Abstract
Background: Hyperadiponectinemia is an indicator of worse outcomes in advanced heart failure (HF), its role in de novo HF is less clear.
Objective: Because this protein is a hormone with starvation properties, we wanted to know its association with nutritional state and its regulator factors in de novo HF.
Methods: Adiponectin circulating levels were determined by ELISA at discharge in patients admitted for de novo HF (n=74). Nutritional status was determined by CONUT score. Univariate and multivariate Cox regression analyses were employed to calculate the estimated hazard ratio (HR) with 95% confidence interval (CI) for death or all-cause readmission. Stromal vascular cells (SVC) of EAT and subcutaneous adipose tissue (SAT) from patients (n=5) underwent heart surgery were induced to adipogenesis for 18 days. Then, cells were cultured with complete or starved medium for 8 hours. At the end, adiponectin expression levels were analysed by real time polymerase chain reaction.
Results: Patients were grouped regarding nutritional status. There was a strong association between high adiponectin levels and failing nutritional status. Those patients with worse nutritional state had the highest adiponectin and proBNP levels at discharge (p<0.01). Both proteins were slightly correlated (p<0.05). However, only high adiponectin levels were independently associated with death or all-cause readmission. Nutrients starvation upregulated adiponectin expression levels in adipogenesis-induced SVC from EAT or SAT.
Conclusions: Worse nutritional state in de novo HF patients is associated with higher adiponectin plasma levels. Their levels were upregulated in adipose cells after being nutrients-starved. These results may help us to understand the adiponectin paradox in HF.
Introduction
Adiponectin is mainly produced by mature adipocytes 1; it has anti-inflammatory 2,3, insulin sensitizing and anti-atherogenic properties 4,5 due to its ability of stimulate the production of nitric oxide in vascular endothelial cells 6. The absence of adiponectin, as it was showed in adiponectin knockout mice, exacerbates myocardial injury and cardiac dysfunction 7. Thus, several studies have showed that low adiponectin levels in plasma and epicardial adipose tissue (EAT) were associated with coronary artery disease (CAD) 8-10 and its risk factors are involved in heart failure (HF) development 11. This is a complex syndrome due to hemodynamic, neurohormonal, inflammation and metabolic disorders 12. However, in a paradoxical way, high adiponectin levels were associated with poor cardiac function, symptomatic clinical status and high mortality in chronic HF patients 13. Therefore, the reduction of adiponectin levels during hospitalization improved the patients' prognosis 14.
In patients with chronic HF and cardiac cachexia, the increment of this protein levels might be related to inflammatory response caused by injury 13 15. Its secretion by adipose tissue may be regulated by plasma brain natriuretic peptide (BNP) and cytokines 16, weight loss 17 or advanced catabolic states like cachexia 18. Other authors have suggested that high levels of adiponectin could be due to the adiponectin resistance in patients with cardiovascular diseases 19 including HF 20. Although adiponectin is produced by adipocytes, paradoxically, malnourished underweight female anorexic patients also contain high adiponectin levels which are declined after refeeding and weight gain 21. Contrary, obese patients have low plasma adiponectin levels which are rise with weight loss 22.
The prognostic value of adiponectin in de novo HF is less known, so we tried to study its relationship with the nutritional state, as well as, its possible regulator factors in this group of patients.
Material and Methods
Study population
This is a retrospective and observational study that included 74 consecutively admitted patients in the Cardiology Department of our hospital between May 2014 and August 2015 with the diagnosis of de novo HF. Heart failure diagnosed was made according to the recommendations of the European Society of Cardiology Guidelines 23. We have excluded patients with decompensated chronic HF, presence of pregnancy, severe chronic liver or renal disease, autoimmune or chronic inflammatory diseases, recent (last 3 weeks) infectious process, recent (last 3 weeks) treatment with corticosteroids or anti-inflammatory drugs, known tumor processes at the time of inclusion in the study, blood disorders and an unknown nutritional status.
The study complies with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of Galicia. All patients signed an informed consent.
At admission, anthropometric and demographic data, cardiac and renal function parameters, glucose and lipid profile, haemogram, pro brain natriuretic peptide (proBNP) values and nutritional status, measured by CONUT score 24 were collected from all included patients. Monocytes subsets were determined as it was described 25.
The CONUT score uses the following three parameters: the serum albumin level (g/dL), total cholesterol level (mg/dL), and lymphocyte count (count/mL). It thus enables evaluation of the protein reserves, calorie depletion, and immune defenses, respectively. We classified the patients according to the CONUT score in normal nutritional status (CONUT 0-1 points), mild malnutrition (CONUT score 2-4 points) and moderate to severe malnutrition (CONUT score > 4 points) 24.
Adiponectin levels
Blood samples were obtained at discharge as we previously described 26 and were centrifuged at 1800 xg for 15 minutes. Isolated plasma was stored at -80 ºC until be used. Adiponectin levels were measured by DuoSet Enzyme-Linked ImmunoSorbent Assay (ELISA) kit with a detection limit of 0.6 ng/mL (R&D Systems, Minneapolis, MN, USA) following manufacture´s protocol.
