Abstract
Background
The aim of this study was to investigate the effects of sulforaphane (SFN), a natural isothiocyanate compound, in a rabbit ascending aortic cerclage model of chronic heart failure (CHF).
Material/Methods
Thirty New Zealand White rabbits were divided into the sham operation group (n=10), the CHF group (n=10), and the CHF + SFN group (n=10) treated with subcutaneous SFN (0.5 mg/kg) for five days per week for 12 weeks. After 12 weeks, echocardiography and biometric analysis were performed, followed by the examination of the rabbit hearts. Enzyme-linked immunosorbent assay (ELISA) and Western blot were used to detect levels of inflammatory cytokines, superoxide dismutase (SOD), and malondialdehyde (MDA).
Results
In the CHF group, compared with the sham operation group, there was an increase in the heart weight to body weight ratio (HW/BW), the left ventricular weight to body weight ratio (LVW/BW), the left ventricular end diastolic diameter (LVEDD), the left ventricular end systolic diameter (LVESD), plasma brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) levels, the cardiac collagen volume fraction (CVF), apoptotic index, expression levels of collagen I, collagen III, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and malondialdehyde (MDA) in the myocardial tissue, and a decrease in the left ventricular shortening fraction (LVFS) and left ventricular ejection fraction (LVEF), and cardiac superoxide dismutase (SOD) activity. These changes were corrected in the SFN-treated group.
Conclusions
In a rabbit model of CHF, treatment with SFN improved cardiac function and remodeling by inhibiting oxidative stress and inflammation.
MeSH Keywords: Heart Failure, Heart Function Tests, Oxidative Stress
Background
Chronic heart failure (CHF) is a complex clinical syndrome that is characterized by a progressive reduction in cardiac output, due to a structural or functional cardiac disorder, and is most commonly due to myocardial ischemia [1]. The failing heart cannot satisfy the metabolic demands of the peripheral tissues and other major organs and is a condition that is one of the major reasons for hospital admission in patients over 65-years-of-age [2]. Despite recent progress in the treatment of CHF, and the implementation of national and international clinical management guidelines, morbidity and mortality from CHF remains high, and remains a global health and economic concern. It has been estimated that approximately 5.7 million patients in the US suffer from CHF, with 26 million patients with CHF globally [3]. For theses reasons, new and more effective prevention, diagnosis, and treatment approaches for CHF should continue to be investigated.
The change from compensated left ventricular hypertrophy to decompensated functional changes and heart failure is multifactorial, but studies have shown that oxidative stress and chronic inflammation have a role in the pathogenesis of heart failure [4–6]. The generation of reactive oxygen species (ROS) during inflammation and ischemic cardiac damage can overwhelm the cardiac antioxidant defense processes and result in chronic oxidative stress, which further damages the heart. The consequences of oxidative stress include cardiac remodeling as fibrosis replaces functioning cardiac myocytes, ultimately leading to the heart failure [7,8]. Chronic inflammation is fundamental to the pathophysiology of heart failure as it contributes to myocardial remodeling, endothelial dysfunction, and peripheral vascular injury [9]. For these reasons, future therapeutic strategies for heart failure may include targeting oxidative stress and inflammation.
Sulforaphane (SFN), is a natural isothiocyanate compound that is found in cruciferous vegetables, such as broccoli, cabbage, and cauliflower and is an antioxidant that has been shown to stimulate the production of intracellular antioxidants as well as phase-II detoxification enzymes [10]. The anti-oxidant effects of SFN have been shown to have a role in the prevention of the progression of chronic diseases of the cardiovascular system, kidney, brain, and also in cancer [11–14]. For example, SFN has been shown to be beneficial in the prevention of diabetes-induced cardiomyopathy by reducing cardiac fibrosis, oxidative stress, and inflammation, and has been shown to upregulate the expression of Nrf2 which provides cellular defense against oxidation [15]. SFN has also been shown to protect the cardiac tissues from ischemic damage by activating the antioxidant pathway and mitochondrial ATP-sensitive potassium channels [16].
However, there have been few studies to investigate the roles of SFN in the myocardium in CHF regarding its effects on inflammation, oxidative stress, and cardiac remodeling or to combine these pathophysiological effects with effects on cardiac function. Therefore, the aim of this study was to investigate the effects of SFN, a natural isothiocyanate compound, in a rabbit model of CHF.
Material and Methods
Establishment of a chronic heart failure (CHF) rabbit model
The purchase of 30 healthy New Zealand white rabbits of both sexes, at 6-months of age, and weighing between 2.5–3.5 kg, was made from the Experimental Animal Center of Nantong University. Approval for the use of animals in the experiments was received from the Institutional Animal Care and Use Committee of Nantong University.
Three groups of rabbits were established for this study: the sham operation group (sham group) (n=10), the chronic heart failure (CHF) group (n=10), and the chronic heart failure with SFN treatment group (CHF + SFN group) (n=10). The rabbit model of CHF used in this study was based on an established model in which cerclage, or constriction, of the ascending aorta was performed, as previously described [17,18]. For the sham operation, a thoracotomy only was performed.
Subcutaneous administration of sulforaphane (SFN) (Sigma-Aldrich) (0.5 mg/kg) given for five days every week for 12 weeks; the sham operation group and CHF group were treated with the same volume of control vehicle consisting of 1% dimethyl sulfoxide (DMSO) in phosphate-buffered saline (PBS). The dose of SFN used was determined from previously published animal studies [15].
