Abstract
Rab8 is a GTPase involved in membrane trafficking. In photoreceptor cells, rab8 is proposed to participate in the late stages of delivery of rhodopsin-containing post-Golgi membranes to the plasma membrane near the base of the connecting cilium. To test the function of rab8 in vivo, we generated transgenic Xenopus laevis expressing wild-type, constitutively active (Q67L), and dominant negative (T22N) forms of canine rab8 in their rod photoreceptors as green fluorescent protein (GFP) fusion proteins. Wild-type and constitutively active GFP-rab8 proteins were primarily associated with Golgi and post-Golgi membranes, whereas the dominant negative protein was primarily cytoplasmic. Expression of wild-type GFP-rab8 had minimal effects on cell survival and intracellular structures. In contrast, GFP-rab8T22N caused rapid retinal degeneration. In surviving peripheral rods, tubulo-vesicular structures accumulated at the base of the connecting cilium. Expression of GFP-rab8Q67L induced a slower retinal degeneration in some tadpoles. Transgene effects were transmitted to F1 offspring. Expression of the GFP-rab8 fusion proteins appears to decrease the levels of endogenous rab8 protein. Our results demonstrate a role for rab8 in docking of post-Golgi membranes in rods, and constitute the first report of a transgenic X. laevis model of retinal degenerative disease.
INTRODUCTION
Vertebrate rod photoreceptors are highly polarized neurons. They possess a light-detecting organelle, the rod outer segment (OS), which is separated from the cell body (the inner segment [IS]) by a connecting cilium (CC) and is composed of a stack of rhodopsin-containing membranous disks. OS membranes are continuously renewed (Young and Droz, 1968). Amphibian rods synthesize photosensitive membranes at an extremely high rate, estimated at 3.2 μm2/min/cell for Xenopus laevis (Besharse et al., 1977; Besharse, 1986). Amphibian rods are an excellent system for studying neuronal membrane transport, because the components involved are likely to be hypertrophied to accommodate high synthetic rates. Furthermore, rods are sensitive to trafficking disruptions; mutations in the rhodopsin gene that disrupt a sorting signal result in IS accumulation of rhodopsin and cause retinitis pigmentosa (Berson 1993; Sung et al., 1994; Deretic et al., 1998; Tam et al., 2000).
Eukaryotic cells possess complex mechanisms to control membrane trafficking between intracellular compartments. Rab proteins are highly conserved GTPases involved in trafficking, although their role is obscure. The human genome contains genes encoding at least 60 rab family members (Bock et al., 2001), each of which may participate in a different trafficking pathway (Novick and Zerial, 1997). Rab8 and rab6, as well as other GTP-binding proteins, are associated with rhodopsin-containing post-Golgi membranes isolated from frog photoreceptors (Deretic et al., 1995; Deretic and Papermaster, 1993). Peptides derived from the effector region of rab6 (Deretic, 1998) and the C terminus of rhodopsin (Deretic et al., 1998) inhibit the formation of rhodopsin-bearing post-Golgi membranes in vitro. Rab8 may participate in a subsequent step, because it is localized near the base of the CC (Deretic et al., 1995). By analogy with other cell types and the yeast homologue sec4p, rab8 is likely to be involved in transport between the TGN and the plasma membrane (Goud et al., 1988; Huber et al., 1993a,b).
Rhodopsin is synthesized within the IS and transported on membranes that bud from the TGN and fuse with the plasma membrane near the CC (Papermaster et al., 1985, 1986; Deretic and Papermaster 1991). In amphibians, this region of membrane is folded into ridges and grooves, forming the periciliary ridge complex (PRC; Peters et al., 1983). Amphibian rods may require this membrane modification to accommodate high rates of membrane turnover. After docking at the PRC, rhodopsin is immediately transported to the OS, most likely via the CC plasma membrane; IS plasma membrane concentrations of rhodopsin are normally low (Papermaster et al., 1985).
An in vitro assay for rab8 function in rods would be difficult to construct, requiring isolation of large quantities of PRC membrane, with cytoplasmic surfaces accessible to assay reagents. Therefore, we decided to study rab8 function in ROS membrane trafficking in vivo, by expressing enhanced green fluorescent protein (GFP)-rab8 fusion proteins in X. laevis rods under control of the X. laevis opsin promoter (Batni et al., 1996). In addition to a wild-type rab8, we used dominant negative (rab8T22N) and constitutively active (rab8Q67L) mutants of canine rab8 previously characterized in cultured BHK fibroblasts (Peränen et al., 1996). GFP-rab8T22N is predicted to have a higher affinity for GDP than GTP (Peränen et al., 1996), whereas GFP-rab8Q67L is predicted to have decreased GTPase activity (Walworth et al., 1992; Richardson et al., 1998). We expected that canine proteins would function adequately in frog retinas because the rab8 gene is highly conserved. We chose X. laevis photoreceptors because rhodopsin transport has been rigorously studied in amphibians, X. laevis rods are large and easily visualized by light microscopy, and transgenic X. laevis are easily generated (Kroll and Amaya 1996; Knox et al., 1998; Moritz et al., 1999).
Numerous transgenic X. laevis tadpoles expressing each fusion protein in rods were generated. Several were raised to sexual maturity, which led to the analysis of transgene effects on F1 offspring. We used histologic techniques, including confocal, electron, and immuno-electron microscopy to study the effects of GFP-rab8 fusion protein expression. Finally, the effect of rab8 fusion protein expression on endogenous rab8 expression was investigated by Western blot. The results demonstrate a significant role for rab8 in docking of rhodopsin-bearing vesicles to the PRC membranes.
MATERIALS AND METHODS
Constructs.
Plasmids for generating transgenic X. laevis were constructed from the previously described canine rab8 mutant and wild-type cDNAs (Peränen et al., 1996). The cDNAs were cloned into the EcoRI/XhoI sites of the previously described vector XOP-eGFP-C1 (Moritz et al., 1999), which uses a 5.5-kb fragment of the X. laevis rhodopsin promoter (Batni et al., 1996) to drive expression of GFP fusion proteins in rod photoreceptors. The resulting fusion proteins consisted of rab8 fused to the C terminus of GFP.
Transgenic Frogs and Tadpoles.
Transgenic X. laevis were generated by the method of Kroll and Amaya (1996), modified as previously described (Moritz et al., 1999). Transgene constructs were linearized with the restriction enzyme NotI. We used microscopes equipped with standard epifluorescence optics, GFP filter sets, and a digital camera to identify transgenic tadpoles by their green fluorescent eyes and to obtain images of their eyes. Tadpoles were raised at 18°C on a 12 h:12 h light:dark cycle and initially were housed 1 per well in standard 24-well dishes in 0.1× Gerhardt's ringers (Wu and Gerhart, 1991). After 14 d, tadpoles were transferred to 4-liter tanks containing 0.1× Gerhardt's ringers and fed a 50:50 mixture of powdered NASCO frog brittle (Fort Atkinson, WI) and powdered frog chow (Rangen, Buhl, ID).