Endpoint definition and follow-up
The endpoints were death from any cause and rehospitalization for all cause. Follow-up information was recorded from medical history. The mean of follow-up was 521 (9-820) days.
Adiponectin expression levels regulation by nutrients starvation
Epicardial and subcutaneous stromal cells were obtained from adipose tissue biopsies of five patients after signing inform consent and undergoing open heart surgery, as it was described previously 27. Then, cells were maintained and subcultured twice in medium M199 (Lonza Biologics Porriño S.L., Pontevedra, Spain) supplemented with 10% fetal bovine serum (FBS) (Promega Biotech Ibérica S.L., Madrid, Spain). Fifteen thousand of cells per well were seeded in 24 well-culture plates and adipogenesis was induced with adipogenic factors (Sigma-Aldrich Quimica S.L., Madrid, Spain) with three replacements a week, as it was previously described 27.
After three or four weeks, mature adipocytes were visualized by microscopy. A group of cells were starved with medium M199 and saline solution (1:1) supplemented with 1% of FBS, other group was maintained with medium M199 and 1% of FBS, other group with starved medium supplemented with 10% FBS and the last group was the control with medium M199 and 10% FBS. The starvation was made for 8 hours. Then, RNA was extracted by RNeasy Plus MiniKit (Qiagen GmbH, Hilden, Germany) as manufacture´s protocol recommendations. RNA was retrotranscripted into cDNA using the Maxima First Strand cDNA Synthesis Kit (Thermo Scientific, Madrid, Spain) in a thermocycler (25°C for 10 minutes, 50°C for 15 minutes, and then 85°C for 5 minutes). Two µL of cDNA was amplified in a total volume of 20 µL with FastStart SYBR Green Master (Roche Diagnostics S.L., Barcelona, Spain). Real-time polymerase chain reaction (PCR) was performed with 2 microliters (µL) of cDNA, SYBR Green and 300 nM of specific primers for adiponectin (ADIPOQ) and β-actin (ACTB), previously described 28. The thermal profile was (95°C for 30 sec and 60°C for 30 sec) for 40 cycles (Stratagene Mx3005P, Agilent Technologies S.L., Las Rozas, Spain). The cycle threshold (Ct) values of ADIPOQ were normalized by the Ct values of ACTB. The differential expression between treated and control points was represented as a fold change by the 2-ΔΔCt algorithm.
Statistical analysis
Clinical characteristics of all patients were expressed as mean±standard deviation (SD) in only continues variables or as percentage (%) in those categorical variables. Differences among groups of patients were expressed at the same manner and their comparison was performed by ANOVA in variables with normal distribution and Kruskal-Wallis test in those skewed. Comparison between treatment and control was analyzed by paired t test. Differences of categorical variables among subgroups were performed by chi-squared test. Non parametric correlation between adiponectin and proBNP was performed by Spearman´s rank coefficient. Pearson correlation coefficient was used for association between adiponectin and total cholesterol levels. Univariate and multivariate Cox regression analyses were employed to calculate the estimated hazard ratio (HR) with 95% confidence interval (CI), where it was appropriate. The variables were entered into a multivariate model for factors with a p value of ≤0.05 in the univariate analysis. The Statistical Package for Social Science (SPSS) for Windows, version 15.0 (software SPSS Inc.; Chicago, Illinois, USA) was used for all statistical analyses. Statistical significance was defined as p<0.05.
Results
Baseline characteristics of the population regarding nutritional state
The classification regarding nutritional state determined 31 patients with normal nutrition, 35 with mild malnutrition and 8 were moderate-severely malnourished. These groups differed respect to haemoglobin, albumin, leukocytes, proBNP and adiponectin levels. Thus, patients with the worst nutritional state had lower levels of haemoglobin and high proBNP and adiponectin levels compared with patients with normal or mild nutritional state (Table 1).
Table 1.
