Abstract
Background and Aim
Statin is a class of medications used to decrease low-density lipoprotein cholesterol level to prevent cardiovascular disease. However, the risk of hepatic damage caused by statin therapy is still controversial. We conducted a systematic review and meta-analysis summarizing the existing evidence of the effect of statin therapy on incidence of liver injury to clarify whether statin therapy could lead to liver function test abnormalities.
Methods
We searched the Cochrane Library, PubMed, and Embase database for the relevant studies update till Jan. 2017 regarding statin therapy and liver injury. Two researchers screened the literature independently by the selection and exclusion criteria. Odds ratios (ORs) and 95% confidence intervals (CIs) were pooled using random effects models, and subgroup analyses were performed by study characteristics. This meta-analysis was performed by STATA 13.1 software.
Results
Analyses were based on 74,078 individuals from 16 studies. The summary OR of statin therapy was 1.18 (95% CI: 1.01–1.39, p = 0.04; I2 = 0.0%) for liver injury. Subgroup analysis indicated that fluvastatin increased the risk of liver injury significantly (OR, 3.50; 95% CI: 1.07–11.53, p = 0.039; I2 = 0.0%) and dose over 40 mg/daily had an unfavorable effect on the liver damage (OR, 3.62; 95% CI: 1.52–8.65, p = 0.004; I2 = 0.0%). The sensitivity analysis indicated that the results were robust.
Conclusion
Our findings confirm that statin therapy substantially increases the risk of liver injury, especially using fluvastatin over 40 mg/d.
1. Introduction
Statins are a group of drugs that inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which is a key enzyme in the rate-limiting step in cholesterol synthesis, and are widely used since introduction in the late 1980s to reduce low-density lipoprotein (LDL) to prevent cardiovascular diseases [1]. European Guidelines on cardiovascular diseases prevention in clinical practice recommend a LDL-c goal of <100 mg/dL (2.6 mmol/L) or a ≥30% reduction in LDL-c among patients with a high risk of cardiovascular diseases [2].
However, there are some unfavorable effects of statin therapy on liver function, especially at high dose [3]. The most common clinical hepatic manifestations caused by statin are elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). It is reported that approximately 3% of patients showed elevations in serum aminotransferase levels exceeding three times the upper limit of normal (ULN) [4, 5]. Though the incidence rate of statin-induced liver injury reported is relatively small, it might be one of the most dangerous complications. Some rare cases even reported that statin therapy could lead to liver failure; therefore it is significantly important to figure out the statin-induced liver injury [6].
Results of the randomized control trials (RCTs) investigating the association between statin therapy and liver injury were inconsistent [7–9]. The different results may be due to the various study population, diverse types of statin, samples size, intervention duration, and dosage of the statin intervention. Previous meta-analyses summarized the effects of statin therapy on liver injury from 13 RCTs involving 49,275 participants, indicating statin therapy (pravastatin, lovastatin, and simvastatin) at low-to-moderate doses was not associated with a significant risk of liver function abnormalities [10]. There have been new RCTs published after the latest meta-analysis mentioned above; therefore, an updated meta-analysis is needed.
Our objective was to perform a comprehensive and update systematic review and meta-analysis of published RCTs, which evaluate the statin therapy (i.e., lovastatin, atorvastatin, simvastatin, pravastatin, rosuvastatin, and fluvastatin) on liver injury (AST, ALT, and ULN).
2. Materials and Methods
This meta-analysis was designed and reported according to the 2009 preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement [11]. All analyses were based on previously published studies. Thus, no ethical approval and patients consent were required.
2.1. Search Strategy
Databases of Cochrane, Medline, PubMed, and Embase were searched (from inception up to Jan 2017) for studies describing the association between statin therapy and liver injury and liver function abnormalities. The search focused on MeSH terms of (atorvastatin OR simvastatin OR rosuvastatin OR fluvastatin OR pravastatin OR pitavastatin OR lovastatin OR cerivastatin OR) AND (liver injury OR liver toxicity OR hepatic injury). References and related citations of articles were manually searched for potentially eligible studies. Unpublished articles were searched from clinicaltrial.gov. All the searches were limited to human studies, and there was no language restriction in this study. Two reviewers (X L and Q W) examined all the articles, and a senior author (QH Z) was consulted when there were discrepancies about the study inclusion [12].
2.2. Study Selections
Trials were selected by two reviewers (X L, Q W) following the inclusion criteria: (1) human RCTs design as either parallel or crossover; (2) investigating the influence of statins on liver function; (3) trials of sample size over 150 included due to the statistical power—to obtain 80% statistical power to investigate the difference at a probability of 0.05, a sample size of 150 was needed; (4) adult participants of studies, who were over 18 years. Exclusion criteria were as follows: (1) observational studies: case-control, cross-sectional, or cohort design; (2) nonclinical studies; (3) transplant recipients; (4) infection with HIV or hepatitis B or C virus and patients with autoimmune diseases and taking immunomodulators. A senior author (QH Z) was consulted when there were discrepancies about the study inclusion.