Confocal Microscopy.
Eyes from transgenic tadpoles were fixed in 0.1 M sodium phosphate, pH 7.5, containing 4% paraformaldehyde. The eyes were embedded in OCT medium and 14-μm sections were cut with a cryostat and collected on glass slides. Sections were permeablized and labeled with Texas red–wheat germ agglutinin conjugate (TR-WGA; Molecular Probes, Eugene OR), and Hoechst 33342 nuclear stain (Sigma-Aldrich, St. Louis, MO) as previously described (Tam et al. 2000). Alternatively, sections were labeled with Hoechst 33342 and antirhodopsin mAb mabE as previously described (Moritz et al. 1999) with a Cy3-conjugated goat anti-mouse secondary antibody (Jackson Immunoresearch, West Grove, PA). Sections were imaged with the use of a Zeiss 410 laser scanning confocal microscope (Carl Zeiss, Thornwood, NY). Digital images were prepared for publication with Adobe Photoshop 5.0 software (Adobe Systems Inc., San Jose, CA). Use of contrast enhancement and manipulation of gamma was avoided.
Electron Microscopy
Epoxy Resin Embedding for Conventional Electron Microscopy.
One hour after light onset, tadpoles were fixed in 1% glutaraldehyde/4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.5, for a minimum of 2 d. The eyes were removed, and postfixed in 1% osmium tetroxide/0.8% potassium ferricyanide in 0.1 M cacodylate buffer, pH 7.4, for 60 min. After en bloc staining with 1% aqueous uranyl acetate for 1 h, the tissue was dehydrated through graded ethanol solutions to absolute ethanol, 50% propylene oxide/50% Polybed 812 epoxy resin (Polysciences, Warrington, PA) and 100% Polybed 812. Blocks were cured at 60°C for 48 h. Sections 100 nm in thickness were mounted on Formvar films and poststained with 2% aqueous uranyl acetate and Sato's lead citrate.
LR White Acrylic Resin Embedding for Immuno-electron Microscopy.
Tadpoles were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for a minimum of 2 d. The eyes were dehydrated in a series of graded ethanol solutions and infiltrated and embedded at 4°C in LR White acrylic resin (Ted Pella, Inc., Irvine, CA) after the manufacturer's procedures.
Immunocytochemistry.
Grids were incubated in 0.1 M Tris buffer, pH 7.5 (TB), for 5 min, followed by a 10-min block in 4% normal goat serum (NGS), and incubated 15 min in 20 mM glycine in TB. Grids were then exposed to either polyclonal anti-GFP antibody (Clontech, Palo Alto, CA) diluted 1:200 or antirhodopsin mAb 11D5 (Deretic and Papermaster, 1991) diluted 1:5000 in 1% NGS in TB for 30 min. Grids were washed in TB and incubated with secondary antibody conjugated to 10 nm colloidal gold (APBiotech, Uppsala. Sweden) (anti-GFP was labeled with goat anti-rabbit IgG; 11D5 with goat anti-mouse IgG). After washing with TB followed by 0.1 M phosphate buffer, bound antibodies were fixed by incubation in 2% glutaraldehyde for 15 min, followed by a distilled water rinse. Grids were poststained with 2% aqueous uranyl acetate and Sato's lead citrate.
Western Blot Analysis.
Western blots were performed on stage 51–54 tadpole retinas. One eye isolated from each animal was triturated in 100 μl of SDS sample buffer with fresh β-mercaptoethanol with the use of a 1-ml pipette tip. After 1 h at room temperature in this buffer, homogenates were centrifuged briefly to remove undissolved tissue. Supernatants were diluted and samples corresponding to 1/8 of a retina were separated by SDS-PAGE and transferred to Immobilon membranes (Millipore, Waltham, MA). Half of the blot was probed with antirab8 antibody (BD Transduction Laboratories, San Diego, CA) diluted 1:500, and the other with anti-GFP antibody (Clontech) diluted 1:1000. Bound antibodies were detected with the use of the ECL Western Blotting Detection System (APBiotech). The expression of detected antigens was quantified with the use of Multianalyst software (Bio-Rad, Hercules, CA).
RESULTS
Transgenic X. laevis Production
In a typical experiment, ∼50 transgenic tadpoles expressing GFP-rab8 fusion proteins in their rod photoreceptors were generated from 2500 injected eggs. This yield paralleled prior experience with tadpoles expressing GFP in rods, a protein that is well tolerated by these cells (Moritz et al., 1999). However, as previously described (Moritz et al., 1999), the majority of tadpoles died before developing into mature frogs, most likely because of genomic damage sustained during the transgenesis procedure. The number of animals that survived to maturity was extremely variable. We obtained 1 sexually mature male that transmitted the transgene encoding the wild-type GFP-rab8 fusion protein (GFP-rab8WT) to its offspring (founder A), 1 mature male and 1 female that transmitted the GFP-rab8Q67L transgene (founders B and C) and 2 mature males that transmitted the GFP-rab8T22N transgene (founders D and E). However, because young transgenic tadpoles were easy to generate and because they develop mature retinas by 2–3 weeks after fertilization, much of the characterization of each transgene's effects was done with the use of primary transgenics, before the availability of the breeding adults.
In Vivo Observation of Fusion Protein Expression
As judged by the fluorescence emitted from the eyes of the transgenic tadpoles, the expression levels of GFP-rab8WT and GFP-rab8Q67L encompassed a similar broad range. However, relative to GFP-rab8WT and GFP-rab8Q67L, the fluorescence emitted from the eyes of the GFP-rab8T22N animals was always extremely faint. Fluorescence first appeared in the eyes at developmental stage 42 (Nieuwkoop and Faber, 1994) or 5 d postfertilization (dpf). By stage 44 (6 dpf) fluorescence peaked in the GFP-rab8T22N eyes. When the focus of the dissecting microscope was altered such that the individual photoreceptors were visible through the lens of the eye (Moritz et al., 1999), the GFP-rab8T22N retinas always had a nonuniform pattern of fluorescence. Fluorescence gradually decreased until it was undetectable by this method in 90% of the GFP-rab8T22N eyes by stage 48 (14 dpf), suggesting rod cell death was occurring. In contrast, the fluorescence emitted from the eyes of GFP-rab8WT tadpoles was generally uniform and usually seemed to increase slightly and then remain constant over the same period.