Nutritional status (normal; n=31, mild; n=35, moderate; n=8)
| Nutritional state | % | Standard deviation | SEM | Significance | ||
|---|---|---|---|---|---|---|
| Female | Normal | 29 | 0.511 | |||
| Mild | 37 | |||||
| Moderate-Severe | 50 | |||||
|
Age (yrs) |
Normal | 67.23 | 10.91 | 1.96 | .538 | |
| Mild | 67.17 | 12.53 | 2.12 | |||
| Moderate -Severe | 72.00 | 7.96 | 2.82 | |||
|
BMI (kg/m2) |
Normal | 31.27 | 5.61 | 1.01 | 0.731 | |
| Mild | 30.16 | 7.53 | 1.27 | |||
| Moderate-Severe | 31.81 | 7.97 | 2.82 | |||
|
Heart Rate (at discharge) |
Normal | 69.00 | 12.10 | 2.17 | .981 | |
| Mild | 68.66 | 14.20 | 2.40 | |||
| Moderate-Severe | 68.00 | 14.28 | 5.05 | |||
|
LVEF (%) |
Normal | 40.99 | 14.33 | 2.57 | .891 | |
| Mild | 39.43 | 14.32 | 2.42 | |||
| Moderate-Severe | 41.42 | 19.49 | 6.89 | |||
|
Hemoglobin (g/dL) |
Normal | 14.45 | 1.54 | .28 | .000*** | |
| Mild | 13.56 | 1.88 | .32 | |||
| Moderate-Severe | 11.76 | 1.21 | .43 | |||
|
Albumin (g/dL) |
Normal | 4.20 | .37 | .07 | .000*** | |
| Mild | 3.84 | .37 | .06 | |||
| Moderate-Severe | 3.55 | .50 | .18 | |||
| HTA | Normal | 77 | 0.856 | |||
| Mild | 71 | |||||
| Moderate-Severe | 75 | |||||
| T2DM | Normal | 39 | 0.768 | |||
| Mild | 37 | |||||
| Moderate-Severe | 25 | |||||
| Previous MI | Normal | 0 | 0.007** | |||
| Mild | 20 | |||||
| Moderate-Severe | 37 | |||||
| CAD | Normal | 13 | 0.201 | |||
| Mild | 31 | |||||
| Moderate-Severe | 25 | |||||
|
Glucose (mg/dL) |
Normal | 147.55 | 48.49 | 8.71 | .568 | |
| Mild | 141.20 | 58.51 | 9.89 | |||
| Moderate-Severe | 123.57 | 56.19 | 21.24 | |||
|
HbA1c (%) |
Normal | 6.30 | 1.18 | .21 | .977 | |
| Mild | 6.31 | 1.19 | .22 | |||
| Moderate-Severe | 6.40 | 1.19 | .42 | |||
|
Triglycerides (mg/dL) |
Normal | 123.58 | 50.97 | 9.15 | .040* | |
| Mild | 104.14 | 44.32 | 7.49 | |||
| Moderate-Severe | 77.43 | 24.59 | 9.30 | |||
|
HDL-cholesterol (mg/dL) |
Normal | 50.04 | 16.97 | 3.21 | .010** | |
| Mild | 38.74 | 14.27 | 2.56 | |||
| Moderate-Severe | 35.43 | 12.58 | 4.75 | |||
|
LDL-cholesterol (mg/dL) |
Normal | 109.36 | 31.85 | 6.02 | .001*** | |
| Mild | 88.45 | 32.33 | 5.81 | |||
| Moderate-Severe | 61.86 | 25.71 | 9.72 | |||
|
Creatinin (mg/dL) |
Normal | 1.31 | .51 | .09 | .478 | |
| Mild | 1.18 | .34 | .06 | |||
| Moderate-Severe | 1.30 | .43 | .15 | |||
|
Na+ (meq/l) |
Normal | 141.87 | 2.64 | .47 | .075 | |
| Mild | 141.17 | 4.82 | .82 | |||
| Moderate-Severe | 138.13 | 5.08 | 1.80 | |||
|
K+(meq/l) |
Normal | 4.49 | .69 | .12 | .819 | |
| Mild | 4.52 | .63 | .11 | |||
| Moderate-Severe | 4.65 | .36 | .13 | |||
|
TPI |
Normal | .27 | 1.07 | .21 | .572 | |
| Mild | 14.14 | 74.79 | 13.89 | |||
| Moderate-Severe | .016 | .02 | .01 | |||
|
Lymphocytes (count/mL) |
Normal | 2194.84 | 634.11 | 113.89 | .000*** | |
| Mild | 1365.71 | 506.28 | 85.58 | |||
| Moderate-Severe | 851.25 | 266.16 | 94.10 | |||
|
Classic monocytes (%) |
Normal | 42.44 | 17.33 | 3.16 | .166 | |
| Mild | 46.49 | 18.08 | 3.15 | |||
| Moderate-Severe | 57.50 | 19.38 | 7.91 | |||
|
Intermediate monocytes (%) |
Normal | 13.62 | 10.10 | 1.84 | .543 | |
| Mild | 13.05 | 10.39 | 1.81 | |||
| Moderate | 8.60 | 8.89 | 3.63 | |||
|
Non classical monocytes (%) |
Normal | 15.54 | 8.16 | 1.49 | .576 | |
| Mild | 14.19 | 8.27 | 1.44 | |||
| Moderate | 11.98 | 6.79 | 2.77 | |||
|
ProBNP (pg/mL) |
Normal | 2519.58 | 2669.47 | 479.45 | .001*** | |
| Mild | 4166.65 | 3543.59 | 607.72 | |||
| Moderate-Severe | 9230.50 | 10331.07 | 3652.59 | |||
|
Adiponectin (ug/mL) |
Normal | 7.14 | 4.48 | .80 | .002*** | |
| Mild | 7.93 | 4.06 | .69 | |||
| Moderate-Severe | 13.71 | 6.69 | 2.36 | |||
| Statins | Normal | 64 | 0.541 | |||
| Mild | 51 | |||||
| Moderate-Severe | 62 | |||||
| ACEI-ARBs | Normal | 96 | 0.765 | |||
| Mild | 91 | |||||
| Moderate-Severe | 87 | |||||
| β-blockers | Normal | 87 | 0.633 | |||
| Mild | 80 | |||||
| Moderate-Severe | 75 | |||||
| ARM | Normal | 87 | 0.440 | |||
| Mild | 90 | |||||
| Moderate-Severe | 87 | |||||
| Diuretics | Normal | 100 | 0.151 | |||
| Mild | 97 | |||||
| Moderate-Severe | 87 |
BMI: Body mass index; LVEF: Left ventricle ejection fraction; MI: myocardial infarction; T2DM: Type 2 diabetes mellitus; HTA: Hypertension; HLP: Hyperlipemia; proBNP: pro-Brain natriuretic peptide; ACEIBs: Angiotensin converting enzyme inhibitor-Angiotensin receptor blockers; ARM: antagonist mineralocorticoid receptor; Hb; Hemoglobin.