The laboratory animals were allowed free access to both water and food, and daily bedding changes were undertaken. For the rabbits in the CHF model group, the success of the model was confirmed physiologically and functionally as a left ventricular ejection fraction ≤40%. Following the three-month period of SFN or vehicle treatment, measurement of cardiac activity was performed. Thereafter, animals were euthanized with the collection of tissues for further analysis.
Echocardiographic studies
Rabbits were anesthetized with 3% sodium pentobarbital through marginal ear vein at a dose of 30–40 mg/kg, the chest of each animal was shaved, and the rabbits were positioned in a dorsal decubitus position. Transthoracic M-mode echocardiography was carried out with the help of a high-resolution system (ACUSON Sequoia 512, Siemens) that used a 5–10 MHz broadband transducer. Measurement and calculation of left ventricular end diastolic diameter (LVEDD, mm), left ventricular end systolic diameter (LVESD, mm), interventricular septal thickness (IVS, mm), left ventricular posterior wall thickness (LVPW, mm), left ventricular shortening fraction (LVFS,%), and left ventricular ejection fraction (LVEF,%) were taken.
Hemodynamic measurements
Before sacrifice, the rabbits were anesthetized, as described above, and invasive hemodynamic measurements were performed. A microtip catheter with a pressure transducer (Millar Instruments, Houston, TX, USA) was inserted into the right carotid artery and advanced into the left ventricular cavity. After at least 5 minutes of stabilization, the heart rate (HR), left ventricular end-systolic pressure (LVESP), end-diastolic pressure (LVEDP) and mean arterial pressure (MAP) were recorded. The parameters of the maximal rates of increase and decrease in left ventricular pressure (±dP/dtmax) were also determined.
Enzyme-linked immunosorbent assay (ELISA)
Arterial blood was collected into EDTA-coated centrifuge tubes. After centrifugation at 3000 rpm for 15 min at 4ºC, the plasma samples were separated and stored at −80°C for future analysis. Plasma brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) were then detected by enzyme-linked immunosorbent assay (ELISA) using commercial ELISA kits (Hermes Criterion Biotechnology, Canada) and following the manufacturer’s instructions.
Tissue preparation and histological examination
At the end of the cardiac and other physiological studies, the rabbits were euthanized by pentobarbital overdose, and immediate isolation of the hearts was undertaken. Measurements of the heart weight, as well as left ventricular measurements, were taken. Calculation of the ratio of heart weight to body weight (HW/BW) together with the ratio of left ventricular weight to body weight (LVW/BW) was also performed. Sampling of the left ventricular myocardial tissues was performed with tissue sample storage in liquid nitrogen for future biochemical assays. Some of the cardiac tissue was fixed in 4% paraformaldehyde for histological analysis.
The fixed cardiac tissues were dehydrated in 70% alcohol, embedded in paraffin wax, and sectioned onto glass slides. Masson’s trichrome histochemical staining of the cardiac tissue sections was performed for the evaluation of cardiac fibrosis, as described previously [19]. Staining of the sections was performed using Masson’s trichrome staining kit (Dako Sciences, Glostrup, Denmark) following the manufacturer’s introductions. On light microscopy, the myocardium was stained red, and the collagen was stained blue. Calculation of the collagen volume fraction (CVF) was performed as the aggregate of the area of collagen divided by the total area, using the Image ProPlus 6.0 software (Media Cybernetics, MD, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
The expression of mRNA for collagen I and collagen III in the myocardium was determined following isolation and purification of mRNA from left ventricular myocardial tissues with the use of the TRIzol reagent (Invitrogen, USA). Quantitative real-time PCR was performed using the SYBR Green PCR Kit (Takara, Japan) on the ABI 7500 Fast Real-Time PCR system, in accordance with the guidelines of the manufacturer.
The premier sequences were: collagen I (forward: GGAGATGATG GGGAAGCTG and reverse: AATCCACGAGCACCCTGA), collagen III (forward): GGAATGGAGCAAGACAGTCTTTG and reverse: TGCGAT ATCTATGATGGGTAGTCTCA).
The use of β-actin was used as an internal control. Calculation of the relative expression of mRNAs was performed with the application of the 2−ΔΔCt technique.
Western blot analysis
The proteins from the frozen cardiac tissues stored in liquid nitrogen were extracted in RIPA lysis buffer. Determination of the protein concentration was made using the BCA protein assay reagent kit (Beyotime Institute of Biotechnology, Shanghai, China). Specimens were fractionated using a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes. Incubation of the membranes with primary antibodies, including collagen I (1: 800, Abcam), collagen III (1: 1000, Abcam), Bcl-2 (1: 500, Abcam), caspase-3 (1: 1000, Abcam) and GAPDH (1: 1500, Abcam). The GAPDH was used as a control. Following incubation with horseradish peroxidase (HRP)-conjugated secondary antibody, detection of the protein bands was performed with the use of the improved chemiluminescence (ECL) system, with quantification performed using the Image ProPlus 6.0 software.
Assessment of apoptosis
Myocardial cell apoptosis was detected using the terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay, following the guidelines provided by the manufacturer (Roche Applied Science, Germany). Calculation of the apoptosis index (AI) was performed as a percentage of TUNEL-positive apoptotic cells in the total number of cells.
Assessment of oxidative stress
Evaluation of superoxide dismutase (SOD) levels and malondialdehyde (MDA) levels in myocardial tissues was made for the evaluation of oxidative stress, using commercial kits (Nanjing Jiancheng Bioengineering Company, China) following the guidelines of the manufacturer.
