Abstract
Airborne particulate matter can cause oxidative stress, inflammation, and premature skin aging. Marine plants such as Ecklonia cava Kjellman contain high amounts of polyphenolic antioxidants. The purpose of this study was to examine the antioxidative effects of E. cava extract in cultured keratinocytes exposed to airborne particulate matter with a diameter of <10 μm (PM10). After the exposure of cultured HaCaT keratinocytes to PM10 in the absence and presence of E. cava extract and its constituents, cell viability and cellular lipid peroxidation were assessed. The effects of eckol and dieckol on cellular lipid peroxidation and cytokine expression were examined in human epidermal keratinocytes exposed to PM10. The total phenolic content of E. cava extract was the highest among the 50 marine plant extracts examined. The exposure of HaCaT cells to PM10 decreased cell viability and increased lipid peroxidation. The PM10-induced cellular lipid peroxidation was attenuated by E. cava extract and its ethyl acetate fraction. Dieckol more effectively attenuated cellular lipid peroxidation than eckol in both HaCaT cells and human epidermal keratinocytes. Dieckol and eckol attenuated the expression of inflammatory cytokines such as tumor necrosis factor- (TNF-) α, interleukin- (IL-) 1β, IL-6, and IL-8 in human epidermal keratinocytes stimulated with PM10. This study suggested that the polyphenolic constituents of E. cava, such as dieckol, attenuated the oxidative and inflammatory reactions in skin cells exposed to airborne particulate matter.
1. Introduction
Air pollution of natural and artificial origins presents a significant concern. Particulate matter of less than 10 micrometers (PM10) suspended in the atmosphere has become a major threat to human health [1, 2]. Because PM10 is too small to be caught in the nasal cavity and bronchial cilia, it can deeply penetrate the lungs and cause inflammation, asthma, chronic bronchitis, and airway obstruction [3, 4]. PM10 can infiltrate blood vessels and then circulate throughout the body, which may cause cardiovascular and cerebrovascular diseases [5]. The particles can also attack eyes, causing various diseases such as allergic conjunctivitis and dry eye syndrome [6]. PM10 penetrates the skin through pores and other inflamed sites, aggravating skin diseases such as atopic dermatitis, acne, and psoriasis [7]. Furthermore, airborne particles are associated with premature skin aging [8].
The best strategy for the prevention and alleviation of airborne particle-induced diseases may be to reduce air pollution and the chance of exposure to air pollution. It is recommended that people minimize outdoor activity and wear a hat, mask, glasses, and long sleeves to protect the body from airborne particles. Although the skin is considered to be a physical barrier to the outer environment, the size and reactivity of PM10 are such that it can clog skin pores and induce inflammation. Therefore, dermatological and cosmetic approaches are needed to attenuate the oxidative and inflammatory reactions that arise from PM10 exposure.
Natural polyphenolic compounds may mitigate oxidative stress and inflammation as a result of exposure to PM10. In a previous study, chocolate reduced cardiac inflammation in mice exposed to urban air pollution [9]. The extract of Eucheuma cottonii attenuated inflammation and oxidative stress induced by chronic exposure to coal dust in rats [10]. Pomegranate peel extract and punicalagin attenuated the PM10-induced adhesion of THP-1 cells to endothelial cells, which was indicative of its anti-inflammatory activity against particulate matter [11].
Marine plants have emerged as a potential resource of bioactive compounds for the development of cosmeceutical ingredients [12]. Marine algae such as Ecklonia cava Kjellman have been shown to inhibit cellular melanin synthesis in murine melanoma B16/F10 cells [13, 14]. In our previous study, the extract of Phyllospadix iwatensis Makino was shown to inhibit human tyrosinase activity and melanin synthesis in human dermal melanocytes [15]. However, little is known about the antioxidant effects of marine plant extracts in skin cells that have been exposed to air pollution. In our preliminary experiments, 50 marine plants indigenous to Korea were analyzed to quantify the total phenol content (TPC). Among these plants, the E. cava extract was found to have the highest TPC. Therefore, we examined the antioxidant effects of E. cava extract in keratinocytes exposed to PM10.
2. Materials and Methods
2.1. Reagents
A standardized fine dust (PM10-like) (European Reference Material ERM-CZ120) and gallic acid (purity > 98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Eckol (purity > 98%) and dieckol (purity > 98%) were purchased from National Development Institute of Korean Medicine (Gyeongsangbuk-do, Republic of Korea).
2.2. Plant Extracts
The extracts of 50 different marine plants, extracted with 80% (v/v) aqueous ethanol, were purchased from Jeju Biodiversity Research Institute of Jeju Technopark (Jeju, Korea). The list of the plant extracts has been previously reported [15].
2.3. Determination of TPC
The TPC of each plant extract was determined using the Folin-Ciocalteau method. The extract was dissolved in 95% ethanol at 1.0 mg mL−1, and 20 μL aliquots were added to a 96-well microplate. A calibration curve using gallic acid (100–500 μg mL−1) was established. Folin-Ciocalteau reagent (Sigma-Aldrich) was diluted 10-fold in water and 100 μL was added to each well, which was reacted at 18–21°C (room temperature) for 5 min. Subsequently, 80 μL of 7.5% Na2CO3 solution was added to the mixture and then allowed to stand for 60 min in the dark. The absorbance of the mixture was measured at 765 nm using a SPECTROstar Nano microplate reader (BMG LABTECH GmbH, Ortenberg, Germany) and the calculated TPC value was expressed as μg gallic acid equivalent (GAE) per mg extract (μg GAE mg−1 extract).