Major factors associated with high adiponectin levels
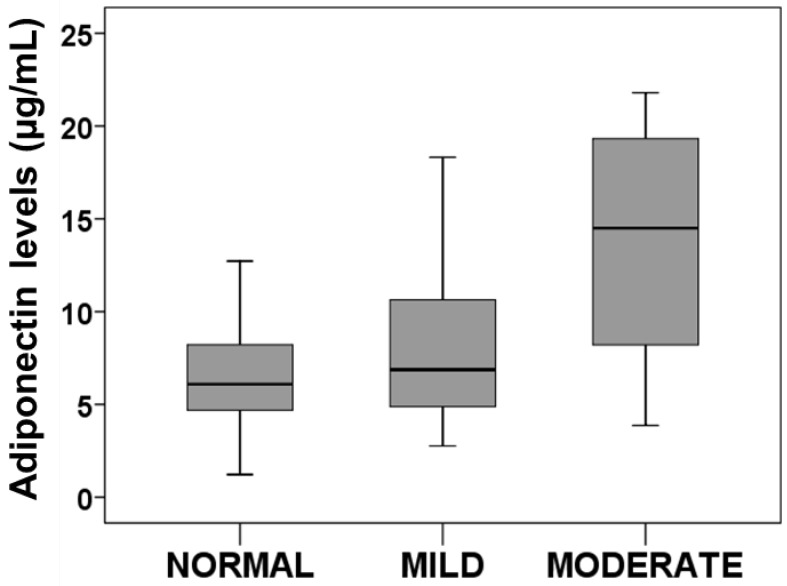
Our results showed that the normal nutritional state is characterized by low adiponectin levels at discharge (7.14 ± 4.48 µg/mL; n=31). Those patients with mild nutritional state had a slight increment (7.93 ± 4.06 µg/mL; n=35). Finally, patients with moderate- severe nutritional state presented the highest adiponectin levels (13.71 ± 6.96; µg/mL; n=8). These differences among groups reached the statistical significance (P<0.01) (Figure 1).
Figure 1.
Adiponectin levels regarding nutritional state. Box plots represents plasma adiponectin levels at discharge in patients with normal, mild, moderate nutritional state. Anova test determined statistical significance p<0.01.
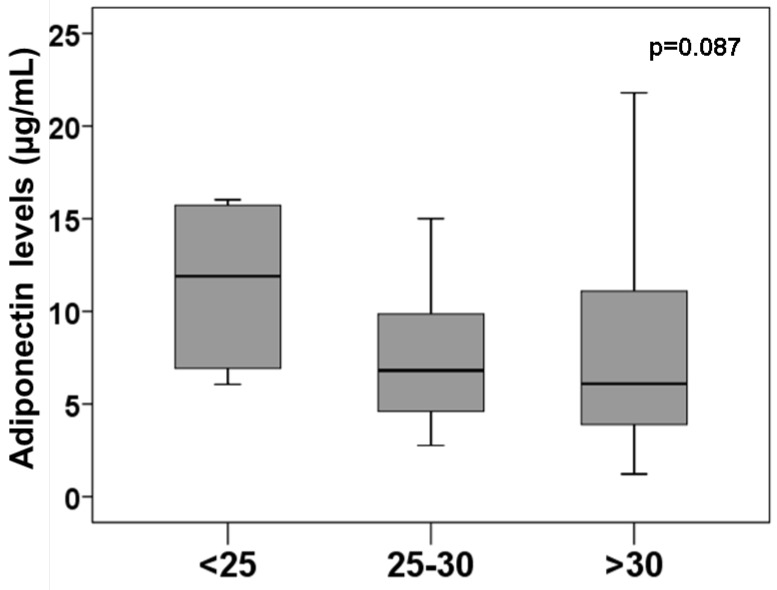
Our data confirmed the association between proBNP and nutritional state or adiponectin levels (Supp. Figure 1). Although patients with overweight or obese presented the lowest adiponectin levels, the differences didn´t reach the statistical significance (Figure 2). Thus, 11.61 ± 4.06 µg/mL of adiponectin was found in patients with BMI<25 kg/m2, 7.77 ± 3.96 µg/mL levels in patients with BMI between 25 and 30 kg/m2 and 8.08 ± 5.82 µg/mL in patients with BMI > 30 kg/m2.
Figure 2.
Adiponectin levels regarding obesity. Box plots represents plasma adiponectin levels at discharge in patients regarding obesity. Non statistical significance among groups.