Localization of Fusion Proteins by Confocal Microscopy
Three tadpoles of each type that reached developmental stage 51–54 (6 weeks old) were selected for further analysis by confocal microscopy. Frozen sections were cut from paraformaldehyde-fixed eyes. Sections were labeled with antirhodopsin mAb mabE, which specifically labels the major rod photoreceptors of the X. laevis retina (Witt et al., 1984) and with Hoechst 33342 to visualize nuclei. As previously described (Knox et al., 1998; Moritz et al., 1999), the X. laevis rhodopsin promoter drove expression of fusion proteins exclusively in photoreceptors that labeled with mabE antirhodopsin (Figure 1,A, B, and G). None of the three retinas of GFP-rab8WT primary transgenics showed signs of retinal degeneration, whereas two of three GFP-rab8Q67L primary transgenics had retinal degeneration at this late stage. GFP-rab8Q67L tadpoles that developed retinal degeneration expressed the fusion protein at higher levels than those without retinal degeneration, as judged by the intensity of fluorescence in cryosections (see also Figure 3). Expression levels in GFP-rab8WT animals were as high or higher than in GFP-rab8Q67L. In the case of the GFP-rab8T22N transgenics, all three tadpoles had retinal degeneration. This retinal degeneration was more pronounced than that seen with GFP-rab8Q67L, extending farther toward the periphery of the retina. A more detailed analysis of these degenerations will be presented elsewhere (Moritz, unpublished results). Some green fluorescent rods survived at the periphery of these retinas. Similar results were subsequently obtained with retinal sections from F1 offspring of mature transgenic frogs mated to wild-type partners.
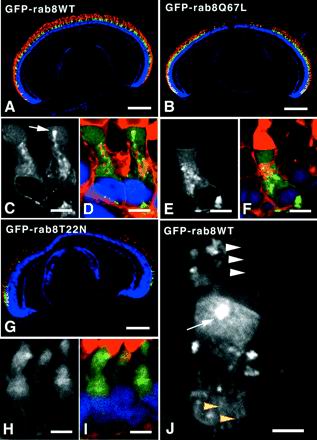
Figure 1.
Expression pattern of rab8WT, rab867L, and rab822N determined by fluorescence microscopy. Frozen sections of eyes from 6-week-old transgenic tadpoles were imaged by confocal microscopy. Selected sections were labeled with the nuclear stain Hoechst 33342 and either antirhodopsin mAb mabE followed by CY3-conjugated secondary antibody (A, B, and G) or TR-WGA (C–F, H, and I). In tri-color pictures, the GFP signal is represented in green, Hoechst stain in blue, and antibody or TR-WGA labeling in red. Grayscale images show only the GFP signal. For all three transgene constructs, green fluorescence was restricted to the major rod photoreceptors, whose OS bound mabE (A, B, and G). GFP fusion proteins were primarily observed in the IS of these rods. GFP-rab8WT did not cause retinal degeneration (A). However, both GFP-rab8Q67L (B) and GFP-rab8T22N (G) caused central retinal rod degeneration as evidenced by the lack of green and red fluorescence in the central retinas in these tadpoles. Retinal degeneration caused by GFP-rab8T22N was more extensive, sparing only a few peripheral rod photoreceptors. At higher magnification, the transgene products GFP-rab8WT and GFP-rab8Q67L appeared to be primarily associated with internal membranes of the IS (C and E), although fluorescence was also observed in the cytoplasm of the IS, and to a lesser extent in the OS. Membrane-associated GFP-rab8WT and GFP-rab8Q67L colocalized with TR-WGA label (D and F), indicating that these fusion proteins were associated with Golgi and post-Golgi membranes. A bright spot of green fluorescence was often observed just below the IS/OS junction near the predicted location of the PRC (white arrows, C and J). In contrast, GFP-rab8T22N appeared to be primarily cytoplasmic (H and I). Imaging of GFP-rab8T22N was more limited because the only surviving rods were the small arched rods confined to the peripheral retina. GFP-rab8WT was also observed along axially aligned structures in the IS (J, yellow arrowheads) and in the calycal processes that surround the OS (J, white arrowheads), but its relative abundance in calyces was low. Bars, A, B, and G, 250 μm; C, D, E, F, H, and I, 5 μm; J, 2.5 μm.
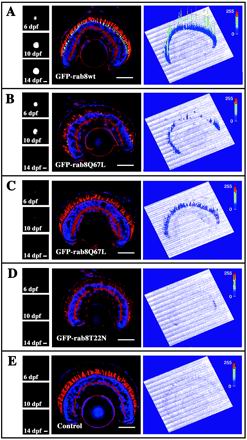
Figure 3.
F1 offspring of transgenic frogs expressing either GFP-rab8T22N or GFP-rab8Q67L have an inherited retinal degeneration; relative toxic effects of the various fusion proteins. F1 offspring expressing the various transgene constructs were obtained from several primary transgenic adults. Eyes of individual F1 tadpoles were imaged at 6, 10, and 14 dpf with the use of a standard fluorescence microscope (left panels). The animals were sacrificed at d 14, and frozen sections of the eyes were stained with TR-WGA (red) and Hoechst nuclear stain (blue). Stained sections were imaged by confocal microscopy with the use of identical laser amplitude and detector gain settings for each image (center panels), avoiding saturation of the GFP signal (green). The GFP fluorescence intensities are therefore comparable between the various images. To the right of the confocal image, the GFP fluorescence is represented as a three-dimensional profile, where the height of the peaks corresponds to the signal intensity in arbitrary fluorescence units. The GFP fluorescence intensity is also represented by a color scale (blue, low intensity; red, high intensity). Retinas of offspring of founder A, which expressed relatively high levels of the GFP-rab8WT fusion protein (A), had little or no degeneration, similar to nontransgenic control animals (E). Significant depletion of rods was seen in the F1 offspring of founder B, which expressed high levels of the GFP-rab8Q67L fusion protein (B), although some central rods survived. The surviving (younger) peripheral rods expressed the fusion protein at levels similar to or lower than the GFP-rab8WT–expressing retinas (A). F1 offspring of founder C expressed GFP-rab8Q67L fusion protein at a lower level (C) and did not have retinal degeneration, demonstrating that lower levels of this fusion protein are less toxic. F1 offspring of founder E expressed GFP-rab8T22N and had severe retinal degeneration with only peripheral rods remaining, despite expressing very low relative levels of fusion protein that were almost undetectable at this level of sensitivity (D). Numerous closely apposed cones were still present, and the layers of the inner retina were of normal thickness. Fusion protein could be easily detected with more sensitive microscope settings (see Figure 1). Interestingly, it was possible to diagnose retinal degeneration in these tadpole eyes by observing the decrease in fluorescence with time (B and D, left panels) in contrast to eyes without degeneration where fluorescence levels increased or remained constant (A and C, left panels). Bar, 100 μm.
