration of 12.5 μmol/L to treat HK-2 cells in the following experiment. Our results showed that BBR promoted the protein expression of CPT1A, PPARα, and PGC1α in the presence or absence of PA treatment (Figure 3C, 3D). Using immunofluorescence staining, we also found that BBR increased the protein expression of CPT1A, the key enzyme in the regulation of FAO, in the presence or absence of PA treatment (Figure 3E, 3F). Taken together, these results suggest that BBR promotes FAO in TECs treated with or without PA. However, there was no statistically significant difference in the protein expression of CPT1A, PPARα, or PGC1α between the PA group and control group (Figure 3C–3F).
Figure 3.
Berberine promoted fatty acid oxidation in HK-2 cells. (A) Representative Western blot analyses of CPT1A, PPARα, and PGC1α protein expression. (B) Densitometric analysis of CPT1A, PPARα, and PGC1α expression in Figure A. (C) Representative Western blot analyses of CPT1A, PPARα, and PGC1α protein expression. (D) Densitometric analysis of CPT1A, PPARα, and PGC1α protein expression in Figure C. (E) Representative immunofluorescence images of CPT1A protein expression (400×). The lowest panels showed enlarged views of the boxed areas in the upper panels. (F) Fluorescence intensities from 5 randomly selected microscopic fields per group in Figure e were measured and analyzed. All the statistical data are presented as the mean ±SEM from 3 independent experiments. * p<0.05 vs. control group. # p<0.05 vs. PA group.
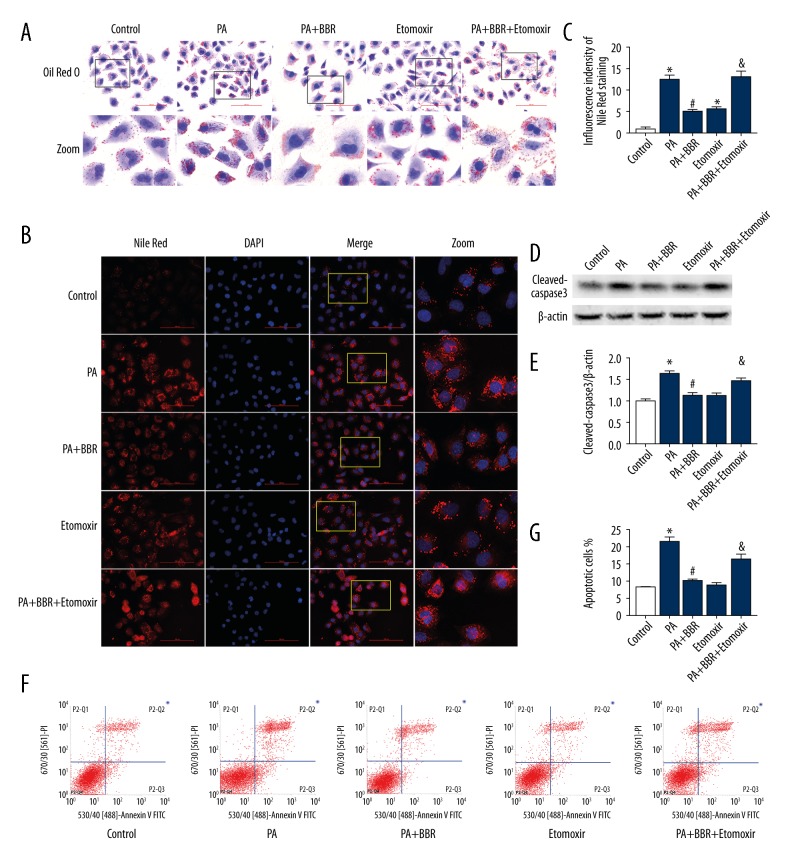
CPT1A inhibitor counteracted the protective effect of BBR against PA-induced lipid accumulation and apoptosis in TECs
To determine whether the protective effect of BBR against PA-induced lipid accumulation and apoptosis is associated with elevating FAO, HK-2 cells were treated with 40 μmol/L CPT1A inhibitor Etomoxir for 48 h. Results showed that Etomoxir induced significantly increased intracellular lipid-droplet formation, accompanied by increased cleaved-caspase3 protein expression and apoptosis in HK-2 cells treated with PA and BBR (Figure 4A–4G), suggesting that inhibition of FAO counteracted the protective effect of BBR.
Figure 4.