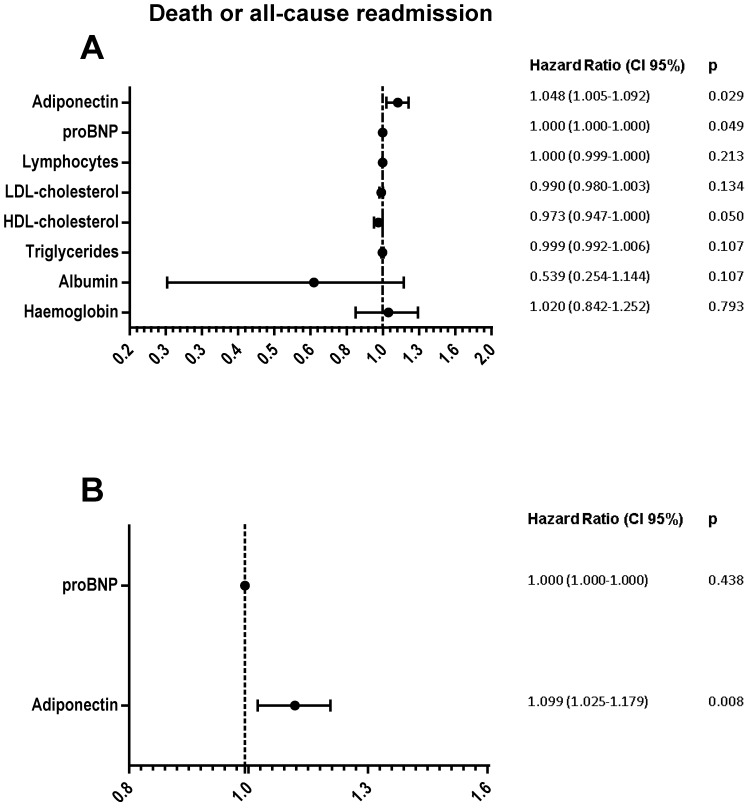
Univariate analysis determined that only proBNP and adiponectin, differential factors associated with nutritional state, were risk factors of death or all-cause readmission (Figure 3A). However, multivariate analysis determined high adiponectin levels as the independent risk factor (Figure 3B).
Figure 3.
Forest plot represents Hazard ratio (black circle) and 95% confidence intervals (horizontal lines) of variables association with death or all-cause readmission. Univariate (A) or multivariate (B) Cox regression analysis.
Adiponectin regulation by nutrients starvation
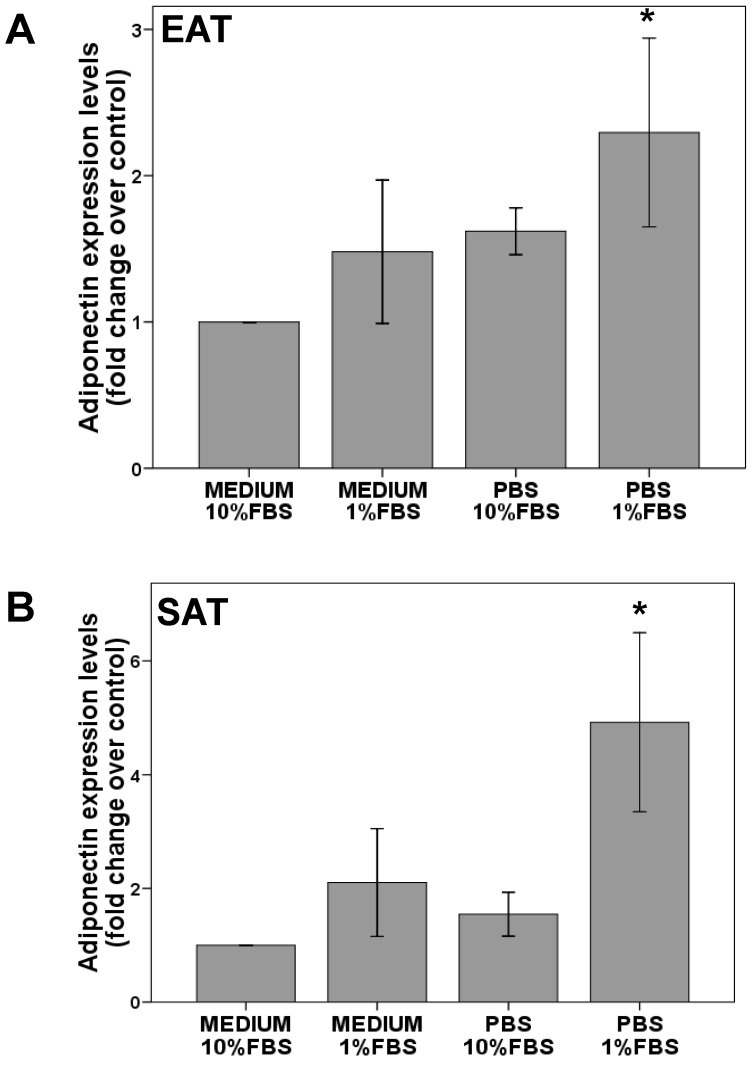
Stromal vascular cells isolation from epicardial or subcutaneous fat pads, were adipogenized until adiponectin expression detection. Finally, cells were starved for 8 hours. The reduction from 10% to 1% of serum (FBS) increased slightly adiponectin levels in EAT (1.5±0.5 fold change over FBS 10%) or SAT cells (2.1±0.9 fold change over FBS 10%). However, this increment reached statistical significance with serum and nutrients starvation in EAT (2.2±0.3 fold change over control; p<0.05) and SAT (8.0 ± 2.3 fold change over control; p<0.05) cells as it is shown in Figure 4.