2.4. Preparation of E. cava Extracts and Fractions
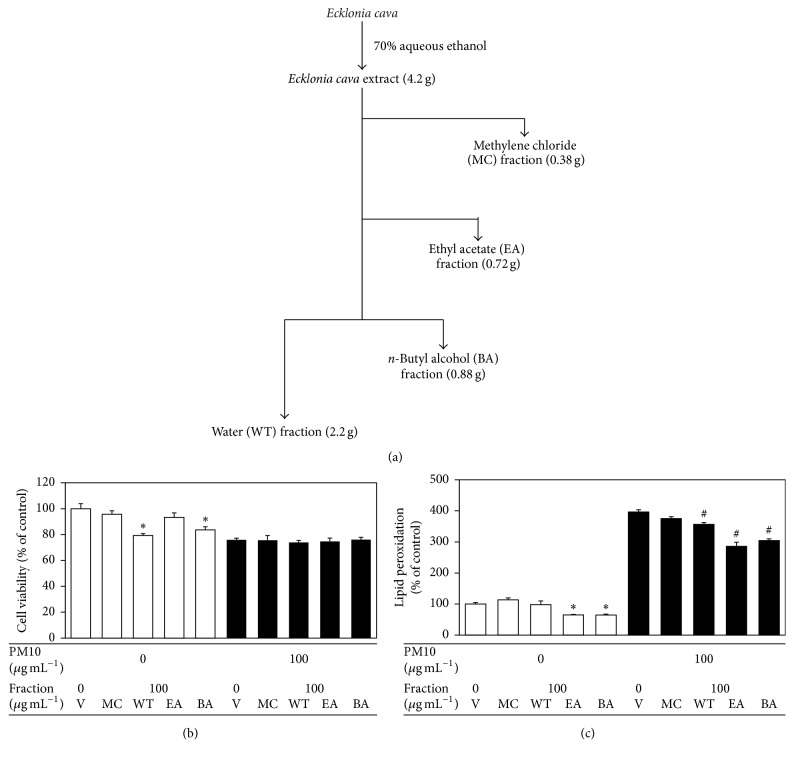
The extract of Ecklonia cava Kjellman was prepared in our laboratory from the plant materials purchased from Jayeoncho (http://www.jherb.com) (Seoul, Korea). The dried E. cava (500 g) was extracted with 80% aqueous ethanol (2.5 L) for 7 days at room temperature. The extract solution was evaporated under reduced pressure to obtain the crude extract (71.5 g). The extract (4.2 g) was suspended in water (WT) (50 mL) and partitioned sequentially with an equal volume of methylene chloride (MC), ethyl acetate (EA), and n-butyl alcohol (BA). These fractions were evaporated under reduced pressure to yield four fractions: the MC (0.38 g), EA (0.72 g), BA (0.88 g), and WT fractions (2.2 g).
2.5. High-Performance Liquid Chromatography (HPLC)
HPLC was performed by using a Gilson HPLC system (Gilson, Inc., Middleton, WI, USA) with a 321 pump and UV/VIS 151 detector. The separation was performed on a 5 μm Hector-M C18 column (4.6 mm × 250 mm) (RS Tech Co., Daejeon, Korea) using a mobile phase of 0.1% phosphoric acid (A) and acetonitrile (B). The gradient program was set as follows: 0–30 min, a linear gradient from 0 to 100% B; 30–40 min, 100% B. The flow rate was 0.6 mL min−1 and the detector was set at 280 nm.
2.6. Cell Culture
The cells were cultured in a closed incubator at 37°C in humidified air containing 5% CO2. HaCaT cells (an immortalized human keratinocyte cell line) were grown in DMEM/F‐12 medium (GIBCO‐BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, antibiotics (100 U mL−1 penicillin, 100 μg mL−1 streptomycin, and 0.25 μg mL−1 amphotericin B), and 10 μg mL−1 hydrocortisone. Primary human epidermal keratinocytes from adult human donors (Invitrogen, Waltham, MA, USA) were cultured in EpiLife medium (Gibco BRL) supplemented with 10% EpiLife defined growth supplement (#S0125) and antibiotics.
2.7. Treatments with PM10
The cells were plated onto 6-well culture plates (SPL Life Sciences, Pocheon, Korea) at 8 × 104 cells/well, cultured for 24 h, and then treated with PM10 at various concentrations (3–100 μg mL−1) for 48 h. In some experiments, the cells were treated with a fixed concentration of PM10 (100 μg mL−1) in the absence and presence of varied concentrations of a test material.
2.8. Cell Viability Assay
The cells were plated on 48-well culture plates at 1.6 × 104 cells/well and the cell viability was assessed by an MTT assay, which assesses the cellular metabolic activity of the reduction of 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyl tetrazolium bromide (MTT) to formazan dye. The cells were washed with phosphate-buffered saline (PBS) and incubated in culture medium (200 μL) containing 1 mg mL−1 MTT (Amresco, Solon, OH, USA) for 2 h at room temperature. After the medium was removed, the formazan dye in the cells was solubilized in dimethyl sulfoxide (200 μL). The resulting dye solution was placed in a 96-well plate and the absorbance was determined at 595 nm by using a SPECTROstar Nano microplate reader.
2.9. Assay of Lipid Peroxidation
The cells were plated in 100 mm culture dishes at 5 × 105 cells/dish and the cellular lipid peroxidation was assessed through the measurement of 2-thiobarbituric acid-reactive substances (TBARS) [16]. The cell extracts were prepared by using cell lysis buffer (20 mM Tris-Cl, 2.5 mM EDTA, 1.0% SDS, pH 7.5). The assay mixture consisted of cell extract (300 mg protein), 100 μL lysis buffer, 50 μL m-phosphoric acid, and 350 μL 0.9% 2-thiobarbituric acid (Sigma-Aldrich), which was then heated in a boiling water bath for 45 min. After cooling, 500 μL BA was added to the mixture, which was then vortex-mixed and centrifuged at 10,000 ×g for 15 min to produce two separate layers. The fluorescence intensity of the BA layer (excitation at 540 nm and emission at 590 nm) was measured by using a Gemini EM fluorescence microplate reader (Molecular Devices).