Measurement of inflammatory cytokines
Measurement of the levels of tumor necrosis factor-a (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) in the myocardial tissues was performed using ELISA kits (ELISA, USCN, Wuhan, China) according to the manufacturer’s guidelines. Analysis of the results was performed with the use of a microplate reader (Thermo Scientific, USA).
Statistical analysis
Data were calculated as the mean ± standard deviation (SD). Performance of all of the statistical analysis was done with the use of SPSS 18.0 software. Analysis of the heterogeneity between the groups was performed using one-way ANOVA. Comparison of data between two groups was performed using the least significant difference (LSD) technique.
Ten randomly selected animals were used to determine intra-observer and interobserver variability for the cardiac imaging evaluation by two observers who were blinded to the previous measurements. The time between intra-observer measurements was two weeks. Intra-observer and interobserver variability were expressed by the coefficient of variation (SD/mean). A value of P<0.05 was deemed to be statistically significant.
Results
By the end of the study, 27 rabbits had survived, including ten in the sham group, eight in the chronic heart failure (CHF) group, and nine in the CHF + sulforaphane (SFN) group. The remaining CHF model animals still satisfied the criteria for CHF.
Sulforaphane (SFN) treatment improved cardiac function and remodeling
As shown in Table 1, the left ventricular systolic activity, evaluated by left ventricular ejection fraction (LVEF) as well as left ventricular shortening fraction (LVFS) showed a reduction in the CHF group in comparison with the sham group. Furthermore, SFN treatment resulted in a rapid decrease in both of the parameters.
Table 1.
Comparison of cardiac function and structure parameters between experimental groups.
| Sham (n=10) | CHF (n=8) | CHF+SFN (n=9) | |
|---|---|---|---|
| LVEF (%) | 72.54±6.42 | 37.25±3.27* | 55.06±4.86*# |
| LVFS (%) | 44.16±4.39 | 23.35±2.31* | 32.67±3.08*# |
| LVESD (mm) | 8.68±0.54 | 11.75±0.82* | 9.85±0.68*# |
| LVEDD (mm) | 11.28±0.91 | 14.76±1.02* | 12.80±0.69*# |
| IVS (mm) | 2.02±0.24 | 2.32±0.28 | 2.14±0.18 |
| LVPM (mm) | 2.15±0.21 | 2.08±0.14 | 2.11±0.15 |
| HW/BW (g/kg) | 2.34±0.16 | 3.36±0.24* | 2.75±0.26*# |
| LVW/BW (g/kg) | 1.89±0.12 | 2.82±0.23* | 2.35±0.10*# |
LVEF indicates left ventricular ejection fraction; LVFS – left ventricular shortening fraction; LVESD – left ventricular end-systolic diameter; LVEDD – left ventricular end-diastolic diameter; IVS – interventricular septal thickness; LVPM – left ventricular posterior wall thickness; HW – heart weight; LVW – left ventricular weight; BW – body weight; SFN – sulforaphane. Data are presented as mean ±SD,
P<0.05 versus Sham (sham operation) group,
P<0.05 versus CHF (chronic heart failure) group.
The left ventricular end-systolic diameter (LVESD) and the left ventricular end-diastolic diameter (LVEDD) were increased in rabbits with CHF; whereas LVESD and LVEDD were reduced following the 12 weeks of treatment with SFN. No differences were observed in interventricular septal thickness (IVS) or left ventricular posterior wall thickness (LVPW) between the animal groups. Variation coefficients of intra-observer and interobserver variability in parameters calculated from ultrasound imaging were less than 10% (Table 2). There was good interobserver and intra-observer reproducibility for measurements.
Table 2.
Inter and intra-observer variability of parameters from ultrasound evaluation.
| Coefficient of variation (%) | |
|---|---|
| Inter-observer | |
| LVEF | 5.58±1.24 |
| LVFS | 1.75±0.62 |
| LVESD | 4.25±3.14 |
| LVEDD | 6.96±1.68 |
| IVS | 0.79±0.24 |
| LVPM | 4.55±2.38 |
| Intra-observer | |
| LVEF | 7.33±1.19 |
| LVFS | 2.75±2.12 |
| LVESD | 0.91±0.36 |
| LVEDD | 4.42±2.98 |
| IVS | 6.57±1.45 |
| LVPM | 3.82±2.49 |
LVEF indicates left ventricular ejection fraction; LVFS – left ventricular shortening fraction; LVESD – left ventricular end-systolic diameter; LVEDD – left ventricular end-diastolic diameter; IVS – interventricular septal thickness; LVPM – left ventricular posterior wall thickness. Data are presented as mean ±SD.
The heart rate (HR) was relatively stable after 12 weeks of treatment with SFN in all groups of animals. As expected, CHF caused significant reduction of mean arterial pressure (MAP), left ventricular end systolic pressure (LVESP), ±dP/dtmax, but no obvious improvement was observed after 12 weeks of treatment with SFN. The left ventricular end diastolic pressure (LVEDP) was increased in the CHF group when compared with the sham group and was slightly decreased in the CHF + SFN group when compared with the CHF group (Table 3).
Table 3.