CPT1A inhibitor counteracted the protective effect of BBR against PA-induced lipid accumulation and apoptosis in HK-2 cells. HK-2 cells were pretreated with or without 40 μmol/L Etomoxir for 24 h, and then treated with 12.5 μmol/L BBR in the presence or absence of 450 μmol/L PA for 24 h. Intracellular lipid content was detected by Oil Red O staining (400×) (A) and Nile Red staining (400×) (B). The lower panels showed enlarged views of the boxed areas in the upper panels in Figure A. The rightmost panels showed that enlarged views of the boxed areas in the left panels in Figure B. (C) Fluorescent intensity from 5 randomly selected microscopic fields per group in Figure B was captured and analyzed. (D) Representative Western blot analyses of cleaved-caspase3 protein expression. (E) Densitometric analysis of cleaved-caspase3 protein expression in Figure D. (F) Representative cytograms of apoptosis. (G) Quantification of cell apoptosis. All the statistical data are shown as the mean ±SEM, n=3. * p<0.05 vs. control group. # p<0.05 vs. PA group. & p<0.05 vs. PA+BBR group.
Discussion
Clinical and experimental evidence indicates that dyslipidemia contributes to CKD progression by inducing inflammatory, oxidative stress, and endoplasmic reticulum stress, and lipid-lowering agents might protect renal function [18]. Excess lipid, especially triglycerides, beyond that needed for cellular structures and ATP generation is stored as lipid droplets in adipocytes. However, too great of an excess will overburden these cells and cause ectopic deposition of lipids in non-adipose cells such as proximal tubular cells. Lipid droplets are often seen in proximal tubular cells in nephrotic syndrome [19]. When the lipid-storage capacity of non-adipose cells is exceeded, triglycerides are hydrolyzed to produce excess free fatty acids (FFAs), which can increase reactive oxygen species production and endoplasmic reticulum stress, leading to cell dysfunction and injury, collectively termed lipotoxicity [5,20]. Apoptosis is one of major types of cell death, a key and active process to keep tissue homeostasis and to clear potentially harmful cells away in multicellular organisms [21], and inappropriate (either too much or too little) apoptosis is widely involved in the pathophysiology of human diseases [22]. Kinetic experiments using mouse models of focal and segmental glomerulosclerosis showed that apoptosis of TECs is the main cause of tubular atrophy, which has been confirmed as an important predictor of CKD progression [23]. In the present study, we demonstrated that PA treatment increased intracellular lipid deposition, including triglycerides deposition, accompanied by elevated apoptosis in TECs, indicating that excessive intracellular lipid leads to lipotoxicity and therefore induces TECs apoptosis.
Intracellular lipid accumulation is attributed to the imbalance between uptake and oxidation of fatty acids [8]. FAO requires fatty acids to translocate into mitochondria, which is a rate-limited step regulated by CPT1 in the oxidation of long-chain fatty acids [24]. CPT1A, the primary isoform expressed in the kidneys [25], is responsible for transporting acyl CoA into mitochondria [26]. PPARα promotes the protein expression of CPT1A with the cooperation of PGC1α [27], and consequently increases FAO [28]. Recently, it was proposed that the cooperation of PPARα and PGC1α plays a critical role in matching FAO capacity to substrate availability [29]. Therefore, in general, if FFA supply increases, the expression of PPARα and PGC1α will correspondingly increase and thus up-regulate CPT1A expression. Otherwise, the imbalance between high FFA level and relatively low FAO level will induce excessive intracellular lipid accumulation and then cause lipotoxicity, leading to cell dysfunction and apoptosis. In the present study we investigated the protein expression of CPT1A, PPARα, and PGC1α in TECs after PA treatment. However, the expression of key factors regulating FAO did not correspondingly increase, while FFA supply increased remarkably, suggesting that PA induces defective FAO in TECs, which might be responsible for intracellular lipid accumulation and apoptosis. D’Agati et al. found that renal triglyceride accumulation can be caused by defective FAO, which is mediated by PPARα and its target enzymes, including CPT1 [28]. However, the exact mechanism by which high lipid levels induce defective FAO in TECs is still uncertain and is the subject of ongoing investigations.