2.10. Quantitative Reverse-Transcriptase Polymerase Chain Reaction (qRT-PCR) Analysis
The mRNA levels of TNF-α, IL-1β, IL-6, and IL-8 were determined by qRT-PCR. Cellular RNA was extracted with an RNeasy kit (Qiagen, Valencia, CA, USA) and was used for the preparation of cDNA by using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). Gene-specific primers were purchased from Macrogen (Seoul, Korea). The sequences of the primers were as follows: TNF-α (TNF, NM_000594.3), 5′-TGCTCCTCACCCACACCAT-3′ (forward) and 5′-GAGATAGTCGGGCCGATTGA-3′ (reverse); IL-1β (IL1B, NM_000576.2), 5′-CCTGTCCTGCGTGTTGAAAGA-3′ (forward) and 5′-TGTCCTGCAGCCACTGGTTC-3′ (reverse); IL-6 (IL6, NM_001318095.1, NM_000600.4), 5′-AAGCCAGAGCTGTGCAGATGAGTA-3′ (forward) and 5′-TGTCCTGCAGCCACTGGTTC-3′ (reverse); IL-8 (IL8, NM_000584.3), 5′-CTGCGCCAACACAGAAATTA-3′ (forward) and 5′-ACTTCTCCACAACCCTCTGC-3′ (reverse); glyceraldehyde 3-phosphate dehydrogenase (GAPDH, NM_002046.3), 5′-ATGGGGAAGGTGAAGGTCG-3′ (forward) and 5′-GGGGTCATTGATGGCAACAA-3′ (reverse). The qRT-PCR was conducted with a StepOnePlus™ Real-Time PCR System (Applied Biosystems). The reaction mixture (20 μL) consisted of SYBR® Green PCR Master Mix (Applied Biosystems), cDNA (60 ng), and gene-specific primer sets (2 pmole). The thermal cycling parameters to be used for the PCR reactions were 50°C for 2 min, 95°C for 10 min, 40 amplification cycles of 95°C for 15 s and 60°C for 1 min, and a dissociation step. The mRNA levels of each gene were calculated relative to that of GAPDH by using the comparative threshold cycle method.
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
The protein levels of TNF-α, IL-1β, IL-6, and IL-8 in the culture medium were determined using respective ELISA kits (K0331131, K0331800, K0331194, and K0331216; Koma Biotech, Seoul, Korea). Briefly, cell culture media or standard protein solutions (100 μL) were transferred to microplate wells containing immobilized antibodies for each protein, followed by the incubation for 2 h at room temperature. The wells were then washed and incubated for 2 h with biotinylated antibodies for each protein and incubated for 45 min with horseradish peroxidase-conjugated streptavidin. The wells were washed and 3,3′,5,5′-tetramethylbenzidine substrate solution was added to initiate enzymatic color development. After 30 min, a stop solution was added to terminate the reaction and the absorbance was measured at 450 nm. The concentrations of cytokines were estimated from each standard curve.
2.12. Statistical Analysis
The data were presented as mean ± standard errors (SE) of three or more independent experiments. The significance of the differences between groups was determined by using Student's t-test and value of p < 0.05 was considered statistically significant.
3. Results
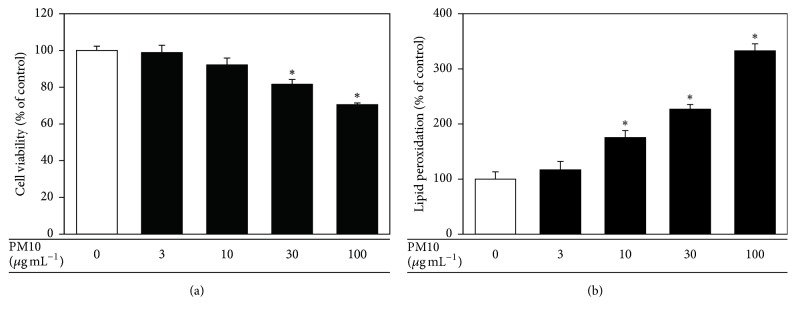
When human HaCaT keratinocytes were treated with PM10 (3 to 100 μg mL−1 for 48 h), their viability decreased in a dose-dependent manner. In addition, PM10 increased lipid peroxidation, as determined by the levels of TBARS (Figure 1(b)).
Figure 1.
The effects of PM10 on viability of and lipid peroxidation in cultured HaCaT keratinocytes. The cells were treated with PM10 at the indicated concentrations for 48 h, followed by the determination of the cell viability (a) and cellular lipid peroxidation (b). The data are presented as a percentage of the control values (mean ± SE, n = 3). ∗p < 0.05 versus control.
Of the 50 plant extracts, the extract of Ecklonia cava Kjellman had the highest TPC (89 μg GAE mg−1 extract), followed by Distromium decumbens (Okamura) Levring, Acrosorium yendoi Yamada, Eisenia bicyclis (Kjellman) Setchell, and Martensia denticulata Harvey, as shown in Table 1. Thus, further studies focused on E. cava extract.
Table 1.
Total phenol contents (TPC) of marine plants extracts used in this study.