Comparison of hemodynamic parameters pre and after treatment with SFN between experimental groups.
| Sham (n=10) | CHF (n=8) | CHF+SFN (n=9) | |
|---|---|---|---|
| HR (bpm) | 307.53±9.21 | 290.28±10.15 | 297.34±8.65 |
| MAP (mmHg) | 96.18±10.32 | 80.20±11.14* | 85.38±9.42* |
| LVESP (mmHg) | 110.62±7.25 | 94.80±9.23* | 98.06±6.54* |
| LVEDP (mmHg) | 4.35±1.20 | 16.42±1.92* | 12.58±1.65* |
| +dP/dtmax (mmHg/s) | 7253±356 | 4658±287* | 5012±294* |
| −dP/dtmax (mmHg/s) | 6645±332 | 4126±285* | 4563±305* |
HR – heart rate; MAP – mean arterial pressure; LVESP – left ventricular end-systolic pressure; LVEDP – left ventricular end-diastolic pressure; ±dP/dtmax – the maximal rates of increase and decrease in left ventricular pressure; SFN – sulforaphane. Data are presented as mean ±SD,
P<0.05 versus Sham (sham operation) group.
The heart weight (HW) to body weight (BW) ratio was observed to increase in the CHF rabbits when compared with the sham-operated rabbits. Also, the ratio of left ventricular weight (LVW) to BW was also increased in the CHF rabbits when compared with the sham-operated rabbits. The increase in HW/BW ratio, as well as the LVW/BW ratio, was reduced following SFN treatment (Table 1).
Effects of SFN treatment on plasma brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP)
The plasma brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) levels, as markers of CHF, were significantly increased in the CHF group and the CHF + SFN group, compared with the sham group. While SFN treatment reduced the levels of both BNP and ANP compared with the CHF group (Table 4).
Table 4.
Comparison of plasma BNP and ANP levels between experimental groups.
| Sham (n=10) | CHF (n=8) | CHF+SFN (n=9) | |
|---|---|---|---|
| BNP (pg/mL) | 36.18±6.35 | 75.72±12.24* | 59.4±10.60*# |
| ANP (pg/mL) | 62.45±8.62 | 158.20±17.31* | 115.33±11.58*# |
BNP – B-type natriuretic peptide; ANP – atrial natriuretic peptide; SFN – sulforaphane. Data are presented as mean ±SD;
P<0.05 versus Sham (sham operation) group,
P<0.05 versus CHF (chronic heart failure) group.
Effects of SFN treatment on cardiac fibrosis
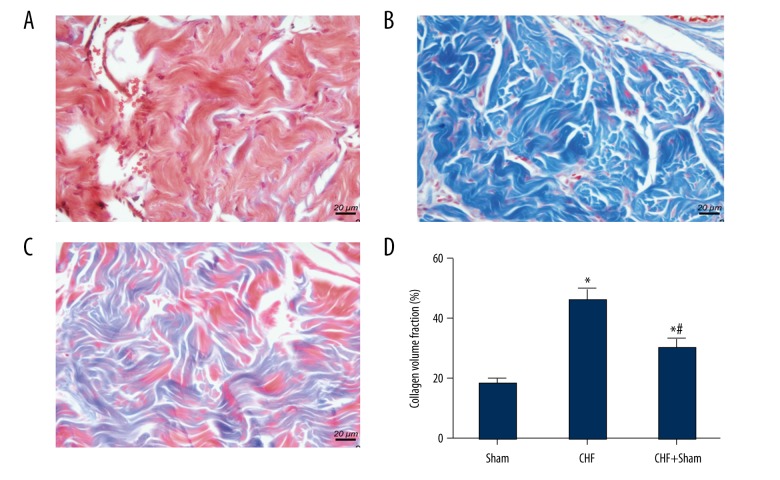
For the purpose of assessing the changes in cardiac fibrosis, Masson’s trichrome staining with CVF was carried out. As shown in Figure 1A, a massive collagen accumulation was presented in left ventricular tissues in the CHF group, in comparison with the sham group. Significant increase was also observed in CVF in the CHF group (Figure 1B). In addition, subsequent to 12 weeks of SFN treatment, collagen areas of heart tissues were significantly reduced, while the remarkable decrease in CVF was observed as well (Figure 1C,1D).
Figure 1.
Effect of sulforaphane (SFN) treatment on cardiac fibrosis. Evaluation of the degree of cardiac fibrosis in the Sham group (A), the chronic heart failure (CHF) group (B), and the CHF + SFN group (C) were performed by Masson’s trichrome staining. (D) The collagen volume fraction (CVF) was presented as the sum of collagen areas (blue) divided by the total area. * P<0.05 versus sham operation group, # P<0.05 versus the chronic heart failure (CHF) group.
Effect of SFN treatment on the expression of collagen I and collagen III
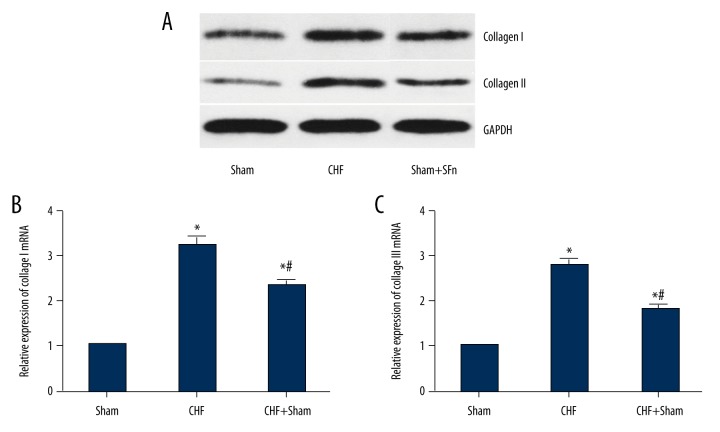
Collagen I and collagen III comprise most of the intracardiac collagen. The findings from quantitative real-time PCR as well as Western blot showed that mRNA and protein levels, collagen I and collagen III were considerably increased in the CHF group compared with the sham group. Also, there was a reduction in expression of both collagen I and collagen III in CHF rabbits treated with SFN (Figure 2A–2C).