2.3. Data Extraction
A standard data extraction form was used by two authors independently to collect the information, which included the first author' name, year of publication, study design, inclusion/exclusion criteria, participants' number of intervention and control group, participants' characteristics (e.g., gender, mean age), intervention details (e.g., dosage, type, frequency, and duration), placebo, the outcomes information of LFTs (e.g., ALT, AST, and ULN), case number, and compliance. A third reviewer (QH Z) was consulted when there were disagreements.
2.4. Quality Assessment
The quality assessment was performed by two researchers (X L, Q H) by the Cochrane Collaboration's tool [12], which included randomization procedures, allocation concealment, blinding of participants, researchers and outcome assessors, incomplete outcome data, nonelective reporting, other bias, and compliance. If all features were adequate, the quality of the studies was a low risk of bias. If one or more features were unclear, the risk of bias was unclear. If one or more features were inadequate or negative, it was at high risk of bias [12].
2.5. Statistical Analysis
A random effects model (using the DerSimonian-Laird method) and the generic inverse variance method were used to derive pooled estimates across studies [10]. Heterogeneity across studies was evaluated by the Cochrane Q statistic (significant p < 0.1) and the I2 statistic. An I2 statistic greater than 25% and less than 50% is considered to represent low heterogeneity. Values above 50% and less than 75% represent moderate heterogeneity and values over 75% illustrate high heterogeneity [13]. The publication bias was evaluated by Harbord method [14]. A p value less than 0.05 was deemed significant. Sensitivity analysis was compared between random effects model and fixed-effect model. Influence analysis method was used to perform the sensitivity test, omitting one study once till all the studies were picked out during the sensibility test. Metaregression was conducted to investigate the potential heterogeneity.
2.6. Subgroup Analysis
To explore the causes of inconsistency and subgroup treatment interactions, a priori subgroup analyses were designed to be conducted according to type of statin (lovastatin, atorvastatin, simvastatin, pravastatin, rosuvastatin, and fluvastatin), dose of statin (low dose, ≤20 mg/daily, moderate dose, >20 mg/daily and ≤40 mg/daily, and high dose, >40 mg/daily) [3], and duration of intervention (short-term response, less than 2 years, long-term intervention, and more than 2 years) [10]. All tests were performed using statistical software package STATA version 13.1 and two-sided p value < 0.05 was considered statistically significant.
3. Results
3.1. Study Identification and Studies Characteristics
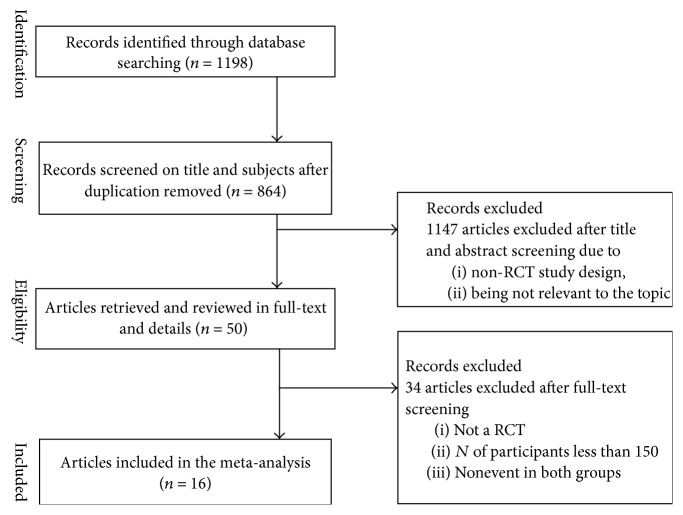
We identified 74,078 participants from 16 studies in this meta-analysis [7–9, 15–27]. 1,198 published articles were found after the initial search. After the titles and abstracts screening, there were 50 articles left. Studies were excluded due to the non-RCTs study design or duplication or irrelevant references. After the full-text screening, four articles were excluded according to the fact that no LFTs abnormal events happened in both statin group and control group. Seven articles were excluded by non-RCTs study design. 23 studies were excluded because the number of participants was less than 150. The details of the studies selection process were shown in Figure 1. The statin type of this intervention included the lovastatin (n = 3), atorvastatin (n = 1), simvastatin (n = 5), pravastatin (5), rosuvastatin, and fluvastatin (n = 2). The dose of intervention in the studies ranged from 10 mg/daily to 80 ml/daily, and the duration of statin administration ranged from 3 months to 6.1 years. Description of the characteristics of the trials included is presented in Table 1. The trials reported compliance rate of 80%–100%.