| Marine plant name | Catalogue number | Parts used | TPC∗ (μg GAE mg−1 extract) |
|---|---|---|---|
| Acrosorium yendoi Yamada | JBRI-20419 | Whole plants | 42.70 |
| Agarum cribrosum Bory de Saint-Vincent | JBRI-20086 | Leaves | 19.31 |
| Amphiroa anceps (Lamarck) Decaisne | JBRI-20373 | Whole plants | 8.16 |
| Asparagopsis taxiformis (Delile) Trevisan | JBRI-20407 | Whole plants | 17.76 |
| Bonnemaisonia hamifera Hariot | JBRI-10278 | Leaves | 7.18 |
| Callophyllis japonica Okamura | JBRI-20365 | Whole plants | 10.35 |
| Callophyllis crispata Okamura | JBRI-10353 | Leaves | 3.28 |
| Carpopeltis affinis (Harvey) Okamura | JBRI-10376 | Leaves | 5.63 |
| Champia parvula (C. Agardh) Harvey | JBRI-20408 | Whole plants | 7.70 |
| Chondracanthus tenellus (Harvey) Hommersand | JBRI-10270 | Leaves | 18.79 |
| Chondria crassicaulis Harvey | JBRI-10264 | Leaves | 3.74 |
| Chondrus ocellatus Holmes | JBRI-10267 | Leaves | 5.81 |
| Cladophora wrightiana Harvey | JBRI-20396 | Whole plants | 16.67 |
| Codium fragile (Suringar) Hariot | JBRI-20092 | Leaves | 1.95 |
| Colpomenia sinuosa (Mertens ex Roth) Derbes et Solier | JBRI-10355 | Leaves | 2.41 |
| Costaria costata (C. Agardh) Saunders | JBRI-20020 | Leaves | 2.76 |
| Desmarestia tabacoides Okamura | JBRI-20087 | Leaves | 7.24 |
| Dictyopteris dichotoma (Hudson) Lamouroux | JBRI-20096 | Leaves | 6.95 |
| Dictyota okamurae (Dawson) Hötning, Schnetter, et Prud'homme van Reine | JBRI-20435 | Whole plants | 12.07 |
| Distromium decumbens Okamura | JBRI-20161 | Leaves | 52.36 |
| Ecklonia cava Kjellman | JBRI-10282 | Leaves | 89.43 |
| Eisenia bicyclis (Kjellman) Setchell | JBRI-20022 | Leaves | 39.14 |
| Galaxaura falcata Kjellman | JBRI-20367 | Whole plants | 6.72 |
| Gelidium amansii (Lam.) Lamouroux | JBRI-10244 | Leaves | 7.30 |
| Gloiopeltis complanata (Harvey) Yamada | JBRI-10527 | Leaves | 33.10 |
| Gracilaria verrucosa (Hudson) Papenfuss | JBRI-10698 | Leaves | 5.23 |
| Grateloupia crispata (Okamura) Lee | JBRI-20366 | Whole plants | 5.29 |
| Grateloupia elliptica Holmes | JBRI-10263 | Leaves | 3.22 |
| Hydroclathrus clathratus (C. Agardh) Howe | JBRI-10366 | Leaves | 0.29 |
| Hypnea charoides Lamouroux | JBRI-20378 | Whole plants | 9.31 |
| Ishige okamurae Yendo | JBRI-10276 | Leaves | 29.20 |
| Laurencia intermedia Lamouroux | JBRI-10351 | Leaves | 3.68 |
| Leathesia difformis (Linnaeus) Areschoug | JBRI-20346 | Whole plants | 4.20 |
| Lomentaria hakodatensis Yendo | JBRI-10266 | Leaves | 4.028 |
| Martensia denticulata Harvey | JBRI-20221 | Leaves | 36.72 |
| Myagropsis myagroides (Martens ex Turner) Fensholt | JBRI-20370 | Whole plants | 2.36 |
| Myelophycus simplex (Harvey) Papenfuss | JBRI-10262 | Leaves | 2.30 |
| Pachydictyon coriaceum (Holmes) Okamura | JBRI-20110 | Leaves | 5.58 |
| Padina arborescens Holmes | JBRI-10172 | Leaves | 5.00 |
| Petalonia binghamiae (J. Agardh) Vinogradova | JBRI-10173 | Leaves | 8.28 |
| Phyllospadix iwatensis Makino | JBRI-10700 | Leaves | 23.79 |
| Plocamium telfairiae (Harvey) Harvey | JBRI-20039 | Leaves | 11.67 |
| Prionitis cornea (Okamura) Dawson | JBRI-10272 | Leaves | 5.12 |
| Pterocladia capillacea (Gmelin) Bornet | JBRI-20027 | Leaves | 6.38 |
| Sargassum fusiformis (Harvey) Okamura | JBRI-10495 | Leaves | 2.93 |
| Scytosiphon gracilis Kogame | JBRI-10517 | Leaves | 3.10 |
| Ulothrix flacca (Dillwyn) Thuret | JBRI-20359 | Whole plants | 1.38 |
| Ulva fasciata Delile | JBRI-20025 | Leaves | 4.37 |
| Undaria pinnatifida (Harvey) Suringar | JBRI-20051 | Leaves | 1.27 |
| Zostera marina L. | JBRI-20093 | Leaves | 4.43 |
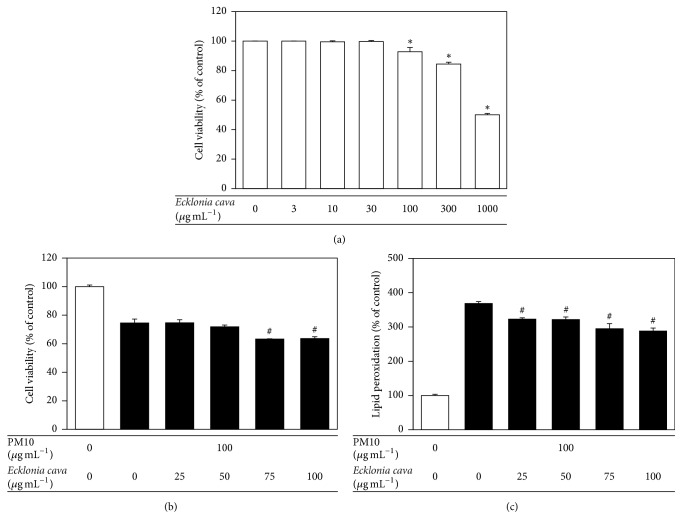
E. cava extract was cytotoxic to HaCaT cells at concentrations above 100 μg mL−1 (Figure 2(a)). In the following experiments, the cells were treated with E. cava extract at 25, 50, 75, or 100 μg mL−1 and then exposed to 100 μg mL−1 PM10 for 48 h. At 25~50 μg mL−1, E. cava extract had no significant effect on the viability of HaCaT cells exposed to PM10 (Figure 2(b)), but significantly attenuated cellular lipid peroxidation (Figure 2(c)). At higher concentrations, E. cava extract attenuated cellular lipid peroxidation more effectively (Figure 2(c)) but it also decreased the cell viability (Figure 2(b)).
Figure 2.
The effects of E. cava extract on viability of and lipid peroxidation in HaCaT keratinocytes exposed to PM10. In (a), the cells were treated with E. cava extract at the indicated concentrations for 48 h. In (b) and (c), the cells were exposed to PM10 (100 μg mL−1) for 48 h in the absence and presence of E. cava extract at the indicated concentrations. The cell viability (a, b) and cellular lipid peroxidation (c) were determined. The data are presented as the percentage of the control value (mean ± SE, n = 3). ∗p < 0.05 versus control and #p < 0.05 versus PM10 control.