Cryosections were also labeled with TR-WGA, which labels glycosylated membranes, including Golgi, post-Golgi, and plasma membranes of the IS and rod OS membranes (Virtanen et al., 1980,;Wood and Napier-Marshall, 1985). The distribution of GFP-rab8WT was nonuniform and appeared to be associated with a subset of intracellular membranes. Overlapping signals from GFP-rab8WT and TR-WGA confirmed that GFP-rab8WT was localized to Golgi and post-Golgi membranes, although a portion of the fusion protein remained in the cytoplasm, but did not materially enter nuclei (Figure 1, C and D). This type of distribution would be expected for rab8, which is membrane associated via a geranyl geranyl moiety (Joberty et al., 1993) and is thought to cycle between membrane-associated and cytoplasmic forms. In many cells, a bright spot of GFP-rab8WT fluorescence was observed just below the OS, near the base of the CC and the PRC. GFP-rab8WT was also observed in the interior of calycal processes (Figure 1J), actin-containing microvillar structures that surround the base of the OS of amphibian photoreceptors. Some GFP-rab8WT localized to axially aligned structures in the IS, also consistent with association with actin microfilaments (Deretic et al., 1995).
The TR-WGA signal and GFP-rab8Q67L were prominently colocalized over the Golgi and TGN (Figure 1, E and F). It was our impression that the proportion of cytoplasmic fusion protein was generally somewhat higher than for GFP-rab8WT; however, any differences were subtle. GFP-rab8T22N was more difficult to visualize, because the only surviving rods were the small, arched rods located at the periphery of the retina. The majority of GFP-rab8T22N fusion protein appeared to be cytoplasmic, but did not enter nuclei (Figure 1, H and I).
Rab822N Partially Blocks Docking of Post-Golgi Membranes
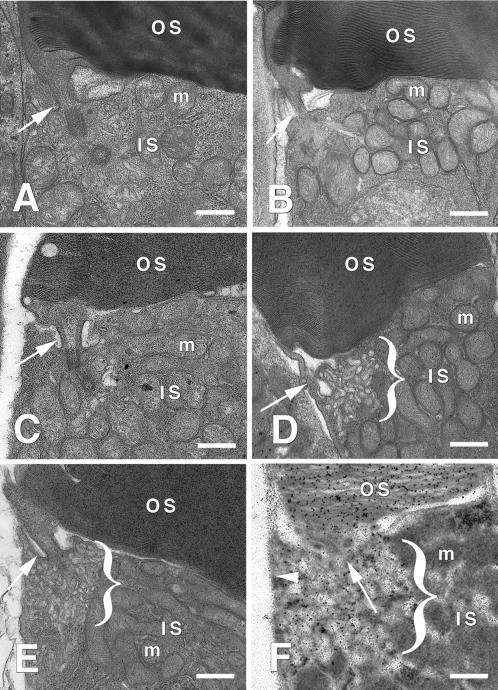
Transgenic tadpole photoreceptors were examined by electron microscopy. Rods expressing GFP-rab8WT and GFP-rab8Q67L resembled rods from nontransgenic retinas and had similar levels of intracellular vesicles (Figure 2, A–C). We did not observe any gross morphological changes that might be caused by cytoskeletal rearrangements, as previously observed when rab8 and rab8Q67L were transfected and expressed in cultured fibroblasts (Peränen et al., 1996). In contrast, the surviving peripheral rods expressing GFP-rab8T22N showed a large accumulation of tubulo-vesicular membranes adjacent to the PRC (Figure 2, D and E). These most likely represent post-Golgi rhodopsin-bearing transport membranes accumulating in this region that were unable to dock with the plasma membrane. To evaluate this interpretation, fixed eyes were also embedded in LRW resin for EM immunocytochemical analysis with monoclonal antirhodopsin C-terminal antibody 11D5 (Deretic et al., 1991) and a gold-conjugated secondary antibody. The accumulated tubulo-vesicular membranes were heavily labeled by antirhodopsin, indicating that rhodopsin was present in these membranes at high density (Figure 2F). We did not observe any increase in antirhodopsin labeling of the IS plasma membrane, which typically contains very low concentrations of rhodopsin.
Figure 2.
Expression of GFP-rab8T22N results in a dramatic increase in tubulo-vesicular membranes adjacent to the PRC. Rod photoreceptors throughout the retina were examined by electron microscopy, except in the case of GFP-rab8T22N-expressing retinas, in which only peripheral rods survived. (A) The IS/OS junction of a rod from a nontransgenic retina, showing the PRC region and CC (white arrows). (B and C) Similar regions of photoreceptors from retinas expressing high levels of GFP-rab8WT (B) and GFP-rab8Q67L (C). The few vesicles found adjacent to the PRC regions resembled those seen in nontransgenic rods. (D and E) Junctional regions of photoreceptors from retinas expressing GFP-rab8T22N. Abundant tubulo-vesicular membranes accumulated in the region beneath the CC (indicated by brackets). (F) Similar region of a photoreceptor from a retina expressing GFP-rab8T22N processed for immuno-electron microscopy and labeled with antirhodopsin mAb 11D5, followed by a secondary antibody conjugated to 10-nm gold particles. The tubulo-vesicular membranes and OS membranes are heavily labeled by antirhodopsin. Note that the adjacent IS plasma membrane (arrowhead) is relatively unlabeled. IS, inner segment; OS, outer segment; m, mitochondria. Bar, 500 nm
Expression of rab8T22N and rab8Q67L Kills Rod Photoreceptors
We further investigated the effects of the transgene expression on rod cell viability by examining the retinas of at least 10 primary transgenic tadpoles of each type, as well as nontransgenic animals. F1 offspring of transgenic founders were also examined, after screening for GFP expression. Tadpole eyes were fixed, paraffin embedded, and processed for routine hematoxylin and eosin staining. Tadpoles were killed at 14 dpf, except in the case of GFP-rab8T22N primary transgenics, where two animals were examined at 9 dpf, six animals at 12 dpf, and four at 14dpf, because preliminary fluorescence results (see above) led us to believe degeneration occurred very early in these animals. Retinal degeneration was scored when we observed a major reduction or complete absence of rods in areas of a retina in several adjacent sections. The results are summarized in Table 1. Degeneration was observed in 20% of GFP-rab8WT–expressing primary transgenics, 55% of GFP-rab8Q67L–expressing primary transgenics, and 100% of GFP-rab8T22N primary transgenics. GFP-rab8WT eyes with retinal degeneration were described as very high level expressers on initial screening. Degeneration was not observed in offspring of nontransgenic adults or F1 littermates that did not inherit the transgenes. We did not observe retinal degeneration in the F1 offspring of the GFP-rab8WT founder A, or GFP-rab8Q67L founder C. However, retinal degeneration was seen in fluorescence-positive F1 offspring of GFP-rab8Q67L founder B, and both GFP-rab8T22N founders (D and E).
Table 1.