Figure 2.
Effect of sulforaphane (SFN) treatment on the expressions of collagen type I and collagen type III. (A) Collagen I and collagen III protein expression in different experimental groups were detected by Western blot. (B) Collagen I and collagen III mRNA expression were examined by the quantitative real-time polymerase chain reaction (qRT-PCR). * P<0.05 versus Sham (sham operation) group, # P<0.05 versus chronic heart failure (CHF) group.
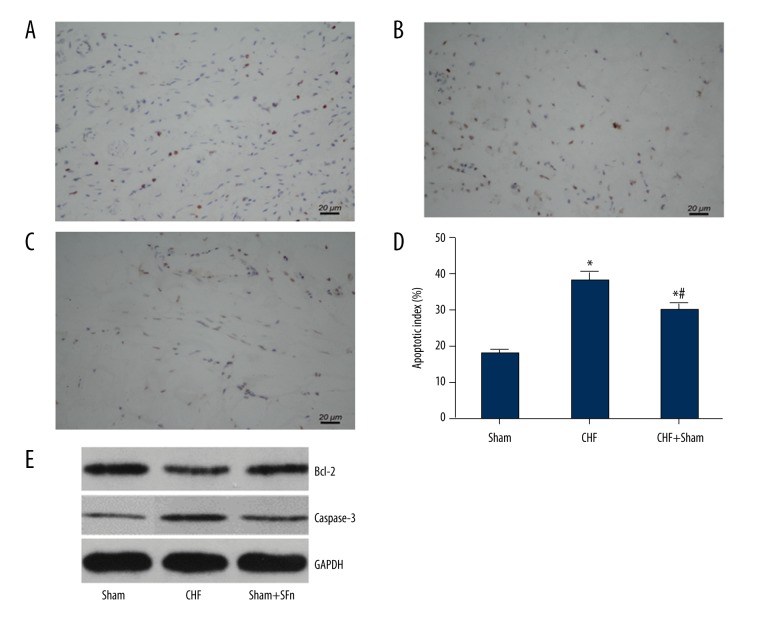
SFN treatment inhibited cardiomyocyte apoptosis
Findings from the TUNEL assay for cell apoptosis are shown in Figure 3A–3D. The percentage of TUNEL-positive cells was greater in the CHF group compared with the sham group. SFN treatment resulted in a reduction in the percentage of apoptotic cardiomyocytes when compared with the CHF group. The evaluation of protein expression levels of Bcl-2 and caspase-3 in cardiac tissues using Western blot showed that Bcl-2 expression was decreased and caspase-3 expression was increased in the CHF group, while these changes were reversed with SFN treatment (Figure 3E).
Figure 3.
Effect of sulforaphane (SFN) treatment on cardiomyocyte apoptosis. The cardiomyocyte apoptosis levels of the Sham group (A), chronic heart failure (CHF) group (B) and the CHF +S FN group (C) were detected by using the terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay. (D) The apoptotic index was presented as the ratio of apoptotic nuclei (brown-stained) to total nuclei counted. (E) Bcl-2 and caspase-3 protein expression of different experimental groups were detected by Western blot. * P<0.05 versus sham operation group, # P<0.05 versus chronic heart failure (CHF) group.
SFN treatment reduced cardiac oxidative stress
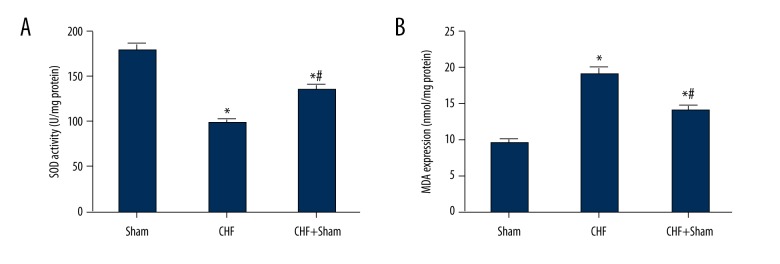
The activity of superoxide dismutase (SOD) was significantly decreased in heart tissues of CHF rabbits compared with the sham-operated rabbits, and the malondialdehyde (MDA) activity was significantly increased in CHF rabbits when compared with the sham-operated rabbits. SFN treatment increased the activity of SOD and reduced the activity of MDA when compared with the CHF group (Figure 4).
Figure 4.
Effect of sulforaphane (SFN) treatment on cardiac oxidative stress. (A) Superoxide dismutase (SOD) activity was significantly decreased in the chronic heart failure (CHF) group compared with the sham operation group, while sulforaphane (SFN) treatment significantly increased SOD activity. (B) Malondialdehyde (MDA) expression was significantly increased in the chronic heart failure (CHF) group compared with the sham operation group, and sulforaphane (SFN) administration decreased MDA expression. * P<0.05 versus sham operation group, # P<0.05 versus chronic heart failure (CHF) group.