Cells were treated with various solvent fractions of E. cava extract at 100 μg mL−1 and then exposed to 100 μg mL−1 PM10 for 48 h. In the absence of PM10, the water and n-butyl alcohol fraction of E. cava extract showed significant cytotoxicities, whereas the ethyl acetate fraction had no effect on the cell viability in comparison with the control (Figure 3(b)). All solvent fractions had no effects on the viabilities of the cells exposed to PM10 (Figure 3(b)). In the absence of PM10, the ethyl acetate and n-butyl alcohol fraction decreased the basal levels of lipid peroxidation (Figure 3(c)). The ethyl acetate fraction attenuated the PM10-stimulated cellular lipid peroxidation more effectively compared with other solvent fractions (Figure 3(c)).
Figure 3.
The effects of various solvent fractions of E. cava extract on viability of and lipid peroxidation in HaCaT keratinocytes exposed to PM10. In (a), E. cava extract was fractionated into the methylene chloride (MC), ethyl acetate (EA), n-butyl alcohol (BA), and water (WT) fractions. In (b) and (c), the cells were exposed to PM10 (100 μg mL−1) for 48 h in the absence and presence of each fraction (100 μg mL−1). The cell viability (b) and cellular lipid peroxidation (c) were determined. The data are presented as the percentage of the control values (mean ± SE, n = 3). ∗p < 0.05 versus control and #p < 0.05 versus PM10 control.
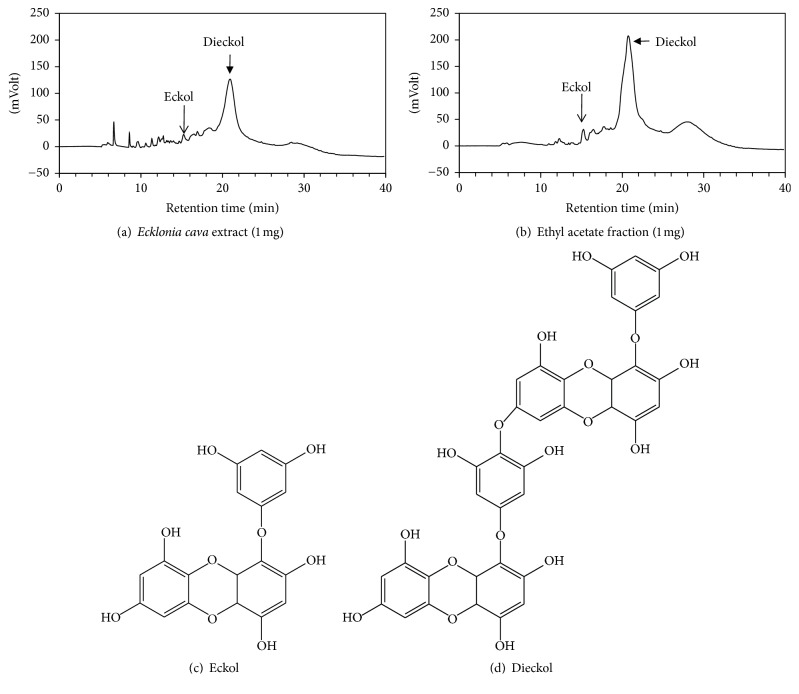
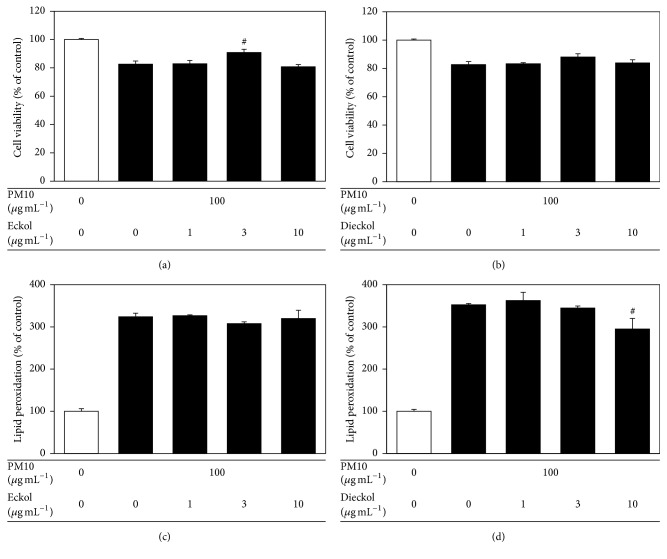
E. cava contains various polyphenolic compounds, including eckol and dieckol [17]. As shown in Figures 4(a) and 4(b), E. cava extract contained eckol and dieckol; these compounds were enriched in the ethyl acetate fraction. When tested in HaCaT cells exposed to PM10, eckol rescued cell viability at 3 μg mL−1 and dieckol attenuated lipid peroxidation at 10 μg mL−1 (Figure 5).
Figure 4.
HPLC of Ecklonia cava extract and the chemical structures of eckol and dieckol. Typical HPLC patterns of E. cava extract (a) and its ethyl acetate fraction (b). The peaks of eckol and dieckol were identified by the comparison of the retention times with those of standard compounds. The chemical structures of eckol and dieckol are shown in (c) and (d).
Figure 5.
The effects of eckol and dieckol on viability of and lipid peroxidation in HaCaT keratinocytes exposed to PM10. The cells were exposed to PM10 (100 μg mL−1) for 48 h in the absence and presence of eckol or dieckol at different concentrations (1–10 μg mL−1). The cell viability (a, b) and cellular lipid peroxidation (c, d) were determined. The data are presented as a percentage of the control values (mean ± SE, n = 3). #p < 0.05 versus PM10 control.