Figure 1.
Flowchart of study selection.
Table 1.
The characteristic of the 16 RCTs included in our study.
| Study | Year | N | Age | Male (%) | Statin type | Dose (mg) | Duration (year) | Compliance |
|---|---|---|---|---|---|---|---|---|
| Keech et al. | 1994 | 415 | 63 | 85 | Simvastatin | 20 | 3.4 | 93% |
| Salonen et al. | 1995 | 424 | 57 | 100 | Pravastatin | 40 | 3.2 | 92% |
| Herd et al. | 1997 | 429 | 59 | 81 | Fluvastatin | 40 | 2.5 | 90% |
| Patrick et al. | 2002 | 1677 | 60 | 84 | Fluvastatin | 80 | 3.9 | 97% |
| Downs et al. | 1998 | 6605 | 58 | 85 | Lovastatin | 30 | 5.2 | 99% |
| Shepherd et al. | 1995 | 6595 | 55 | 100 | Pravastatin | 40 | 4.9 | 70% |
| Pedersen et al. | 1994 | 4444 | 59 | 81 | Simvastatin | 27 | 5.4 | 99% |
| Tonkin | 1998 | 9014 | 62 | 83 | Pravastatin | 40 | 6.1 | 87% |
| Sacks et al. | 1996 | 4159 | 59 | 86 | Pravastatin | 40 | 5.0 | 90% |
| Shepherd et al. | 2002 | 5804 | 75 | 48 | Pravastatin | 40 | 3.2 | 86% |
| Armitage et al. | 2009 | 20536 | 64 | 83 | Simvastatin | 40 | 5.0 | 85% |
| Bays et al. | 2004 | 1526 | 55 | 48 | Simvastatin | 60 | 0.25 | 90% |
| Baigent et al. | 2005 | 448 | 53 | 79 | Simvastatin | 20 | 1.0 | 80% |
| Furberg et al. | 1994 | 919 | 62 | 52 | Lovastatin | 30 | 2.8 | 95% |
| Newman et al. | 2008 | 2838 | 62 | 68 | Atorvastatin | 10 | 3.9 | 90% |
| Bradford et al. | 1991 | 8245 | 56 | 59 | Lovastatin | 80 | 1.0 | 97% |
3.2. Risk Bias of Assessment
The Cochrane Collaboration's tool for assessing the risk of bias included the adequate sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and another risk of bias. The accurate information of the quality of the studies was demonstrated in Table 2. Two studies were probably not allocation concealed. All of the studies have reported the exact information of the loss to follow-up and were explained in detail. Two of the studies were double-blinded properly. None of the studies was defined as a high risk of bias.
Table 2.
Quality assessment of the included studies.
| Study | Random sequence generation |
Allocation concealment |
Blinding of participants personnel and outcome assessors | Incomplete outcome data |
Selective outcome reporting |
Other sources of bias |
|---|---|---|---|---|---|---|
| Keech et al. 1994 | L | L | H | L | L | L |
| Salonen et al. 1995 | L | L | L | L | L | L |
| Herd et al. 1997 | U | U | L | L | L | U |
| Patrick et al. 2002 | U | U | L | L | L | U |
| Downs et al. 1998 | U | U | L | L | L | L |
| Shepherd et al. 1995 | U | U | L | L | L | L |
| Pedersen et al. 1994 | L | L | L | L | L | L |
| Tonkin 1998 | U | U | L | L | L | L |
| Sacks et al. 1996 | L | U | L | L | L | L |
| Shepherd et al. 2002 | L | L | L | L | L | L |
| Armitage et al. 2009 | L | L | L | L | L | L |
| Bays et al. 2004 | U | U | H | L | L | U |
| Baigent et al. 2005 | U | U | L | L | L | U |
| Furberg et al. 1994 | U | U | L | L | L | L |
| Newman et al. 2008 | U | U | L | L | L | H |
| Bradford et al. 1991 | U | U | L | L | L | U |
L: low risk of bias; H: high risk of bias; U: unclear risk of bias.