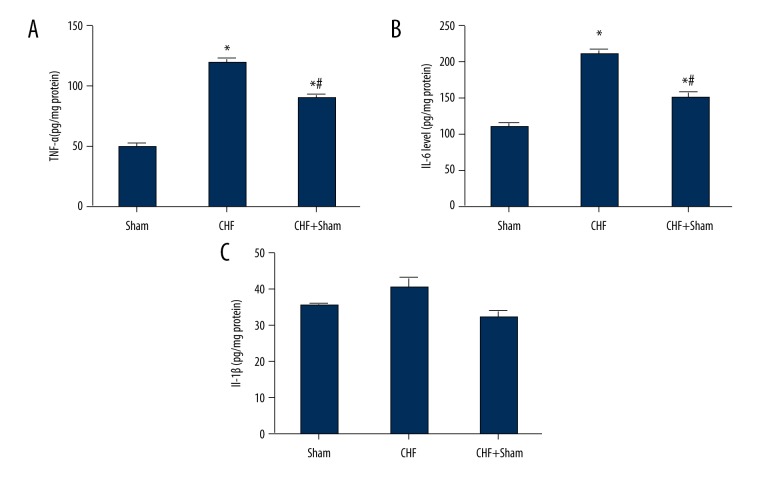
SFN treatment reduced cardiac inflammatory cytokines levels
As shown in Figure 5, the expression levels of the inflammatory cytokines TNF-α as well as IL-6 in heart tissues were greater in the CHF group compared with the sham group. SFN treatment significantly reduced both TNF-α and IL-6 expression. However, no significant difference was observed in IL-1β expression between the experimental groups.
Figure 5.
Effect of sulforaphane (SFN) treatment on the expression levels of cardiac inflammatory cytokines. The levels of the inflammatory cytokines tumor necrosis factor-α (TNF-α) (A) and interleukin (IL)-6 (B) were markedly increased in the chronic heart failure (CHF) group compared with the Sham group. Sulforaphane (SFN) treatment significantly reduced the tumor necrosis TNF-α and IL-6 levels. (C) There was no significant difference in the IL-1β levels between experimental groups. * P<0.05 versus sham operation group, # P<0.05 versus chronic heart failure (CHF) group.
Discussion
In this study, the effects of sulforaphane (SFN) in a rabbit model of chronic heart failure (CHF) included improved cardiac function and left ventricular remodeling, with inhibition of oxidative stress and inflammation. The key novel observations made in this study were that SFN treatment increased systolic cardiac function and reduced left ventricular remodeling and that SFN treatment significantly reduced cardiac fibrosis, apoptosis, oxidative stress, and inflammation in rabbits with CHF.
SPN is a naturally occurring compound that is found in vegetables, and can be extracted and used as a nutraceutical, which is food, or part of a food, that can provide medical or health benefits, including prevention and treatment of a disease. For example, Scicchitano et al. reported that nutraceuticals might be effective in the prevention of atherosclerosis and coronary heart disease [20]. Nutraceuticals have a role in the reduction hyperlipidemia, which is considered to be a risk factor for coronary heart disease and its sequelae [21]. However, the biochemical, physiological, and molecular mechanisms of action of many nutraceuticals remain poorly understood.
SFN has been used recently, in some countries, for the prevention and treatment of cardiovascular disease. Studies have shown that SFN has pharmacological effects on the reduction of fibrosis, oxidative stress, and inflammation. Fernandes et al. have shown that SFN reduced the rate of progression of cardiac remodeling post-infarction through its effects on reducing cardiac fibrosis and oxidative stress [11]. Other research groups have shown that SFN activates the transcriptional cofactor, PPAR-gamma coactivator 1-alpha (PGC1-α), which may be a mechanism by which SFN reduces oxidative stress and cell apoptosis [22]. Xu et al. have shown that SFN reduces diabetes-induced cardiac dysfunction, hypertrophy, and fibrosis via Nrf2 activation in mice [23]. Li et al. have shown that SFN prevented doxorubicin-induced oxidative stress, as well apoptosis, in H9c2 rat myoblasts [24]. These previously published findings support the findings of the present study, but the mechanism of action of SFN and the possible molecular pathways involved, remain to be clarified.
There have been no previous studies on the impact of SFN on cardiac activity and remodeling in heart failure. In this study, we established the rabbit model of CHF as confirmed by left ventricular dilation, decreased left ventricular shortening fraction (LVFS) and left ventricular ejection fraction (LVEF) measurements, increased heart weight to body weight (HW/BW) ratios in the CHF + SFN group, as well as plasma BNP and ANP levels. Also, this study has shown that, in the rabbit CHF model, treatment with SFN improved cardiac function and remodeling, reduced cardiomyocyte apoptosis, oxidative stress, and inflammation. To the best of our knowledge, this study appears to be the first to demonstrate the effects of SFN in an animal model of CHF.
In this study, cardiac fibrosis was evaluated because of its known importance in the pathogenesis of CHF and cardiac remodeling [25,26]. The use of the collagen volume fraction (CVF) in CHF rabbits was evaluated by histochemical staining and light microscopy and the findings associated with SFN treatment of reduced collagen deposition were supported by the use of quantitative real-time PCR and Western blot for collagen I and collagen III expression.
Oxidative stress can be caused by a variety of stimuli and results in the production of reactive oxygen species (ROS), which lead to cellular oxidative injury [27]. Oxidative stress contributes to cardiac remodeling and fibrosis, ultimately leading to heart failure [8,28]. Yoshioka et al. have shown that sepiapterin could prevent left ventricular hypertrophy and dilatory remodeling induced by pressure overload by inhibiting oxidative stress [29]. In this study, we detected myocardial oxidative stress through the measurement of SOD function as well as MDA content; SOD is a key cellular defense against superoxide, whereas MDA is capable of causing the toxic cellular stress [30]. In this study, a significant decrease in SOD activity, together with an increase in the MDA was found in the myocardium of CHF rabbits when compared with the sham-operated rabbits, but SFN treatment reduced cardiac oxidative stress, which may explain its effects on reduction of cardiac fibrosis and remodeling in CHF.