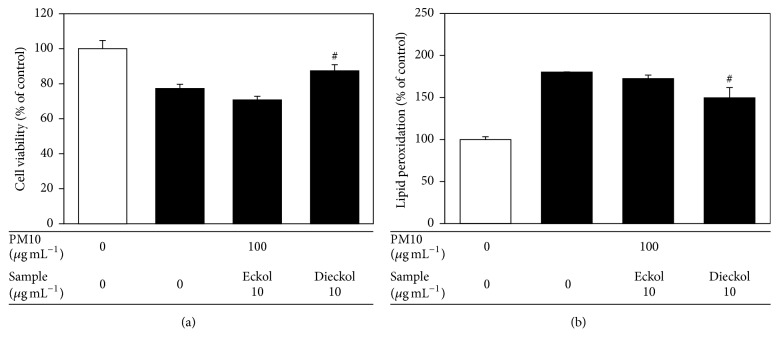
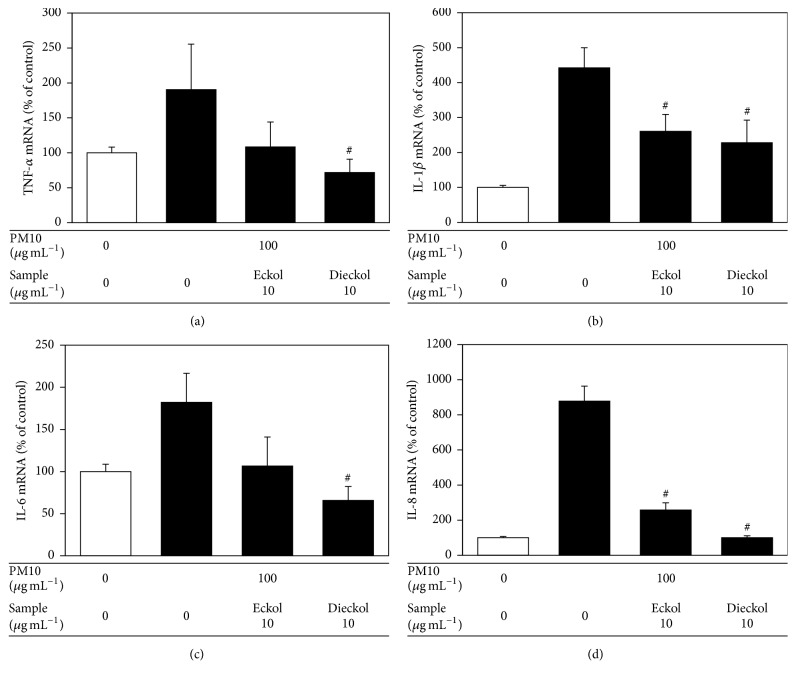
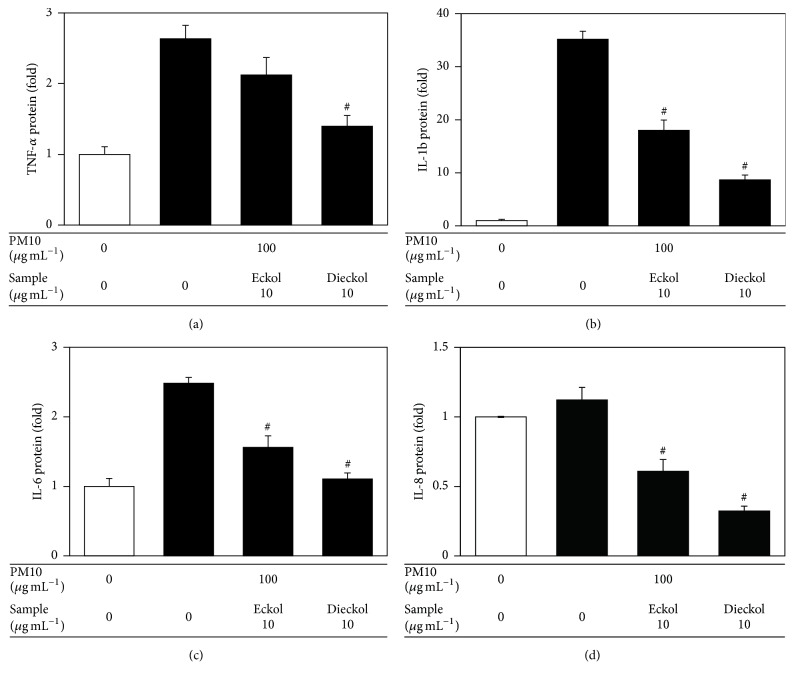
Human epidermal keratinocytes were also used to examine the antioxidant and anti-inflammatory effects of eckol and dieckol. Dieckol rescued cell viability and attenuated cellular lipid peroxidation in human epidermal keratinocytes exposed to PM10 (Figure 6). In the PM10-exposed human epidermal keratinocytes, dieckol attenuated the expression of inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8 at the mRNA and protein levels, more effectively than eckol (Figures 7 and 8).
Figure 6.
The effects of eckol and dieckol on viability of and lipid peroxidation in human epidermal keratinocytes exposed to PM10. The cells were exposed to PM10 (100 μg mL−1) for 48 h in the absence and presence of eckol or dieckol (10 μg mL−1). The cell viability (a) and cellular lipid peroxidation (b) were determined. The data are presented as the percentage of the control values (mean ± SE, n = 3). #p < 0.05 versus PM10 control.
Figure 7.
The effects of eckol and dieckol on the expression of inflammatory cytokines in human epidermal keratinocytes exposed to PM10. The cells were exposed to PM10 (100 μg mL−1) for 24 h in the absence and presence of eckol or dieckol (10 μg mL−1). The mRNA levels of TNF-α (a), IL-1β (b), IL-6 (c), and IL-8 (d) were analyzed by qRT-PCR and normalized to those of GAPDH, a housekeeping gene. The data are presented as the percentage of the control value (mean ± SE, n = 3). #p < 0.05 versus PM10 control.
Figure 8.
The effects of eckol and dieckol on the expression of inflammatory cytokines in human epidermal keratinocytes exposed to PM10. The cells were exposed to PM10 (100 μg mL−1) for 48 h in the absence and presence of eckol or dieckol (10 μg mL−1). The protein levels of TNF-α, IL-1β, IL-6, and IL-8 in the culture medium were analyzed by ELISA and normalized to the total protein content of the cells. Data are presented as fold changes compared to the control value (mean ± SE, n = 3). #p < 0.05 versus PM10 control.