3.3. Meta-Analysis
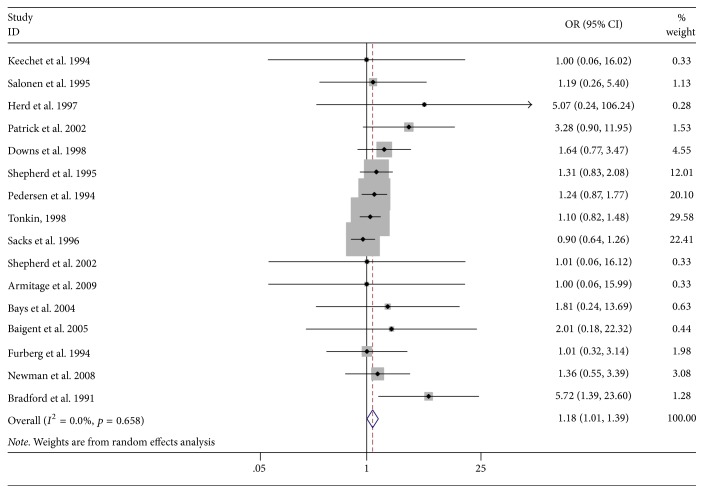
The pooled odds ratio of 16 studies was 1.18 (95% CI 1.01 to 1.39, p = 0.04; I2 = 0.0%), indicating that statin therapy could increase the liver injury, which is shown in Figure 2.
Figure 2.
Meta-analysis of the association between statin therapy and incidence of liver injury. The area of each square is proportional to the inverse of the variance of the log relative risks. Horizontal lines represent the 95% confidence intervals (CIs). Diamonds represent pooled estimates from an inverse variance-weighted random effects model. OR = odds ratio.
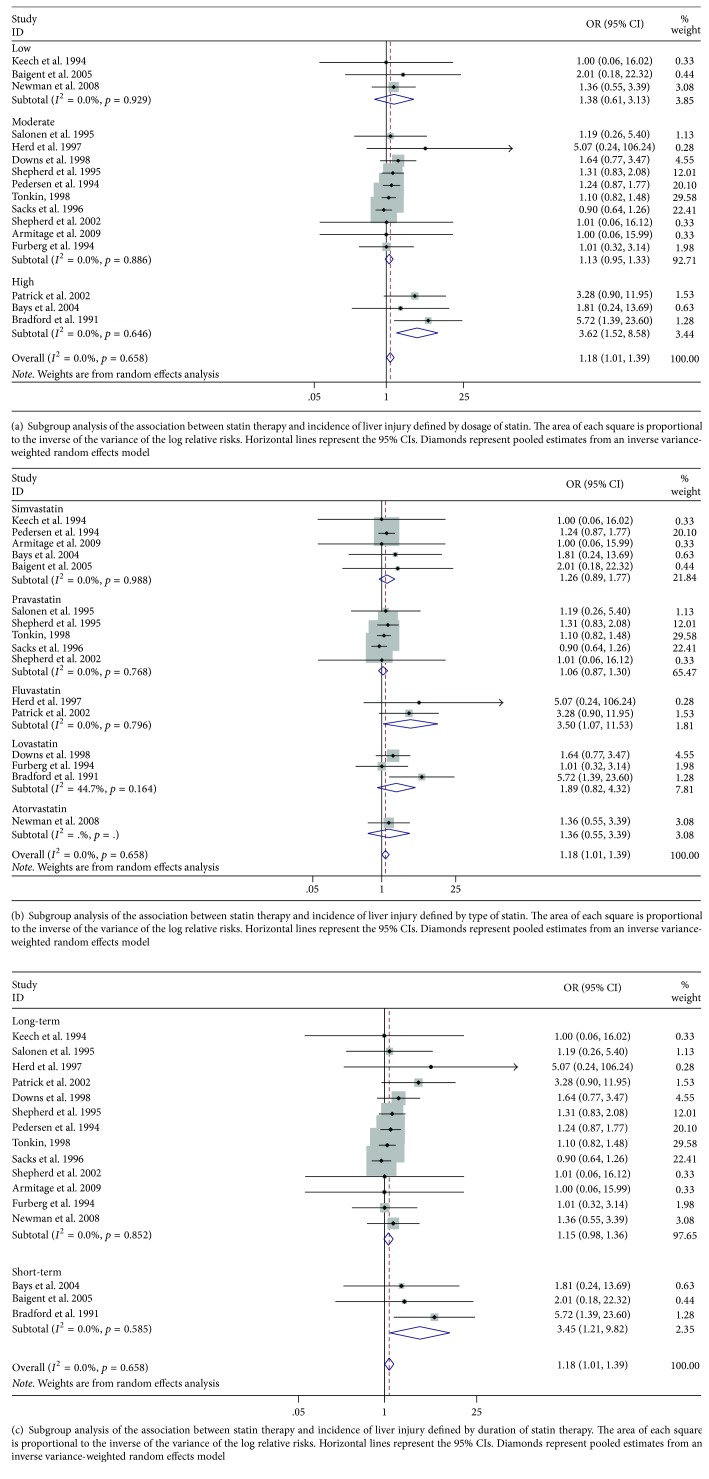
3.4. Subgroup Analysis
To further validate the robustness of overall analysis, subgroup analyses were carried out and are shown in Figures 3(a), 3(b), and 3(c). Fluvastatin showed an unfavorable effect on increasing the risk of liver injury (OR, 3.50; 95% CI 1.07–11.53, p = 0.039), while other types of statin did not show the negative effects. Daily high dose of statin therapy (over 40 mg/daily) was found to increase the risk of liver injury (OR, 3.62; 95% CI 1.52–8.58, p = 0.004). Moderate and low dose of the statin had no effects on liver damage. Short-term duration (less than two years) had adverse effects on liver injury rather than long-term statin therapy (OR, 3.45; 95% CI 1.21–9.82, p = 0.02).