In this study, inflammatory cytokines were evaluated in the animal model studies as inflammation is another important factor involved in the pathophysiology of CHF [31], and serum levels of IL-6 level have been shown to be increased in cardiac hypertrophy in rabbits [32]. Sriramula et al. have shown that TNF-α contributed to angiotensin II-induced hypertension and cardiac remodeling [33]. ROS generation in oxidative stress is also likely to have a role in the modulation of inflammatory mechanism in CHF [34]. In the present study, inflammatory cytokines TNF-α, as well as IL-6 levels, in the myocardium increased in the in CHF group when compared with the sham group, and levels of these cytokines were reduced following treatment with SFN.
Previous studies have shown that cardiac myocyte apoptosis occurs in CHF and the percentage of apoptotic cardiac myocytes in the failing human heart increase with increasing severity of the condition [35]. Moe et al. have shown that early and consistent activation of myocardial apoptosis and pro-apoptotic factors contributed to the progression of HF in canine cardiac pacing-induced cardiomyopathy [36]. Inhibition of cardiac myocyte apoptosis is capable of improving the symptoms of HF [37]. A recent study in a rat model of myocardial ischemia has shown that SFN can reduce stress-induced cardiomyocyte apoptosis through the activation of the SIRT1 signaling pathway [38]. Collectively, these previous studies support the findings of the present study and serve as an incentive to investigate further the mechanisms of action of SFN on the heart.
Conclusions
In a rabbit model of chronic heart failure, treatment with sulforaphane, a natural isothiocyanate compound, improved cardiac function and remodeling by inhibiting oxidative stress and inflammation.
Footnotes
Source of support: Departmental sources
References
- 1.Wong LL, Wang J, Liew OW, et al. MicroRNA and Heart Failure. Int J Mol Sci. 2016;17(4):502. doi: 10.3390/ijms17040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alla F, Zannad F, Filippatos G. Epidemiology of acute heart failure syndromes. Heart Fail Rev. 2007;12(2):91–95. doi: 10.1007/s10741-007-9009-2. [DOI] [PubMed] [Google Scholar]
- 3.Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63(12):1123–33. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 4.Kotur-Stevuljevic J, Memon L, Stefanovic A, et al. Correlation of oxidative stress parameters and inflammatory markers in coronary artery disease patients. Clin Biochem. 2007;40(3–4):181–87. doi: 10.1016/j.clinbiochem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93(8):903–7. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briasoulis A, Androulakis E, Christophides T, Tousoulis D. The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail Rev. 2016;21(2):169–76. doi: 10.1007/s10741-016-9533-z. [DOI] [PubMed] [Google Scholar]
- 7.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301(6):H2181–90. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 8.Tham YK, Bernardo BC, Ooi JY, et al. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch Toxicol. 2015;89(9):1401–38. doi: 10.1007/s00204-015-1477-x. [DOI] [PubMed] [Google Scholar]
- 9.Szczurek W, Szygula-Jurkiewicz B. Oxidative stress and inflammatory markers – the future of heart failure diagnostics? Kardiochir Torakochirurgia Pol. 2015;12(2):145–49. doi: 10.5114/kitp.2015.52856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angeloni C, Leoncini E, Malaguti M, et al. Modulation of phase II enzymes by sulforaphane: Implications for its cardioprotective potential. J Agric Food Chem. 2009;57(12):5615–22. doi: 10.1021/jf900549c. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes RO, De Castro AL, Bonetto JH, et al. Sulforaphane effects on postinfarction cardiac remodeling in rats: Modulation of redox-sensitive prosurvival and proapoptotic proteins. J Nutr Biochem. 2016;34:106–17. doi: 10.1016/j.jnutbio.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Townsend BE, Johnson RW. Sulforaphane induces Nrf2 target genes and attenuates inflammatory gene expression in microglia from brain of young adult and aged mice. Exp Gerontol. 2016;73:42–48. doi: 10.1016/j.exger.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cekauskas A, Bruns H, Manikas M, et al. Sulforaphane decreases kidney injury after transplantation in rats: Role of mitochondrial damage. Ann Transplant. 2013;18:488–96. doi: 10.12659/AOT.884013. [DOI] [PubMed] [Google Scholar]
- 14.Fuentes F, Paredes-Gonzalez X, Kong AT. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3,3′-diindolylmethane: Anti-oxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr Pharmacol Rep. 2015;1(3):179–96. doi: 10.1007/s40495-015-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai Y, Cui W, Xin Y, et al. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol. 2013;57:82–95. doi: 10.1016/j.yjmcc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Piao CS, Gao S, Lee GH, et al. Sulforaphane protects ischemic injury of hearts through antioxidant pathway and mitochondrial K(ATP) channels. Pharmacol Res. 2010;61(4):342–48. doi: 10.1016/j.phrs.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Dang Y, Li Y, et al. Cardiac contractility modulation attenuate myocardial fibrosis by inhibiting TGF-beta1/Smad3 signaling pathway in a rabbit model of chronic heart failure. Cell Physiol Biochem. 2016;39(1):294–302. doi: 10.1159/000445624. [DOI] [PubMed] [Google Scholar]