4. Discussion
E. cava is a brown alga (Lessoniaceae) found in the coastal area of Korea and Japan and has been used as food and traditional medicine [18]. It is a rich source of phlorotannins and fucoidans that possesses a wide range of biological activities [17]. Phlorotannins are produced by the polymerization of phloroglucinol and are found only in marine brown algae. E. cava contains various phlorotannins, such as eckol, dieckol, 6,6′-bieckol, eckstolonol, and triphlorethol-A [19]. The biological effects of E. cava identified so far include antioxidant, anti-inflammatory, antibacterial, antidiabetic, and anticancer activities [20–25].
The present study demonstrated that the antioxidant effects of E. cava extract mitigated cellular lipid peroxidation in HaCaT keratinocytes exposed to PM10. In addition, one of its constituent compounds, dieckol, attenuated cellular lipid peroxidation and the expression of inflammatory cytokines in human epidermal keratinocytes exposed to PM10. Although the antioxidant and anti-inflammatory effects of E. cava extract have been reported in other experimental models, the present study was the first to describe the antioxidant and anti-inflammatory behavior in keratinocytes exposed to PM10.
The E. cava extract was found to attenuate PM10-induced cellular lipid peroxidation in a dose-dependent manner. Of the solvent fractions of E. cava extract, the ethyl acetate fraction attenuated the cellular lipid peroxidation most effectively. Water n-butyl alcohol fractions exhibited cytotoxicity. Thus, purification steps are necessary to improve the activity and safety profiles of E. cava extracts.
Eckol and dieckol are known polyphenolic constituents of E. cava. Dieckol is a dimeric form of eckol, and it is of interest to observe the different effects of these two compounds in cells. Compared with eckol, dieckol was more active against the PM10-induced cellular lipid peroxidation in both HaCaT and human epidermal keratinocytes. In addition, dieckol inhibited the expression of inflammatory cytokines in human epidermal keratinocytes, more strongly than eckol did.
The composition of airborne PM10 varies depending on the locations and seasons. PM10 usually contains miscellaneous toxic compounds, including transition metals, endotoxins, and ultrafine components, and induces oxidative stress and inflammation in various organs [26, 27]. It stimulates cellular reactive oxygen (ROS) production [28–30] and the expression of inflammatory cytokines such as TNF-α and IL-1β [31, 32]. In the present study, it was confirmed that PM10 increased cellular lipid peroxidation and induced the gene expression of the inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-8. In addition, the PM10-induced lipid peroxidation and cytokine expression were attenuated by dieckol and eckol in human epidermal keratinocytes.
Previous studies have used cell culture models and reconstructed human epidermis models to investigate the harmful effects of airborne pollution on the skin [33–35]. The keratinocytes culture model used in the present study was useful in identifying potential antioxidants which might protect the skin exposed to air pollution. Further studies are needed to validate its antioxidative and anti-inflammatory effects against PM10 in vivo.
In conclusion, this study suggested that the polyphenolic constituents of E. cava, such as dieckol, attenuated the oxidative and inflammatory reactions in skin cells exposed to airborne particulate matter.
Acknowledgments
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2016R1D1A1B03932501), Republic of Korea.
Disclosure
Parts of this study were presented as a poster at SfRBM's 24th Annual Meeting in Baltimore, 2017, and the abstract was published in SfRBM's 24th Annual Meeting Program and Abstracts (https://doi.org/10.1016/j.freeradbiomed.2017.10.029).
Conflicts of Interest
The authors have no conflicts of interest to declare with regard to the publication of this article.
References
- 1.Sacks J. D., Stanek L. W., Luben T. J., et al. Particulate matter-induced health effects: who is susceptible? Environmental Health Perspectives. 2011;119(4):446–454. doi: 10.1289/ehp.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson J. O., Thundiyil J. G., Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. Journal of Medical Toxicology. 2012;8(2):166–175. doi: 10.1007/s13181-011-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donaldson K., Stone V., Clouter A., Renwick L., MacNee W. Ultrafine particles. Occupational and Environmental Medicine. 2001;58(3):211–216. doi: 10.1136/oem.58.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X. Y., Gilmour P. S., Donaldson K., MacNee W. Free radical activity and pro-inflammatory effect of particulate air pollution (PM10) in vivo and in vitro. Thorax. 1996;51(12):1216–1222. doi: 10.1136/thx.51.12.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo L., Zhu N., Guo Z., et al. Particulate matter (PM10) exposure induces endothelial dysfunction and inflammation in rat brain. Journal of Hazardous Materials. 2012;213-214:28–37. doi: 10.1016/j.jhazmat.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 6.Hwang S. H., Choi Y.-H., Paik H. J., RyangWee W., KumKim M., Kim D. H. Potential importance of ozone in the association between outdoor air pollution and dry eye disease in South Korea. JAMA Ophthalmology. 2016;134(5):503–510. doi: 10.1001/jamaophthalmol.2016.0139. [DOI] [PubMed] [Google Scholar]
- 7.Kim K. E., Cho D., Park H. J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sciences. 2016;152:126–134. doi: 10.1016/j.lfs.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 8.Vierkötter A., Schikowski T., Ranft U., et al. Airborne particle exposure and extrinsic skin aging. Journal of Investigative Dermatology. 2010;130(12):2719–2726. doi: 10.1038/jid.2010.204. [DOI] [PubMed] [Google Scholar]
- 9.Villarreal-Calderon R., Reed W., Palacios-Moreno J., et al. Urban air pollution produces up-regulation of myocardial inflammatory genes and dark chocolate provides cardioprotection. Experimental and Toxicologic Pathology. 2012;64(4):297–306. doi: 10.1016/j.etp.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saputri R. K., Setiawan B., Nugrahenny D., Kania N., Sri Wahyuni E., Widodo M. A. The effects of Eucheuma cottonii on alveolar macrophages and malondialdehyde levels in bronchoalveolar lavage fluid in chronically particulate matter 10 coal dust-exposed rats. Iranian Journal of Basic Medical Sciences. 2014;17(7):541–545. [PMC free article] [PubMed] [Google Scholar]