Summary of retinal degeneration observed in transgenic X. laevis
| Type of transgenic tadpole | GFP expression | Number examined | Proportion with degeneration (%) |
|---|---|---|---|
| GFP-rab8WT primary transgenic | + | 10 | 20 |
| GFP-rab8Q67L primary transgenic | + | 11 | 55 |
| GFP-rab8T22N primary transgenic | + | 12 | 100 |
| GFP-rab8WT F1 (founder A) | + | 8 | 0 |
| GFP-rab8Q67L F1 (founder B) | + | 6 | 100 |
| GFP-rab8Q67L F1 (founder B) | − | 4 | 0 |
| GFP-rab8Q67L F1 (founder C) | + | 4 | 0 |
| GFP-rab8T22N F1 (founder D) | + | 11 | 100 |
| GFP-rab8T22N F1 (founder D) | − | 4 | 0 |
| GFP-rab8T22N F1 (founder E) | + | 6 | 100 |
| Offspring of nontransgenics | − | 8 | 0 |
Summary of results from H&E histology of primary transgenic animals and F1 offspring. All animals were sacrificed at 14 dpf, except for GFP-rab8T22N primary transgenics, where two animals were sacrificed at 9 dpf, six animals at 12 dpf, and four at 14 dpf. Retinal degeneration was observed only in animals expressing fusion protein (GFP expression +) and not in nonexpressing offspring of transgenic animals (GFP expression −), or offspring of nontransgenic animals.
To demonstrate relative expression levels of the fusion proteins, eyes from F1 offspring screened for GFP fluorescence were fixed at 14 dpf, cryosectioned, and examined by confocal microscopy as described above. Confocal settings were held constant for all animals. Representative sections from F1 offspring are shown in Figure 3. The results confirmed that GFP-rab8WT is relatively nontoxic, because the F1 offspring of founder A expressed the highest levels of fusion protein observed in this group, but had no degeneration (Figure 3A). Low levels of GFP-rab8Q67L (founder C, Figure 3C) were also nontoxic, but in F1 offspring of founder B, GFP-rab8Q67L caused degeneration at expression levels below the GFP-rab8WT threshold for degeneration (Figure 3B). In contrast, even very low relative levels of GFP-rab8T22N expression seen in offspring of founder E resulted in dramatic rod death (Figure 3D). Expression of high levels of GFP in X. laevis rods is not detrimental to photoreceptor viability and has not been observed to cause rapid retinal degeneration in other studies (Knox et al. 1998; Moritz et al. 1999; Tam et al. 2000). To demonstrate the capacity to identify retinal degeneration in these animals before killing, we also photographed the eyes of these tadpoles at 6, 10, and 14 dpf (Figure 3). Disappearance of fluorescence (Figure 3, B and D) correlated with retinal degeneration.
Transgenic Expression of rab8 Reduces Expression of Endogenous rab8
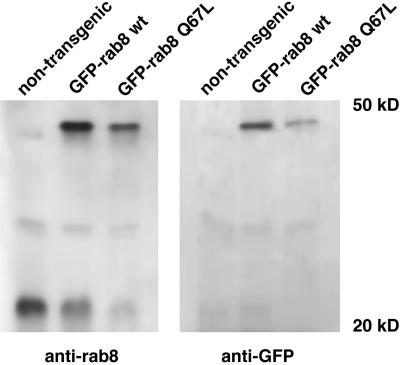
The relative levels of expression of the fusion proteins and the endogenous rab8 in these retinas were compared by Western blot analysis. Right eyes from stage 51–54 primary transgenic animals expressing GFP-rab8WT and GFP-rab8Q67L were bisected, and the retinas were removed and solubilized. Proteins were separated by SDS-PAGE and transferred to nylon membranes. Western blots were analyzed with the use of anti-GFP and anti-rab8 antibodies (Figure 4). The expression of the fusion proteins GFP-rab8Q67L or GFP-rab8WT decreased the levels of the endogenous rab8 protein. The experiment was not attempted with eyes expressing GFP-rab8T22N, because the level of fusion protein expression was low and the extremely rapid rod death would most likely have confounded the results. The corresponding left eyes were sectioned and examined by microscopy. The GFP-rab8Q67L eyes were found to have a slight degeneration, but the GFP-rab8WT eyes had none, indicating that the observed decrease of intrinsic rab8 was not entirely due to fewer photoreceptors in the transgenic retina.
Figure 4.
Expression of GFP-rab8WT and GFP-rab8Q67L downregulates the expression of endogenous rab8 in transgenic tadpoles. Equivalents of 1/8 of the retinas isolated from stage 51 tadpoles were subjected to SDS-PAGE and immunoblotted with anti-rab8 antibody (left panel) and anti-GFP antibody (right panel). In addition to a 23-kDa protein recognized by anti-rab8 antibody, transgenic retinas contain a 50-kDa protein that is also recognized by anti-GFP. The expression of GFP-rab8WT fusion protein under the control of the X. laevis rhodopsin promoter does not exceed that of endogenous rab8 as measured in the nontransgenic retina. However, the amount of the endogenous rab8 in these tadpoles is <50% of the nontransgenic animal. Therefore, the total content of rab8 (endogenous plus the fusion protein) is ∼1.5- to 2-fold higher than in the nontransgenic animals. In GFP-rab8Q67L tadpoles endogenous rab8 is also reduced to similar levels (<50% of control) as in GFP-rab8WT animals, and the fusion protein is in a threefold excess over the endogenous rab8. Because of the progressive retinal degeneration in GFP-rab8Q67L animals, the contribution of rod photoreceptors is lower than that in the retinas expressing GFP-rab8WT protein.
DISCUSSION
To study the role of rab8 in rod photoreceptor rhodopsin transport, we expressed GFP-rab8 fusion proteins in transgenic X. laevis rods. Expression of mutant rab8 proteins caused photoreceptor cell death. To determine whether GFP-rab8WT or mutant variants interfered with normal transport processes, we examined transgenic retinas by electron microscopy. Surviving peripheral rods expressing the GFP-rab8T22N dominant negative mutant developed a dramatic increase in tubulo-vesicular membranous structures immediately adjacent to the PRC. These structures contained rhodopsin and probably represent rhodopsin-bearing post-Golgi membranes that did not fuse with the PRC. However, we did not observe any increase in rhodopsin levels in the IS plasma membrane, a phenotype observed with C-terminal mutations of rhodopsin (Sung et al., 1994; Tam et al., 2000; Green et al., 2000). These experiments did not reveal transport abnormalities in GFP-rab8Q67L or GFP-rab8WT expressing cells. Only GFP-rab8T22N had dramatic effects on intracellular compartments.
Previously, Peränen et al. (1996) observed alterations in cellular morphology when rab8WT or rab8Q67L were overexpressed in cultured BHK fibroblasts. Comparable changes in photoreceptors did not develop. In fibroblasts, microtubules and actin filaments were reorganized, resulting in cellular protrusions. VSV-G protein, normally targeted basolaterally in polarized epithelia, was preferentially delivered to the protrusions. Neither membrane protrusions nor other alterations in the plasma membrane of transgenic photoreceptors were evident by fluorescence or electron microscopy. Furthermore, when stained with fluorescent phalloidin, the prominent actin cytoskeleton of the photoreceptors appeared unchanged. This may reflect a fundamental difference in the ability of photoreceptors and fibroblasts to reorganize their cellular architecture.