BBR has recently been demonstrated to have great effects on correcting lipid metabolism disorders [12,13]. In vivo and in vitro experiments suggest BBR can ameliorate PA-induced fat deposition in hepatocytes, and suppress inflammation, oxidative stress, and triglyceride accumulation in the liver of HFD-fed mice, thus inhibiting the progression of hepatic steatosis to steatohepatitis [30–33]. Moreover, it has been shown that BBR protects HFD-induced kidney damage in a mouse model [34], but the underlying mechanism has not been well defined. Our study shows that BBR can effectively protect TECs against PA-induced intracellular lipid accumulation and apoptosis, indicating that BBR protects the kidneys from lipid nephrotoxicity by reducing intracellular lipid accumulation. We further found that BBR greatly elevated CPT1A, PPARα, and PGC1α expressions in TECs. In HFD-fed fish, it is reported that BBR also increased hepatic CPT1A and PPARα mRNA expression, and decreased fat accumulation, alleviating oxidative stress and apoptosis in the liver [35]. We speculated that increasing FAO might be the mechanism underlying the protective effect of BBR against PA-induced intracellular lipid accumulation and apoptosis. As CPT1A is the rate-limiting enzyme for FAO, we further inhibited FAO with the CPT1A inhibitor Etomoxir in the present study, and found that Etomoxir counteracted the protective effect of BBR in TECs. All these results demonstrate that BBR can prevent intracellular lipid accumulation and therefore reduce apoptosis in PA-treated TECs by promoting FAO. In addition, some studies have found that BBR can activate AMP-activated protein kinase (AMPK) in fat and liver cells [14,36]. Moreover, previous studies have demonstrated that activating AMPK inhibits the activity of acetyl CoA carboxylase, and thus reduces malonyl CoA levels, resulting in increased CPT1 expression [37]. However, it remains unclear whether BBR promotes FAO by activating AMPK, which requires further studies in the future.
Conclusions
We have shown that PA can induce intracellular lipid accumulation and apoptosis in TECs, and defective FAO might be the mechanism. In addition, to the best of our knowledge, we provide the first evidence to suggest a new mechanism of BBR action in TECs that involves, sequentially, promoting FAO, lowering PA-induced excess intracellular lipid accumulation, and attenuating apoptosis. However, this was an in vitro study, and further in vivo studies are needed to confirm these results.
Acknowledgements
We are grateful for support from the Chongqing Key Laboratory of Translational Medicine in Major Metabolic Diseases, where our work was done.
Footnotes
Conflicts of interest
None.
Source of support: This work was supported by a grant from the National Natural Science Foundation of China (No. 81370816), grants from the Natural Science Foundation of Chongqing Science and Technology Commission of China (No. cstc2012jjA10136) and the Chongqing Municipal Health Bureau of China (No. 2011-1-016) to X. Du, a grant from the National Natural Science Foundation of China (No. 31171619), and grant from the Natural Science Foundation of Chongqing Science and Technology Commission of China (No. cstc2012jjjq10001) to H. Huang
References
- 1.Keane WF, Tomassini JE, Neff DR. Lipid abnormalities in patients with chronic kidney disease: Implications for the pathophysiology of atherosclerosis. J Atheroscler Thromb. 2013;20:123–33. doi: 10.5551/jat.12849. [DOI] [PubMed] [Google Scholar]
- 2.Pandya V, Rao A, Chaudhary K. Lipid abnormalities in kidney disease and management strategies. World J Nephrol. 2015;4:83–91. doi: 10.5527/wjn.v4.i1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samouilidou EC, Karpouza AP, Kostopoulos V, et al. Lipid abnormalities and oxidized LDL in chronic kidney disease patients on hemodialysis and peritoneal dialysis. Ren Fail. 2012;34:160–64. doi: 10.3109/0886022X.2011.641515. [DOI] [PubMed] [Google Scholar]
- 4.Moorhead JF, Chan MK, El-Nahas M, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2:1309–11. doi: 10.1016/s0140-6736(82)91513-6. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg JM. Lipotoxicity. Kidney Int. 2006;70:1560–66. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 6.Kang HM, Ahn SH, Choi P, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decleves AE, Zolkipli Z, Satriano J, et al. Regulation of lipid accumulation by AMP-activated kinase [corrected] in high fat diet-induced kidney injury. Kidney Int. 2014;85:611–23. doi: 10.1038/ki.2013.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15:805–12. doi: 10.1016/j.cmet.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau CW, Yao XQ, Chen ZY, et al. Cardiovascular actions of berberine. Cardiovasc Drug Rev. 2001;19:234–44. doi: 10.1111/j.1527-3466.2001.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 10.Taylor CE, Greenough WB., 3rd Control of diarrheal diseases. Annu Rev Public Health. 1989;10:221–44. doi: 10.1146/annurev.pu.10.050189.001253. [DOI] [PubMed] [Google Scholar]