Figure 3.
3.5. Publication Bias
Potential publication bias was not presented according to the funnel plots, which is shown in Figure 4. The Harbord tests for the studies are included in the primary analysis (p = 0.07), which illustrated that the small study did not contribute to the overall estimate.
Figure 4.
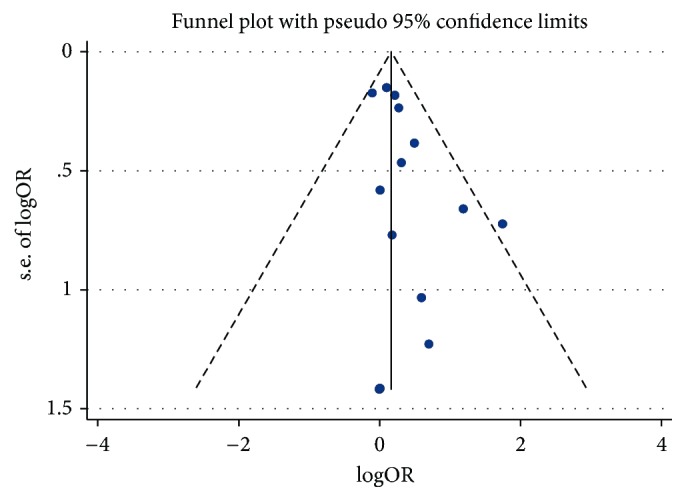
Funnel plot for statin therapy and incidence of liver injury.
3.6. Sensitivity Analyses and Metaregression
In sensitivity analyses the estimates of effects did not differ significantly comparing with fixed effects model (OR, 1.28; 95% CI, 1.07–1.54; I2 = 0.0%; S Figure 4) and random effects model. Influence analysis showed that one study was excluded at a time from each analysis; the results appeared to be robust to the influence of individual studies, which is shown in S Figure 1. The estimate of effect was 1.28 (95% CI, 1.07–1.54) and 1.22 (95% CI, 1.01–1.47) after excluding the most influential studies (Frank et al. and Tonkin et al.), respectively, shown in S Figures 2 and 3. There was little evidence of heterogeneity between any of these subgroups with metaregression (p > 0.05 by metaregression).
4. Discussion
The findings from this meta-analysis, based on 74,078 participants from 16 studies, illustrated that statin therapy has unfavorable effects on liver injury; the risk of liver injury was increased by 22% among patients with statin therapy. Subgroup analysis showed fluvastatin increased liver injury significantly rather than other types of statins. The results were similar to0 the previous meta-analysis conducted by Denus et al. in 2004, including 13 trials involving 49,275 patients [10]. However, there were some limitations of Denus et al.'s results. Denus et al. only included the studies with the sample size over 400 in order not to overestimate the safety of statins. Meanwhile, after calculating the statistical power of the trial as 80%, sample size of 150 is sufficient for the study. Therefore, Denus et al.'s study may overexclude some trials in the meta-analysis. Denus et al. did not perform sensitive analysis, which may not explain the heterogeneity and confirm the robustness of the results. In addition, Denus et al. only performed the subgroup analysis defined by types of statins, without addressing the issues related to the dosages and durations of statin therapy. Our comprehensive and updated meta-analysis included more trials and involved more participants. Our study did subgroup analysis according to dose of the statin and length of the intervention, which illustrated that statin over 40 mg was associated with increasing risk of liver injury. And there was no significant evidence that long-term statin intervention will increase the risk of liver injury.
Previous RCTs have indicated that daily high dose of fluvastatin was highly linked to abnormal liver enzymes [28, 29]. There was a relationship between increasing statin dose and reduction in the level of elevated triglyceride, which has been shown to be independently associated with an incidence rate of cardiovascular diseases. According to the previous studies, the incidence rate of elevation was dose dependent, with rates of 0.2% with 10 mg versus 2.3% with 80 mg [30]. Patients receiving 20 mg/daily atorvastatin showed a trend of higher transaminases than those were treated with 10 mg/daily [31]. Our study illustrates that 40 mg/daily statin therapy increases the risk of liver injury by more than 200%. Patients under statin therapy should be cautious about the dosage of intervention. Previous studies reported that there was no association between cumulative exposure and cumulative dose of statin and risk of liver injury, which illustrated that long-term use of statin might not lead to liver injury [32]. Our results also demonstrated that the negative effects were significant within 2 years rather than more than 2-year intervention. Masana et al. reported that the therapy safety and efficacy were similar between the 12th week and 48th week. The liver side effects may occur when the statins started to reduce low-density lipoprotein cholesterol within three weeks. Therefore, statin therapy should be monitored in the beginning of the intervention.