Peränen et al. (1996) found little or no effects with the rab8T22N mutant on cultured fibroblasts. By contrast, this mutant generated exaggerated phenotypes in frog rods, consisting of accumulation of post-Golgi membranes and cell death. This difference may be due to the relatively enormous flow of membrane to the OS. Based on the previous study (Peränen et al., 1996), overexpression of rab8WT or rab8Q67L might be expected to accelerate transport. However, rhodopsin transport to the photoreceptor OS may be close to physical limits, and therefore little effect was seen. In contrast, inhibition of docking at the PRC by rab8T22N had dramatic and disastrous consequences.
The GFP-rab8WT fusion protein was relatively nontoxic to rods and probably retained rab8 function. Photoreceptors were able to survive in these retinas for weeks to months. Furthermore, we observed reduction of endogenous rab8 in GFP-rab8WT–expressing retinas, which in other respects appeared normal, suggesting that GFP-rab8WT was able to substitute for the missing endogenous protein or that diminished levels of endogenous rab8 were sufficient for normal function. It is not clear why levels of endogenous rab8 were reduced; possible explanations include a feedback loop regulating rab8 expression at the promoter level, as previously reported for genes such as Period and Gal (Lakin-Thomas 2000, Lohr et al., 1995). Overexpression of fusion proteins may have suppressed the activity of the rab8 promoter, whereas fusion protein expression from the rhodopsin promoter was unaffected. Alternatively, excess rab8 not involved in function-related interactions may have been targeted for proteolytic degradation. It will be interesting to determine whether similar effects are seen with other fusion proteins in this system.
The intracellular localization of GFP-rab8WT to Golgi and post-Golgi membranes resembled the localization of rab8 previously described by Deretic et al. (1995) in Rana berlandieri frog rods, where rab8 is found on intracellular membranes and is associated with actin microfilaments of the inner segment and calycal processes. However, Deretic et al. found that endogenous rab8 was relatively abundant in calycal processes. We found GFP-rab8WT in calycal processes, but levels were low relative to internal membranes. This discrepancy may be due to overexpression of GFP-rab8WT in the transgenic frogs, producing a somewhat aberrant distribution, subtle differences in the properties of rab8 and GFP-rab8WT, or differences in the capacity of antibody labeling to detect Golgi vs. calycal rab8. Despite minor differences, the distribution of GFP-rab8WT resembled the previously described localization of endogenous rab8 in frog rods and other cell types (Huber et al., 1993a).
The intracellular distribution of the fusion protein supports the interpretation that GFP-rab8WT retained rab8 function. Rab proteins are associated with various (but not all) membrane compartments, yet the mechanism of membrane attachment (geranyl-geranylation of conserved C-terminal cysteine residues) is probably the same for all rabs (Khosravi-Far et al., 1991; Kinsella and Maltese 1991; Joberty et al., 1993). GFP-rab8WT was not found associated with membranes of the ER, OS, mitochondria, or nuclear envelope. This suggests that GFP-rab8WT does not bind indiscriminately, but interacts with specific membrane components and that this specificity is not altered by fusion of rab8 to the C terminus of GFP. Similar findings were reported for a GFP-rab6 fusion protein (White et al., 1999).
The intracellular distribution and toxicity of the mutant fusion proteins differed from GFP-rab8WT, suggesting that the mutations altered their interactions with membranes as well as their functional capabilities. GFP-rab8WT was membrane associated to a large extent and caused degeneration only at very high levels of expression. Like GFP-rab8WT, GFP-rab8Q67L was associated with Golgi and post-Golgi membranes, although possibly to a lesser extent than GFP-rab8. By contrast, GFP-rab8T22N appeared completely cytoplasmic. Furthermore, the mutant fusion proteins caused more aggressive retinal degeneration than GFP-rab8WT, again suggesting functional defects in both mutants. The fact that their intracellular distributions also differed suggests rab8 function is intimately associated with intracellular membrane localization.
Our observation that photoreceptors expressing GFP-rab8Q67L were relatively long-lived and did not accumulate post-Golgi membranes at the PRC suggests that cycling of rab8 between GTP- and GDP-bound forms may not be crucial to its intracellular trafficking functions. Rather, this may be the mechanism for recycling rab8 between membrane compartments, as previously suggested by others (Richardson et al., 1998). The role of rab8 in trafficking of post-Golgi membranes may not be dependent on hydrolysis of GTP, but is dependent on membrane association. Continuous synthesis of sufficient quantities of fusion protein in GFP-rab8Q67L retinas may have made recycling of rab8 unnecessary. It is also possible that residual GTPase activity in GFP-rab8Q67L was sufficient to allow relatively normal function when GFP-rab8Q67L was overexpressed. The equivalent mutations in sec4p and ypt1 reduce maximal GTPase activity to 30% and 4% of wild type, respectively (Walworth et al., 1992; Richardson et al., 1998). However, there was clearly some defect in GFP-rab8Q67L, as evidenced by the more aggressive cell death relative to GFP-rab8WT retinas. One possibility is that a factor necessary for normal cell function that interacts with multiple targets (including rab8-GTP) binds to GFP-rab8Q67L, thereby preventing other interactions crucial for cell survival. A similar mechanism could be invoked to explain the retinal degeneration caused by high-level expression of GFP-rab8WT.
Because GFP-rab8T22N was mostly cytoplasmic, the relative defect in membrane fusion at the PRC may result from the inability of GFP-rab8T22N to couple to membranes. Repression of endogenous rab8 expression might then account for the defect that resulted in accumulation of tubulo-vesicular membranes adjacent to the PRC. Alternatively, a rab8-interacting factor may have bound to the cytoplasmic fusion protein, thereby preventing its participation in membrane-trafficking events. Such a factor might be rab-GDP dissociation inhibitor, which functions in rab recycling, binding to rab-GDP to prevent association with membranes. Another possibility is that a rab8-interacting protein, such as a guanine nucleotide exchange factor, was titrated into the cytoplasm by GFP-rab8T22N, preventing interaction with endogenous rab8 and inhibiting its function.
The retinal degenerations induced by GFP-rab8T22N and GFP-rab8Q67L are the first amphibian models of inherited retinal degeneration. The degenerations differ in their severity, but are similar in that they affect the central retina, whereas peripheral rods are spared. This central-to-peripheral pattern of degeneration most likely reflects the need for the X. laevis eye and retina to increase substantially in size as the animal grows, by adding new photoreceptors at the periphery (Hollyfield, 1971). The surviving rods in these degenerations are therefore the youngest in the retina.