- 11.Lan J, Zhao Y, Dong F, et al. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol. 2015;161:69–81. doi: 10.1016/j.jep.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 12.Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Tong Q, Shou JW, et al. Gut microbiota-mediated personalized treatment of hyperlipidemia using berberine. Theranostics. 2017;7:2443–51. doi: 10.7150/thno.18290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni WJ, Ding HH, Tang LQ. Berberine as a promising anti-diabetic nephropathy drug: An analysis of its effects and mechanisms. Eur J Pharmacol. 2015;760:103–12. doi: 10.1016/j.ejphar.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Xue M, Zhang L, Yang MX, et al. Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int J Nanomedicine. 2015;10:5049–57. doi: 10.2147/IJN.S84565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao N, Zhao TY, Li XJ. The protective effect of berberine on beta-cell lipoapoptosis. J Endocrinol Invest. 2011;34:124–30. doi: 10.1007/BF03347042. [DOI] [PubMed] [Google Scholar]
- 17.Pascual G, Avgustinova A, Mejetta S, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 18.Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol. 2009;5:713–21. doi: 10.1038/nrneph.2009.184. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C, Moore L, Yool A, et al. Renal tubular epithelial vacuoles – a marker for both hyperlipidemia and ketoacidosis at autopsy. J Forensic Sci. 2015;60:638–41. doi: 10.1111/1556-4029.12711. [DOI] [PubMed] [Google Scholar]
- 20.Li LO, Klett EL, Coleman RA. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim Biophys Acta. 2010;1801:246–51. doi: 10.1016/j.bbalip.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7(12) doi: 10.1101/cshperspect.a006080. pii: a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schelling JR. Tubular atrophy in the pathogenesis of chronic kidney disease progression. Pediatr Nephrol. 2016;31:693–706. doi: 10.1007/s00467-015-3169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casals N, Zammit V, Herrero L, et al. Carnitine palmitoyltransferase 1C: From cognition to cancer. Prog Lipid Res. 2016;61:134–48. doi: 10.1016/j.plipres.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 25.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 26.Lundsgaard AM, Fritzen AM, Kiens B. Molecular regulation of fatty acid oxidation in skeletal muscle during aerobic exercise. Trends Endocrinol Metab. 2018;29:18–30. doi: 10.1016/j.tem.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20:1868–76. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Agati VD, Chagnac A, de Vries AP, et al. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12:453–71. doi: 10.1038/nrneph.2016.75. [DOI] [PubMed] [Google Scholar]
- 29.Zhang P, Ma D, Wang Y, et al. Berberine protects liver from ethanol-induced oxidative stress and steatosis in mice. Food Chem Toxicol. 2014;74:225–32. doi: 10.1016/j.fct.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Guo T, Woo SL, Guo X, et al. Berberine ameliorates hepatic steatosis and suppresses liver and adipose tissue inflammation in mice with diet-induced obesity. Sci Rep. 2016;6:22612. doi: 10.1038/srep22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Yuan X, Zhang F, et al. Berberine ameliorates fatty acid-induced oxidative stress in human hepatoma cells. Sci Rep. 2017;7:11340. doi: 10.1038/s41598-017-11860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao L, Cang Z, Sun H, et al. Berberine improves glucogenesis and lipid metabolism in nonalcoholic fatty liver disease. BMC Endocr Disord. 2017;17:13. doi: 10.1186/s12902-017-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Li B, Meng X, et al. Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci Rep. 2016;6:20848. doi: 10.1038/srep20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu U, Cha Y, Huang X, et al. Protective effects of berberine on high fat-induced kidney damage by increasing serum adiponectin and promoting insulin sensitivity. Int J Clin Exp Pathol. 2015;8:14486–92. [PMC free article] [PubMed] [Google Scholar]
- 35.Lu KL, Zhang DD, Wang LN, et al. Molecular characterization of carnitine palmitoyltransferase IA in Megalobrama amblycephala and effects on its expression of feeding status and dietary lipid and berberine. Comp Biochem Physiol B Biochem Mol Biol. 2016;191:20–25. doi: 10.1016/j.cbpb.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Zhao X, Feng X, et al. Berberine alleviates olanzapine-induced adipogenesis via the AMPKalpha-SREBP pathway in 3T3-L1 cells. Int J Mol Sci. 2016;17:1865. doi: 10.3390/ijms17111865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]