Figure 4.
Adiponectin expression levels in adipogenesis-induced EAT (A) or SAT (B) stromal vascular cells. After adipogenesis induction for 14 -18 days, cells were cultured for 24 hours with medium+ (10% or 1%FBS) or starved medium (10% or 1% FBS). Paired t test determined statistical significance p<0.05 between PBS 1%FBS and medium+10%FBS (control). N=5.
Discussion
For the first time, our group has showed that high adiponectin levels are associated with malnutrition and worse prognosis in de novo HF patients. Moreover, we found that starvation upregulates adiponectin expression levels in epicardial and subcutaneous stromal cells after adipogenesis induction. This result contributes to understand the paradoxical behavior of adiponectin in HF patients.
Nowadays malnutrition is a common comorbidity in advanced HF and the nutritional status is considered as a marker and a mediator of the disease´s progression, severity and prognosis 29. However in de novo HF patients its prognostic value is less clear. In our study we found that about 10% of patients with de novo HF are moderate-severely malnourished and we described that this state is characterized by high adiponectin and proBNP and low hemoglobin levels. This result reinforced the need of determining the nutritional status in de novo HF patients.
Adiponectin is mainly produced by adipocytes 1 with cardioprotective properties 3 in vasculature and myocardium cells. Traditionally obesity is associated with an increment of adipocytes but paradoxically exists a downregulation of adiponectin levels 30. This reduction drives up insulin resistance 31 and in consequence cardiovascular disease. Contrary, anorexia nervosa with an adipose tissue mass reduction is associated with hyperadiponectinemia 32, higher insulin sensitivity 32, mild anaemia and moderate leucopoenia 33. Likewise, patients with de novo HF and moderate-severe malnutrition presented the highest adiponectin levels. However, we didn´t find a clear association with BMI. Despite of the adipose tissue is the main adiponectin producer, we didn´t find any correlation between body fat mass and its circulating levels. However, there was a clear inverse association with total cholesterol levels, which is used for CONUT score calculation, as it was previously described 34,35 (Sup. Figure 2). These results suggest that adiponectin levels are more dependent on the nutritional state than BMI in de novo HF.
Some studies highlighted hyperadiponectinemia could be due to an adiponectin resistance in cellular receptors 36, similar to insulin resistance, promoting metabolic dysfunction and HF syndrome 37. However, adiponectin is an insulin sensitizer and we didn´t find any differences regarding diabetes presence (data not shown). This may indicate that another factor is the main cause of high plasma adiponectin levels in de novo HF patients. Thus, we focus our attention on the association between malnutrition and adiponectin. Malnutrition entails energy reduction with catabolic/anabolic imbalance that leads to cachexia 38. Our results showed that starvation of nutrients and circulating factors upregulated adiponectin levels in adipogenesis-induced stromal cells from epicardial or subcutaneous fat cells. The absence of fat stores, as well as in chronic anorexia nervosa and in malnutrition 39, can be the main reason of hyperadiponectinemia in patients and explain its paradoxical behavior in HF.
As it was previously described we found a positive relationship between adiponectin levels and proBNP levels 40. We also described that adiponectin is a prognostic factor in de novo HF (figure 4) independent of the NT-proBNP levels suggesting that high adiponectin levels in these patients could be a mediator of the disease progression.
Our results identified that patients with high adiponectin levels in de novo HF had worse nutritional status and worse outcomes suggesting that they were in a more advanced phase of the disease and efforts should be focus on them to avoid the progression of the disease.
Although more mechanistic insights are necessary to understand the adiponectin regulation in HF, our results contribute to the knowledge of this molecule as indicator of nutritional state. Since a huge increment of adiponectin levels can be mediators of chronic obstructive pulmonary disease 41 and HF, a nutritional intervention in these patients might normalize adiponectin levels, which can modulate cardiometabolic disorders 42 and reduce -HF progression.
Limitations
The main limitation of our study is the number of patients; however the results are consistent and statistically significant. It is a unicentric, retrospective and observational study with the limitations inherent to this type of design. We use the CONUT score which is not best match with the subjective global assessment of nutritional status in HF patients but it is very easy to use in hospitalized patients.
Conclusion
High adiponectin levels in patients with de novo HF are associated with worse nutritional status. Starvation regulates adiponectin expression levels in epicardial and subcutaneous fat cells and may explain its behaviour in HF.
Acknowledgments
We would like to thank patient's participation. The present study was supported by Complejo Hospitalario Universitario de Santiago de Compostela (Santiago de Compostela, Spain), Fondo de Investigaciones Sanitarias (PIE13/00024) and (PI13/01852) from Plan Estatal de I+D+I 2013-2016 and cofounded by ISCIII-Subdirección General de Evaluación y Fomento de la Investigación el Fondo Europeo de Desarrollo Regional (FEDER).