Statin is widely used to prevent cardiovascular diseases for the antithrombotic effects, cholesterol reduction, and lower risk of creatinine kinase (CK-MB) [33, 34]. Statins could reduce cardiovascular-related mortality and morbidity significantly [3]. However, one of the serious side effects of statin is liver toxicity, and it is recommended that statin therapy should be stopped if the transaminase levels are raised more than three times the upper limit of normal [35]. Although rare, statins may occasionally cause severe liver injury, some of which are with autoimmune features and some are predominantly hepatocellular [36, 37]. Statin-associated liver injury has been listed as one of the top 10 drug-associated adverse drug reactions in Taiwan [32]. According to the available guidelines when transaminase levels are raised more than three times the upper limit of normal (>3 ULN) treatment should be stopped. Potential mechanisms could explain the association between statin and liver injury.
5. Mechanism
The elevation of serum aminotransferase concentration is often seen in patients receiving statin therapy, especially in the first 3 months. The pathophysiologic mechanisms of hepatotoxicity are still being explored, and several potential mechanisms could explain the effects of stain on liver injury. Most people believed that the pathophysiologic mechanisms of hepatotoxicity which was caused by statins include both intracellular and extracellular mechanisms. All of these have direct effects on organelles such as the endoplasmic reticulum, mitochondria, the cytoskeleton, the nucleus, or microtubules [38]. Mitochondria dysfunction releases excessive amounts of oxidants that injure hepatic cells. Activation of some enzymes also leads to oxidative stress. They may influence cellular organelles through the activation or inhibition of signaling kinases, gene expression profiles, and transcription factors [39].
6. Strength and Limitation
This meta-analysis was an updated and comprehensive investigation on the effects of statin therapy and liver injury, which included more trials and larger sample size. We searched databases with no language restriction to increase the completeness of the identification of studies. We included RCTs, which avoided the influence of bias of observational studies.
Our study results should be interpreted with considerations of several potential limitations.
Firstly, there is no standard of LFTs and no specific rates of recovery of LFT abnormalities after decreasing doses from the original studies. Further studies focusing on this topic may consider individual patient data to clarify the related issue. Secondly, more than half of the trials included did not mention the methods of randomization and concealment. Two of the studies were single blinded. However, sensitivity analysis showed that the results maintained robustness. Well-designed RCTs are needed to be performed in the future. Thirdly, it has been reported that the old adults are the vulnerable group who are at high risk of cardiovascular diseases [40]. Our meta-analysis did not make a subgroup analysis according to the age. Additionally, our study did not address the issues related to the participants' disease history at baseline, such as patients with the chronic liver disease, patients with acute hepatitis, or patients with abnormal blood glucose [41, 42]. Patients suffering from active liver diseases, especially under inflammations, seem to be more vulnerable to develop impaired liver function, though there is only evidence in animal research [43]. Previous studies have reported that elevation of transaminase enzymes has frequently happened among patients with type 2 diabetes [44]. The studies in the future should analyze the basic diseases of the participants. Salmela et al. illustrated that elevated transaminase enzyme was highly related to poor diabetic management as well [44]. And the abnormal LFTs may be related to the abnormal blood glucose and insulin secretion. Therefore, statin therapy could be the single factor or could be multifactors combining with the underlying diseases, which should be clarified in the future. Finally, some of the studies included were with more than 10% of participants dropping out. However, due to the large sample size, the compliance was acceptable.
7. Further Implication
Although monitoring liver function tests for patients prescribed statins is recommended, the necessity of the monitoring is still questioned due to the inconsistent results.
Our meta-analysis suggests that it is essential to monitor patients receiving statin in high dose and in the beginning of intervention, especially fluvastatin, for the risk of liver injury.
Conflicts of Interest
The authors have no existing conflicts of interest.
Authors' Contributions
Xiao Liang and Qinghua Zhao were responsible for the study concept and design, literature searches, selecting eligible articles, and methodological quality assessment. Xiao Liang and Qin He extracted data from these eligible articles. Qinghua Zhao participated in the analysis and interpretation of the data. Xiao Liang was responsible for the article writing. All authors reviewed and approved the final manuscript.
Supplementary Materials
S. Figure 1: statin therapy and incidence of liver injury; sensitivity analysis excluding one study at a time S. Figure 2: meta-analysis of the association between statin therapy and incidence of liver injury excluding Frank's study. S. Figure 3: meta-analysis of the association between statin therapy and incidence of liver injury excluding Tonkin's study. S. Figure 4: meta-analysis of the association between statin therapy and incidence of liver injury using the fixed-effect model according to the Mantel-Haenszel (M-H) model.