- 18.Ning B, Qi X, Li Y, et al. Biventricular pacing cardiac contractility modulation improves cardiac contractile function via upregulating SERCA2 and miR-133 in a rabbit model of congestive heart failure. Cell Physiol Biochem. 2014;33(5):1389–99. doi: 10.1159/000358705. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Chen A, Song L, et al. Low-level vagus nerve stimulation reverses cardiac dysfunction and subcellular calcium handling in rats with post-myocardial infarction heart failure. Int Heart J. 2016;57(3):350–55. doi: 10.1536/ihj.15-516. [DOI] [PubMed] [Google Scholar]
- 20.Scicchitano P, Cameli M, Maiello M, et al. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. Journal of Functional Foods. 2014;6:11–32. [Google Scholar]
- 21.Ebong IA, Goff DC, Jr, Rodriguez CJ, et al. Association of lipids with incident heart failure among adults with and without diabetes mellitus: Multiethnic Study of Atherosclerosis. Circ Heart Fail. 2013;6(3):371–78. doi: 10.1161/CIRCHEARTFAILURE.112.000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandes RO, Bonetto JH, Baregzay B, et al. Modulation of apoptosis by sulforaphane is associated with PGC-1alpha stimulation and decreased oxidative stress in cardiac myoblasts. Mol Cell Biochem. 2015;401(1–2):61–70. doi: 10.1007/s11010-014-2292-z. [DOI] [PubMed] [Google Scholar]
- 23.Xu Z, Wang S, Ji H, et al. Broccoli sprout extract prevents diabetic cardiomyopathy via Nrf2 activation in db/db T2DM mice. Sci Rep. 2016;6:30252. doi: 10.1038/srep30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Kim DS, Yadav RK, et al. Sulforaphane prevents doxorubicin-induced oxidative stress and cell death in rat H9c2 cells. Int J Mol Med. 2015;36(1):53–64. doi: 10.3892/ijmm.2015.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Z, Zhao W, Zhang X, et al. Scutellarin alleviates interstitial fibrosis and cardiac dysfunction of infarct rats by inhibiting TGFbeta1 expression and activation of p38-MAPK and ERK1/2. Br J Pharmacol. 2011;162(3):688–700. doi: 10.1111/j.1476-5381.2010.01070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Wu Y, Chen J, et al. Pirfenidone attenuates cardiac fibrosis in a mouse model of TAC-induced left ventricular remodeling by suppressing NLRP3 inflammasome formation. Cardiology. 2013;126(1):1–11. doi: 10.1159/000351179. [DOI] [PubMed] [Google Scholar]
- 27.Elnakish MT, Hassanain HH, Janssen PM, et al. Emerging role of oxidative stress in metabolic syndrome and cardiovascular diseases: Important role of Rac/NADPH oxidase. J Pathol. 2013;231(3):290–300. doi: 10.1002/path.4255. [DOI] [PubMed] [Google Scholar]
- 28.Sugiyama K, Sasano T, Kurokawa J, et al. Oxidative stress induced ventricular arrhythmia and impairment of cardiac function in Nos1ap deleted mice. Int Heart J. 2016;57(3):341–49. doi: 10.1536/ihj.15-471. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka K, Otani H, Shimazu T, et al. Sepiapterin prevents left ventricular hypertrophy and dilatory remodeling induced by pressure overload in rats. Am J Physiol Heart Circ Physiol. 2015;309(10):H1782–91. doi: 10.1152/ajpheart.00417.2015. [DOI] [PubMed] [Google Scholar]
- 30.Yu W, Wan Z, Qiu XF, et al. Resveratrol, an activator of SIRT1, restores erectile function in streptozotocin-induced diabetic rats. Asian J Androl. 2013;15(5):646–51. doi: 10.1038/aja.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aukrust P, Yndestad A, Damas JK, Gullestad L. Inflammation and chronic heart failure-potential therapeutic role of intravenous immunoglobulin. Autoimmun Rev. 2004;3(3):221–27. doi: 10.1016/S1568-9972(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 32.Al-Rasheed NM, Al-Oteibi MM, Al-Manee RZ, et al. Simvastatin prevents isoproterenol-induced cardiac hypertrophy through modulation of the JAK/STAT pathway. Drug Des Devel Ther. 2015;9:3217–29. doi: 10.2147/DDDT.S86431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sriramula S, Francis J. Tumor necrosis factor-alpha is essential for Angiotensin II-induced ventricular remodeling: Role for oxidative stress. PLoS One. 2015;10(9):e0138372. doi: 10.1371/journal.pone.0138372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amir O, Paz H, Rogowski O, et al. Serum oxidative stress level correlates with clinical parameters in chronic systolic heart failure patients. Clin Cardiol. 2009;32(4):199–203. doi: 10.1002/clc.20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moe GW, Marin-Garcia J. Role of cell death in the progression of heart failure. Heart Fail Rev. 2016;21(2):157–67. doi: 10.1007/s10741-016-9532-0. [DOI] [PubMed] [Google Scholar]
- 36.Moe GW, Naik G, Konig A, et al. Early and persistent activation of myocardial apoptosis, bax and caspases: Insights into mechanisms of progression of heart failure. Pathophysiology. 2002;8(3):183–92. doi: 10.1016/s0928-4680(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Wu P, Wang Y, et al. Ad-HGF improves the cardiac remodeling of rat following myocardial infarction by upregulating autophagy and necroptosis and inhibiting apoptosis. Am J Transl Res. 2016;8(11):4605–27. [PMC free article] [PubMed] [Google Scholar]
- 38.Li YP, Wang SL, Liu B, et al. Sulforaphane prevents rat cardiomyocytes from hypoxia/reoxygenation injury in vitro via activating SIRT1 and subsequently inhibiting ER stress. Acta Pharmacol Sin. 2016;37(3):344–53. doi: 10.1038/aps.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]