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, Kadowaki T. Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2005;54:3358–3370. doi: 10.2337/diabetes.54.12.3358. [DOI] [PubMed] [Google Scholar]
- 3.Villarreal-Molina MT, Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie. 2012;94:2143–2149. doi: 10.1016/j.biochi.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 4.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 5.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 7.Guo R, Zhang Y, Turdi S, Ren J. Adiponectin knockout accentuates high fat diet-induced obesity and cardiac dysfunction: role of autophagy. Biochim Biophys Acta. 2013;1832:1136–1148. doi: 10.1016/j.bbadis.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 9.Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, Gallo P, di Gioia CR. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Eiras S, Teijeira-Fernandez E, Shamagian LG, Fernandez AL, Vazquez-Boquete A, Gonzalez-Juanatey JR. Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue. Cytokine. 2008;43:174–180. doi: 10.1016/j.cyto.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Lala A, Desai AS. The role of coronary artery disease in heart failure. Heart Fail Clin. 2014;10:353–365. doi: 10.1016/j.hfc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Rame JE. Chronic heart failure: a reversible metabolic syndrome? Circulation. 2012;125:2809–2811. doi: 10.1161/CIRCULATIONAHA.112.108316. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Funayama H, Kubo N, Yasu T, Kawakami M, Saito M, Momomura S, Ishikawa SE. Association of hyperadiponectinemia with severity of ventricular dysfunction in congestive heart failure. Circ J. 2006;70:1557–1562. doi: 10.1253/circj.70.1557. [DOI] [PubMed] [Google Scholar]
- 14.Ohara T, Hashimura K, Asakura M, Ogai A, Amaki M, Hasegawa T, Kanzaki H, Sonoda M, Nishizawa H, Funahashi T, Kitakaze M. Dynamic changes in plasma total and high molecular weight adiponectin levels in acute heart failure. J Cardiol. 2011;58:181–190. doi: 10.1016/j.jjcc.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Celik T, Yaman H. Elevated adiponectin levels in patients with chronic heart failure: an independent predictor of mortality or a marker of cardiac cachexia? Int J Cardiol. 2009;144:319–320. doi: 10.1016/j.ijcard.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Haugen E, Furukawa Y, Isic A, Fu M. Increased adiponectin level in parallel with increased NT-pro BNP in patients with severe heart failure in the elderly: A hospital cohort study. Int J Cardiol. 2008;125:216–219. doi: 10.1016/j.ijcard.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Kopp HP, Krzyzanowska K, Mohlig M, Spranger J, Pfeiffer AF, Schernthaner G. Effects of marked weight loss on plasma levels of adiponectin, markers of chronic subclinical inflammation and insulin resistance in morbidly obese women. Int J Obes (Lond) 2005;29:766–771. doi: 10.1038/sj.ijo.0802983. [DOI] [PubMed] [Google Scholar]
- 18.Szabo T, Scherbakov N, Sandek A, Kung T, von Haehling S, Lainscak M, Jankowska EA, Rudovich N, Anker SD, Frystyk J, Flyvbjerg A, Pfeiffer AF, Doehner W. Plasma adiponectin in heart failure with and without cachexia: catabolic signal linking catabolism, symptomatic status, and prognosis. Nutr Metab Cardiovasc Dis. 2013;24:50–56. doi: 10.1016/j.numecd.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Van Berendoncks AM, Garnier A, Beckers P, Hoymans VY, Possemiers N, Fortin D, Martinet W, Van Hoof V, Vrints CJ, Ventura-Clapier R, Conraads VM. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail. 2010;3:185–194. doi: 10.1161/CIRCHEARTFAILURE.109.885525. [DOI] [PubMed] [Google Scholar]
- 20.Van Berendoncks AM, Garnier A, Beckers P, Hoymans VY, Possemiers N, Fortin D, Van Hoof V, Dewilde S, Vrints CJ, Ventura-Clapier R, Conraads VM. Exercise training reverses adiponectin resistance in skeletal muscle of patients with chronic heart failure. Heart. 2011;97:1403–1409. doi: 10.1136/hrt.2011.226373. [DOI] [PubMed] [Google Scholar]
- 21.Pannacciulli N, Vettor R, Milan G, Granzotto M, Catucci A, Federspil G, De Giacomo P, Giorgino R, De Pergola G. Anorexia nervosa is characterized by increased adiponectin plasma levels and reduced nonoxidative glucose metabolism. J Clin Endocrinol Metab. 2003;88:1748–1752. doi: 10.1210/jc.2002-021215. [DOI] [PubMed] [Google Scholar]
- 22.Manigrasso MR, Ferroni P, Santilli F, Taraborelli T, Guagnano MT, Michetti N, Davi G. Association between circulating adiponectin and interleukin-10 levels in android obesity: effects of weight loss. J Clin Endocrinol Metab. 2005;90:5876–5879. doi: 10.1210/jc.2005-0281. [DOI] [PubMed] [Google Scholar]