References
- 1.Baigent C., Keech A., Kearney P. M. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. The Lancet. 2005;366(9493):1267–1278. doi: 10.1016/s0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 2.Smith S. C., Jr., Benjamin E. J., Bonow R. O., Braun L. T., Creager M. A., Franklin B. A., et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the american heart association and american college of cardiology foundation endorsed by the world heart federation and the preventive cardiovascular nurses association. Journal of the American College of Cardiology. 2011;58(23):2432–2446. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 3.Auer J., Berent R., Weber T., Eber B. Clinical significance of pleiotropic effects of statins: Lipid reduction and beyond. Current Medicinal Chemistry. 2002;9(20):1831–1850. doi: 10.2174/0929867023369024. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41(4):690–695. doi: 10.1002/hep.20671. [DOI] [PubMed] [Google Scholar]
- 5.Clarke A., Mills P. R. Atorvastatin associated liver disease. Digestive and Liver Disease. 2006;38(10):772–777. doi: 10.1016/j.dld.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Grattagliano I., Portincasa P., Palmieri V. O., Palasciano G. Overview on the mechanisms of drug-induced liver cell death. Annals of Hepatology. 2002;1(4):162–168. [PubMed] [Google Scholar]
- 7.Bradford R. H., Shear C. L., Chremos A. N., et al. Expanded clinical evaluation of lovastatin (EXCEL) study results: I. efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia. JAMA Internal Medicine. 1991;151(1):43–49. doi: 10.1001/archinte.1991.00400010067008. [DOI] [PubMed] [Google Scholar]
- 8.Newman C. B., Szarek M., Colhoun H. M., Betteridge D. J., Durrington P. N., Hitman G. A., et al. The safety and tolerability of atorvastatin 10 mg in the Collaborative Atorvastatin Diabetes Study (CARDS) Diabetes & Vascular Disease Research. 2008;5(3):177–183. doi: 10.3132/dvdr.2008.029. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd J., Blauw G. J., Murphy M. B., et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. The Lancet. 2002;360(9346):1623–1630. doi: 10.1016/S0140-6736(02)11600-X. [DOI] [PubMed] [Google Scholar]
- 10.Denus S. D., Spinler S. A., Miller K., Peterson A. M. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24(5):584–591. doi: 10.1592/phco.24.6.584.34738. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000097.e1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J. P. T., Altman D. G., Gøtzsche P. C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. British Medical Journal. 2011;343(7829) doi: 10.1136/bmj.d5928.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalyani R. R., Stein B., Valiyil R., Manno R., Maynard J. W., Crews D. C. Vitamin D treatment for the prevention of falls in older adults: systematic review and meta-analysis. Journal of the American Geriatrics Society. 2010;58(7):1299–1310. doi: 10.1111/j.1532-5415.2010.02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harbord R. M., Egger M., Sterne J. A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine. 2006;25(20):3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 15.Keech A., Collins R., Macmahon S., et al. Three-year follow-up of the oxford cholesterol study: assessment of the efficacy and safety of simvastatin in preparation for a large mortality study. European Heart Journal. 1994;15(2):255–269. doi: 10.1093/oxfordjournals.eurheartj.a060485. [DOI] [PubMed] [Google Scholar]
- 16.Salonen R., Nyyssönen K., Porkkala E., et al. Kuopio Atherosclerosis Prevention Study (KAPS): a population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation. 1995;92(7):1758–1764. doi: 10.1161/01.cir.92.7.1758. [DOI] [PubMed] [Google Scholar]
- 17.Herd J. A., Ballantyne C. M., Farmer J. A., et al. Effects of fluvastatin on coronary atherosclerosis in patients with mild to moderate cholesterol elevations (lipoprotein and coronary atherosclerosis study [LCAS]) American Journal of Cardiology. 1997;80(3):278–286. doi: 10.1016/S0002-9149(97)00346-9. [DOI] [PubMed] [Google Scholar]
- 18.Patrick W. S., de Feyter P., Macaya C., Kokott N., Puel J., Vrolix M., et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. Journal of the American Medical Association. 2002;287(24):3215–3222. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 19.Downs J. R., Clearfield M., Weis S. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. The Journal of the American Medical Association. 1998;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd J., Cobbe S. M., Ford I. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. The New England Journal of Medicine. 1995;333(20):1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen T. R., Kjekshus J., Berg K., Haghfelt T., Faergeman O., Faergeman G., et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Atherosclerosis Supplements. 1994;5(3):81–87. doi: 10.1016/j.atherosclerosissup.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 22.LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The New England Journal of Medicine. 1998;339(19):1349–1357. doi: 10.1056/nejm199811053391902. [DOI] [PubMed] [Google Scholar]