Human retinal degenerations are unlikely to be caused by rab8 mutations, because rab8 is ubiquitously expressed and probably essential for viability. However, it is possible that retina-specific rab8 interaction partners exist, and defects in such proteins could provoke similar cell death. Furthermore, the mechanism of rod death may not be critical in generating a useful disease model. Many inherited retinal degenerations are caused by mutations in rod-specific genes such as rhodopsin. Despite expression of the mutant rhodopsin only in rods, cone photoreceptors also die, usually lagging behind rod death (Berson, 1993). Because cones are responsible for the majority of human visual function, except under conditions of extremely dim illumination, it is the death of cones that causes blindness. The interaction between rod and cone cell death is difficult to study in transgenic mice and rats, which are nocturnal animals and have few cones. In contrast, the X. laevis retina contains large numbers of cones (Peters et al., 1983; Rohlich et al., 1989). Preliminary results suggest that cone death eventually occurs in the GFP-rab8T22N retinas as a late event, and therefore these tadpoles may constitute a useful disease model of secondary cone loss.
Identification of downstream rab effector proteins will be necessary to determine the exact role of rabs in membrane trafficking. Current hypotheses for rab8 function suggest that rabs provide specificity in transport pathways (Gonzalez and Scheller, 1999). SNARE proteins most likely mediate vesicle docking and fusion (Sollner et al., 1993) and can catalyze this process in vitro (Weber et al., 1998). Although unique SNARE combinations are present in different transport pathways, SNARE proteins may not be responsible for the fidelity of vesicle transport. Rather, SNARE proteins appear to determine the specificity of fusion (McNew et al., 2000; Scales et al., 2000). Rab proteins may provide specificity by guiding transport vesicles to appropriate docking sites, and may have multiple effectors. Potential rab8 effectors include cytoskeletal elements, molecular motors involved in vesicle movement, and proteins present at the vesicle docking site, including factors that tether membranes before final docking and fusion catalyzed by SNARE interaction (Waters and Pfeffer, 1999; Guo et al., 2000). Strong candidates for rab8 effectors include a stress-activated protein kinase (Ren et al., 1996) and the vesicle tethering complex known as sec6/8 or exocyst, specifically the sec15p subunit (Guo et al., 1999). In polarized MDCK epithelial cells and neurons, the sec6/8 complex is present at sites of vesicle fusion with the plasma membrane (Grindstaff et al., 1998; Hazuka et al., 1999). Interestingly, the actin network is essential for sec6/8 localization to exocytic sites (Hsu et al., 1999), and in photoreceptors both endogenous rab8 and GFP-rab8WT colocalize with actin filaments (Deretic et al., 1995 and Figure 1J). Consistent with the potential role for sec6/8 in rhodopsin trafficking, the phenotype of GFP-rab8T22N (shown in Figure 2, D–F) suggests a defect in tethering of rhodopsin-bearing post-Golgi membranes. It will be interesting to determine which rab8 interaction partners are crucial in photoreceptors and whether novel interaction partners adapted to high rates of membrane turnover are present. The transgenic X. laevis approach may also prove useful in the study of these proteins.
ACKNOWLEDGMENTS
The authors thank Nancy Ryan for histology services. Confocal microscopy facilities were provided by the Center for Biomedical Imaging Technology at the University of Connecticut Health Center. This research was supported by grants from the Foundation Fighting Blindness and National Institutes of Health (EY-06891, EY-12421). D.D. is a recipient of a Career Development Award from Research to Prevent Blindness.
Abbreviations used:
- CC
connecting cilium
- dpf
days postfertilization
- GFP
green fluorescent protein
- IS
inner segment
- NGS
normal goat serum
- OS
outer segment
- PRC
periciliary ridge complex
- TB
Tris buffer
- TR-WGA
Texas red– conjugated wheat germ agglutinin
REFERENCES
- Batni S, Scalzetti L, Moody SA, Knox BE. Characterization of the Xenopus rhodopsin gene. J Biol Chem. 1996;271:3179–3186. doi: 10.1074/jbc.271.6.3179. [DOI] [PubMed] [Google Scholar]
- Berson EL. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993;34:1659–1676. [PubMed] [Google Scholar]
- Besharse JC. Photosensitive membrane turnover: differentiated membrane domains and cell-cell interaction. In: Adler R, Farber D, editors. The Retina: A Model for Cell Biology Studies. Orlando: Academic Press; 1986. pp. 297–352. [Google Scholar]
- Besharse JC, Hollyfield JG, Rayborn ME. Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. J Cell Biol. 1977;75:507–527. doi: 10.1083/jcb.75.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock JB, Matern HT, Peden AA, Scheller RH. A genomic perspective on membrane compartment organization. Nature. 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- Deretic D. Post-Golgi trafficking of rhodopsin in retinal photoreceptors. Eye. 1998;12:526–530. doi: 10.1038/eye.1998.141. [DOI] [PubMed] [Google Scholar]
- Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS. Rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995;108:215–224. doi: 10.1242/jcs.108.1.215. [DOI] [PubMed] [Google Scholar]
- Deretic D, Schmerl S, Hargrave PA, Arendt A, McDowell JH. Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proc Natl Acad Sci USA. 1998;95:10620–10625. doi: 10.1073/pnas.95.18.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D, Papermaster DS. Polarized sorting of rhodopsin on post-Golgi membranes in frog retinal photoreceptor cells. J Cell Biol. 1991;113:1281–1293. doi: 10.1083/jcb.113.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D, Papermaster DS. Rab6 is associated with a compartment that transports rhodopsin from the trans-Golgi to the site of rod outer segment disk formation in frog retinal photoreceptors. J Cell Sci. 1993;106:803–813. doi: 10.1242/jcs.106.3.803. [DOI] [PubMed] [Google Scholar]
- Gonzalez L, Scheller R. Regulation of membrane trafficking: structural insights from a Rab/effector complex. Cell. 1999;96:755–758. doi: 10.1016/s0092-8674(00)80585-1. [DOI] [PubMed] [Google Scholar]
- Goud B, Salminen A, Walworth NC, Novick PJ. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988;53:753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Green ES, Menz DM, LaVail MM, Flannery JG. Characterization of rhodopsin mis-sorting and constitutive activation in a transgenic rat model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2000;41:1546–1553. [PubMed] [Google Scholar]
- Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Sacher M, Barrowman J, Ferro-Novick S, Novick P. Protein complexes in transport vesicle targeting. Trends Cell Biol. 2000;10:251–255. doi: 10.1016/s0962-8924(00)01754-2. [DOI] [PubMed] [Google Scholar]