- 23.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 24.Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, Rodriguez F, Fernandez G. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45. [PubMed] [Google Scholar]
- 25.Eiras S, Varela-Roman A, Andrade MC, Castro A, Gonzalez-Ferreiro R, Vinuela JE, Fernandez-Trasancos A, Carreira MC, Alvarez E, Casanueva FF, Gonzalez-Juanatey JR. Non classical Monocytes Levels, Increased by Subcutaneous Fat-Secretome, Are Associated with Less Rehospitalization after Heart Failure Admission. J Cardiovasc Transl Res. 2017;10:16–26. doi: 10.1007/s12265-016-9724-y. [DOI] [PubMed] [Google Scholar]
- 26.Agra RM, Varela-Roman A, Gonzalez-Ferreiro R, Vinuela JE, Castro-Pais A, Fernandez-Trasancos A, Diaz-Rodriguez E, Alvarez E, Carreira MC, Casanueva FF, Gonzalez-Juanatey JR, Eiras S. Orosomucoid as prognosis factor associated with inflammation in acute or nutritional status in chronic heart failure. Int J Cardiol. 2017;228:488–494. doi: 10.1016/j.ijcard.2016.11.134. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Trasancos A, Guerola-Segura R, Paradela-Dobarro B, Alvarez E, Garcia-Acuna JM, Fernandez AL, Gonzalez-Juanatey JR, Eiras S. Glucose and Inflammatory Cells Decrease Adiponectin in Epicardial Adipose Tissue Cells: Paracrine Consequences on Vascular Endothelium. J Cell Physiol. 2015;231:1015–1023. doi: 10.1002/jcp.25189. [DOI] [PubMed] [Google Scholar]
- 28.Iglesias MJ, Eiras S, Pineiro R, Lopez-Otero D, Gallego R, Fernandez AL, Lago F, Gonzalez-Juanatey JR. [Gender differences in adiponectin and leptin expression in epicardial and subcutaneous adipose tissue. Findings in patients undergoing cardiac surgery] Rev Esp Cardiol. 2006;59:1252–1260. [PubMed] [Google Scholar]
- 29.Agra Bermejo RM, Gonzalez Ferreiro R, Varela Roman A, Gomez Otero I, Kreidieh O, Conde Sabaris P, Rodriguez-Manero M, Moure Gonzalez M, Seoane Blanco A, Virgos Lamela A, Garcia Castelo A, Gonzalez Juanatey JR. Nutritional status is related to heart failure severity and hospital readmissions in acute heart failure. Int J Cardiol. 2017;230:108–114. doi: 10.1016/j.ijcard.2016.12.067. [DOI] [PubMed] [Google Scholar]
- 30.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 31.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 32.Delporte ML, Brichard SM, Hermans MP, Beguin C, Lambert M. Hyperadiponectinaemia in anorexia nervosa. Clin Endocrinol (Oxf) 2003;58:22–29. doi: 10.1046/j.1365-2265.2003.01702.x. [DOI] [PubMed] [Google Scholar]
- 33.Devuyst O, Lambert M, Rodhain J, Lefebvre C, Coche E. Haematological changes and infectious complications in anorexia nervosa: a case-control study. Q J Med. 1993;86:791–799. [PubMed] [Google Scholar]
- 34.Kosacka M, Korzeniewska A, Jankowska R. The evaluation of body composition, adiponectin, C-reactive protein and cholesterol levels in patients with obstructive sleep apnea syndrome. Adv Clin Exp Med. 2014;22:817–824. [PubMed] [Google Scholar]
- 35.Kojima S, Maruyoshi H, Nagayoshi Y, Kaikita K, Sumida H, Sugiyama S, Funahashi T, Ogawa H. Hypercholesterolemia and hypoadiponectinemia are associated with necrotic core-rich coronary plaque. Int J Cardiol; 2009. [DOI] [PubMed] [Google Scholar]
- 36.Rathmann W, Herder C. Adiponectin and cardiovascular mortality: evidence for "reverse epidemiology". Horm Metab Res. 2007;39:1–2. doi: 10.1055/s-2007-958630. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Doehner W, Frenneaux M, Anker SD. Metabolic impairment in heart failure: the myocardial and systemic perspective. J Am Coll Cardiol. 2014;64:1388–1400. doi: 10.1016/j.jacc.2014.04.083. [DOI] [PubMed] [Google Scholar]
- 39.Brichard SM, Delporte ML, Lambert M. Adipocytokines in anorexia nervosa: a review focusing on leptin and adiponectin. Horm Metab Res. 2003;35:337–342. doi: 10.1055/s-2003-41353. [DOI] [PubMed] [Google Scholar]
- 40.Allison MA, Criqui MH, Maisel AS, Daniels LB, Roberts CK, Polak JF, Cushman M. Adiponectin is independently associated with NT-proBNP: The Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2015;25:780–786. doi: 10.1016/j.numecd.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato K, Shibata Y, Abe S, Inoue S, Igarashi A, Yamauchi K, Aida Y, Nunomiya K, Nakano H, Sato M, Kimura T, Nemoto T, Watanabe T, Konta T, Ueno Y, Kato T, Kayama T, Kubota I. Association between plasma adiponectin levels and decline in forced expiratory volume in 1 s in a general Japanese population: the Takahata study. Int J Med Sci. 2014;11:758–764. doi: 10.7150/ijms.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Jaramillo P. The Role of Adiponectin in Cardiometabolic Diseases: Effects of Nutritional Interventions. J Nutr. 2016;146:422S–426S. doi: 10.3945/jn.114.202432. [DOI] [PubMed] [Google Scholar]