- 11.Park S., Seok J. K., Kwak J. Y., Suh H.-J., Kim Y. M., Boo Y. C. Anti-Inflammatory Effects of Pomegranate Peel Extract in THP-1 Cells Exposed to Particulate Matter PM10. Evidence-Based Complementary and Alternative Medicine. 2016;2016 doi: 10.1155/2016/6836080.6836080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiuru P., Valeria D’Auria M., Muller C. D., Tammela P., Vuorela H., Yli-Kauhaluoma J. Exploring marine resources for bioactive compounds. Planta Medica. 2014;80(14):1234–1246. doi: 10.1055/s-0034-1383001. [DOI] [PubMed] [Google Scholar]
- 13.Cha S.-H., Ko S.-C., Kim D., Jeon Y.-J. Screening of marine algae for potential tyrosinase inhibitor: those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. The Journal of Dermatology. 2011;38(4):354–363. doi: 10.1111/j.1346-8138.2010.00983.x. [DOI] [PubMed] [Google Scholar]
- 14.Heo S.-J., Ko S.-C., Kang S.-M., et al. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food and Chemical Toxicology. 2010;48(5):1355–1361. doi: 10.1016/j.fct.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Kwak J. Y., Seok J. K., Suh H.-J., et al. Antimelanogenic effects of luteolin 7-sulfate isolated from Phyllospadix iwatensis Makino. British Journal of Dermatology. 2016;175(3):501–511. doi: 10.1111/bjd.14496. [DOI] [PubMed] [Google Scholar]
- 16.Uchiyama M., Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Analytical Biochemistry. 1978;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 17.Wijesinghe W. A. J. P., Jeon Y.-J. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: A review. International Journal of Food Sciences and Nutrition. 2012;63(2):225–235. doi: 10.3109/09637486.2011.619965. [DOI] [PubMed] [Google Scholar]
- 18.Kim M.-M., Ta Q. V., Mendis E., et al. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sciences. 2006;79(15):1436–1443. doi: 10.1016/j.lfs.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Li Y., Qian Z.-J., Ryu B., Lee S.-H., Kim M.-M., Kim S.-K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorganic & Medicinal Chemistry. 2009;17(5):1963–1973. doi: 10.1016/j.bmc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Lee H., Kang C., Jung E.-S., Kim J.-S., Kim E. Antimetastatic activity of polyphenol-rich extract of Ecklonia cava through the inhibition of the Akt pathway in A549 human lung cancer cells. Food Chemistry. 2011;127(3):1229–1236. doi: 10.1016/j.foodchem.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Choi J.-G., Kang O.-H., Brice O.-O., et al. Antibacterial activity of Ecklonia cava against methicillin-resistant Staphylococcus aureus and Salmonella spp. Foodborne Pathogens and Disease. 2010;7(4):435–441. doi: 10.1089/fpd.2009.0434. [DOI] [PubMed] [Google Scholar]
- 22.Kang C., Jin Y. B., Lee H., et al. Brown alga Ecklonia cava attenuates type 1 diabetes by activating AMPK and Akt signaling pathways. Food and Chemical Toxicology. 2010;48(2):509–516. doi: 10.1016/j.fct.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Shin H.-C., Hwang H. J., Kang K. J., Lee B. H. An antioxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Archives of Pharmacal Research. 2006;29(2):165–171. doi: 10.1007/BF02974279. [DOI] [PubMed] [Google Scholar]
- 24.Kang S.-M., Cha S.-H., Ko J.-Y., et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environmental Toxicology and Pharmacology. 2012;34(1):96–105. doi: 10.1016/j.etap.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Heo S.-J., Ko S.-C., Cha S.-H., et al. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicology in Vitro. 2009;23(6):1123–1130. doi: 10.1016/j.tiv.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Donaldson K., Stone V. Current hypotheses on the mechanisms of toxicity of ultrafine particles. Ann Ist Super Sanita. 2003;39(3):405–410. [PubMed] [Google Scholar]
- 27.Ishii H., Fujii T., Hogg J. C., et al. Contribution of IL-1β and TNF-α to the initiation of the peripheral lung response to atmospheric particulates (PM10) American Journal of Physiology-Lung Cellular and Molecular Physiology. 2004;287(1):L176–L183. doi: 10.1152/ajplung.00290.2003. [DOI] [PubMed] [Google Scholar]
- 28.Cho D.-Y., Le W., Bravo D. T., et al. Air pollutants cause release of hydrogen peroxide and interleukin-8 in a human primary nasal tissue culture model. International Forum of Allergy & Rhinology. 2014;4(12):966–971. doi: 10.1002/alr.21413. [DOI] [PubMed] [Google Scholar]
- 29.Bedard K., Krause K. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 30.Lassègue B., Griendling K. K. NADPH oxidases: functions and pathologies in the vasculature. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(4):653–661. doi: 10.1161/atvbaha.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bengalli R., Molteni E., Longhin E., Refsnes M., Camatini M., Gualtieri M. Release of IL-1β triggered by milan Summer PM10: molecular pathways involved in the cytokine release. BioMed Research International. 2013;2013:9. doi: 10.1155/2013/158093.158093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujii T., Hayashi S., Hogg J. C., et al. Interaction of alveolar macrophages and airway epithelial cells following exposure to particulate matter produces mediators that stimulate the bone marrow. American Journal of Respiratory Cell and Molecular Biology. 2002;27(1):34–41. doi: 10.1165/ajrcmb.27.1.4787. [DOI] [PubMed] [Google Scholar]
- 33.Lecas S., Boursier E., Fitoussi R., et al. In vitro model adapted to the study of skin ageing induced by air pollution. Toxicology Letters. 2016;259:60–68. doi: 10.1016/j.toxlet.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Soeur J., Belaïdi J.-P., Chollet C., et al. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. Journal of Dermatological Science. 2017;86(2):162–169. doi: 10.1016/j.jdermsci.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Lin Z.-C., Lee C.-W., Tsai M.-H., et al. Eupafolin nanoparticles protect HaCaT keratinocytes from particulate matter-induced inflammation and oxidative stress. International Journal of Nanomedicine. 2016;11:3907–3926. doi: 10.2147/IJN.S109062. [DOI] [PMC free article] [PubMed] [Google Scholar]