- Hazuka CD, Foletti DL, Hsu SC, Kee Y, Hopf FW, Scheller RH. The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J Neurosci. 1999;19:1324–1334. doi: 10.1523/JNEUROSCI.19-04-01324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyfield JG. Dev. Biol. 24, 264–286. 1971. Differential growth of the neural retina in Xenopus laevis larvae. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Hazuka CD, Foletti DL, Scheller RH. Targeting vesicles to specific sites on the plasma membrane: the role of the sec6/8 complex. Trends Cell Biol. 1999;9:150–153. doi: 10.1016/s0962-8924(99)01516-0. [DOI] [PubMed] [Google Scholar]
- Huber LA, de Hoop MJ, Dupree P, Zerial M, Simons K, Dotti C. Protein transport to the dendritic plasma membrane of cultured neurons is regulated by rab8p. J Cell Biol. 1993a;123:47–55. doi: 10.1083/jcb.123.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LA, Pimplikar S, Parton RG, Virta H, Zerial M, Simons K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol. 1993b;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty G, Tavitian A, Zahraoui A. Isoprenylation of Rab proteins possessing a C-terminal CaaX motif. FEBS Lett. 1993;330:323–328. doi: 10.1016/0014-5793(93)80897-4. [DOI] [PubMed] [Google Scholar]
- Khosravi-Far R, Lutz RJ, Cox AD, Conroy L, Bourne JR, Sinensky M, Balch WE, Buss JE, Der CJ. Isoprenoid modification of rab proteins terminating in CC or CXC motifs. Proc Natl Acad Sci USA. 1991;88:6264–6268. doi: 10.1073/pnas.88.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella BT, Maltese WA. rab GTP-binding proteins implicated in vesicular transport are isoprenylated in vitro at cysteines within a novel carboxyl-terminal motif. J Biol Chem. 1991;266:8540–8544. [PubMed] [Google Scholar]
- Knox BE, Schlueter C, Sanger BM, Green CB, Besharse JC. FEBS Lett. 423, 117–121. 1998. Transgene expression in Xenopus rods. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas PL. Circadian rhythms: new functions for old clock genes. Trends Genet. 2000;16:135–142. doi: 10.1016/s0168-9525(99)01945-9. [DOI] [PubMed] [Google Scholar]
- Lohr D, Venkov P, Zlatanova J. Transcriptional regulation in the yeast GAL gene family: a complex genetic network. FASEB J. 1995;9:777–787. doi: 10.1096/fasebj.9.9.7601342. [DOI] [PubMed] [Google Scholar]
- McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Sollner TH, Rothman JE. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Knox BE, Papermaster DS. Fluorescent photoreceptors of transgenic Xenopus laevis imaged in vivo by two microscopy techniques. Invest Ophthalmol Vis Sci. 1999;40:3276–3280. [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. New York: Garland Publishing Inc.; 1994. http://www.biosci.utexas.edu/MCDB/Xenbase/atlas/NF/NF-all.html : ( http://www.biosci.utexas.edu/MCDB/Xenbase/atlas/NF/NF-all.html). ). [Google Scholar]
- Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, Besharse JC. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest Ophthalmol Vis Sci. 1985;26:1386–1404. [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, DeFoe D, Besharse JC. Biosynthesis and vectorial transport of opsin on vesicles in retinal rod photoreceptors. J Histochem Cytochem. 1986;34:5–16. doi: 10.1177/34.1.2934469. [DOI] [PubMed] [Google Scholar]
- Peränen J, Auvinen P, Virta H, Wepf R, Simons K. Rab8 promotes polarized membrane transport through reorganization of actin and microtubules in fibroblasts. J Cell Biol. 1996;135:153–167. doi: 10.1083/jcb.135.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KR, Palade GE, Schneider BG, Papermaster DS. Fine structure of a periciliary ridge complex of frog retinal rod cells revealed by ultrahigh resolution scanning electron microscopy. J Cell Biol. 1983;96:265–276. doi: 10.1083/jcb.96.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Zeng J, De Lemos-Chiarandini C, Rosenfeld M, Adesnik M, Sabatini DD. In its active form, the GTP-binding protein rab8 interacts with a stress-activated protein kinase. Proc Natl Acad SciUSA. 1996;93:5151–5155. doi: 10.1073/pnas.93.10.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson CJ, Jones S, Litt RJ, Segev N. GTP hydrolysis is not important for Ypt1 GTPase function in vesicular transport. Mol Cell Biol. 1998;18:827–838. doi: 10.1128/mcb.18.2.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlich P, Szel A, Papermaster DS. Immunocytochemical reactivity of Xenopus laevis retinal rods and cones with several monoclonal antibodies to visual pigments. J Comp Neurol. 1989;290:105–117. doi: 10.1002/cne.902900107. [DOI] [PubMed] [Google Scholar]
- Scales SJ, Chen YA, Yoo BY, Patel SM, Doung YC, Scheller RH. SNAREs contribute to the specificity of membrane fusion. Neuron. 2000;26:457–464. doi: 10.1016/s0896-6273(00)81177-0. [DOI] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Sung CH, Makino C, Baylor D, Nathans J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J Neurosci. 1994;14:5818–5833. doi: 10.1523/JNEUROSCI.14-10-05818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Hurd LB, Papermaster DS. Identification of a rod outer segment targeting signal in the C-terminus of rhodopsin using transgenic Xenopus laevis. J Cell Biol. 2000;151:1369–1380. doi: 10.1083/jcb.151.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen I, Ekblom P, Laurila P. Subcellular compartmentalization of saccharide moieties in cultured normal and malignant cells. J Cell Biol. 1980;85:429–434. doi: 10.1083/jcb.85.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth NC, Brennwald P, Kabcenell AK, Garrett M, Novick P. Hydrolysis of GTP by Sec4 protein plays an important role in vesicular transport and is stimulated by a GTPase-activating protein in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:2017–2028. doi: 10.1128/mcb.12.5.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Pfeffer SR. Membrane tethering in intracellular transport. Curr Opin Cell Biol. 1999;11:453–459. doi: 10.1016/s0955-0674(99)80065-9. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- White J, Johannes L, Mallard F, Girod A, Grill S, Reinsch S, Keller P, Tzschaschel B, Echard A, Goud B, Stelzer EH. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol. 1999;147:743–760. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt PL, Hamm HE, Bownds MD. Preparation and characterization of monoclonal antibodies to several frog rod outer segment proteins. J Gen Physiol. 1984;84:251–263. doi: 10.1085/jgp.84.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Napier-Marshall L. Cytochemical analysis of oligosaccharide processing in frog photoreceptors. Histochem J. 1985;17:585–594. doi: 10.1007/BF01003198. [DOI] [PubMed] [Google Scholar]
- Wu M, Gerhart J. Raising Xenopus in the laboratory. Methods Cell Biol. 1991;36:3–18. doi: 10.1016/s0091-679x(08)60269-1. [DOI] [PubMed] [Google Scholar]
- Young RW, Droz B. The renewal of protein in retinal rods and cones. J Cell Biol. 1968;39:169–184. doi: 10.1083/jcb.39.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]