- 23.Sacks F. M., Pfeffer M. A., Moye L. A., et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. The New England Journal of Medicine. 1996;335(14):1001–1009. doi: 10.1056/nejm199610033351401. [DOI] [PubMed] [Google Scholar]
- 24.Armitage J., Bowman L., Collins R., Parish S., Tobert J. Effects of simvastatin 40 mg daily on muscle and liver adverse effects in a 5-year randomized placebo-controlled trial in 20,536 high-risk people. BMC Clinical Pharmacology. 2009;9(6) doi: 10.1186/1472-6904-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bays H. E., Ose L., Fraser N., et al. A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clinical Therapeutics. 2004;26(11):1758–1773. doi: 10.1016/j.clinthera.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Baigent C., Landray M., Leaper C., et al. First United Kingdom Heart and Renal Protection (UK-HARP-I) study: biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. American Journal of Kidney Diseases. 2005;45(3):473–484. doi: 10.1053/j.ajkd.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Furberg C. D., Adams H. P., Applegate W. B., et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation. 1994;90(4):1679–1687. doi: 10.1161/01.CIR.90.4.1679. [DOI] [PubMed] [Google Scholar]
- 28.Henkin Y., Johnson K. C., Segrest J. P. Rechallenge with crystalline niacin after drug-induced hepatitis from sustained-release niacin. Journal of the American Medical Association. 1990;264(2):241–243. doi: 10.1001/jama.1990.03450020093033. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson T. A., Amorosa L. F. Combination therapy with fluvastatin and niacin in hypercholesterolemia: A preliminary report on safety. American Journal of Cardiology. 1994;73(14):D25–D29. doi: 10.1016/0002-9149(94)90629-7. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz G. G., Olsson A. G., Ezekowitz M. D., et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes the MIRACL study: A randomized controlled trial. Journal of the American Medical Association. 2001;285(13):1711–1718. doi: 10.1016/S1062-1458(01)00368-3. [DOI] [PubMed] [Google Scholar]
- 31.Hu M., Tomlinson B. Current perspectives on rosuvastatin. Integrated Blood Pressure Control. 2013;6:15–25. doi: 10.2147/IBPC.S34814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G. L., Hsiao F. Y., Dong Y. H., Shen L. J., Wu F. L. Statins and the risk of liver injury: a population-based case-control study. Pharmacoepidemiology and Drug Safety. 2014;23(7):719–725. doi: 10.1002/pds.3646. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann J., Lerman A., Baumgart D., et al. Preprocedural statin medication reduces the extent of periprocedural non-Q-wave myocardial infarction. Circulation. 2002;106(17):2180–2183. doi: 10.1161/01.CIR.0000037520.89770.5E. [DOI] [PubMed] [Google Scholar]
- 34.Sposito A. C., Chapman M. J. Statin therapy in acute coronary syndromes: mechanistic insight into clinical benefit. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22(10):1524–1534. doi: 10.1161/01.ATV.0000032033.39301.6A. [DOI] [PubMed] [Google Scholar]
- 35.Kiortsis D. N., Nikas S., Hatzidimou K., Tsianos E., Elisaf M. S. Lipid-lowering drugs and serum liver enzymes: the effects of body weight and baseline enzyme levels. Fundamental & Clinical Pharmacology. 2003;17(4):491–494. doi: 10.1046/j.1472-8206.2003.00176.x. [DOI] [PubMed] [Google Scholar]
- 36.Russo M. W., Hoofnagle J. H., Gu J., et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60(2):679–686. doi: 10.1002/hep.27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo M. W., Scobey M., Bonkovsky H. L. Drug-induced liver injury associated with statins. Seminars in Liver Disease. 2009;29(4):412–422. doi: 10.1055/s-0029-1240010. [DOI] [PubMed] [Google Scholar]
- 38.Wei M. C., Zong W. X., Cheng E. H. Y., et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplowitz N. Biochemical and cellular mechanisms of toxic liver injury. Seminars in Liver Disease. 2002;22(2):137–144. doi: 10.1055/s-2002-30100. [DOI] [PubMed] [Google Scholar]
- 40.Isaacsohn J., Zinny M., Mazzu A., Lettieri J., Heller A. H. Influence of gender on the pharmacokinetics, safety, and tolerability of cerivastatin in healthy adults. European Journal of Clinical Pharmacology. 2001;56(12):897–903. doi: 10.1007/s002280000225. [DOI] [PubMed] [Google Scholar]
- 41.Lewis J. H., Mortensen M. E., Zweig S. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453–1463. doi: 10.1002/hep.21848. [DOI] [PubMed] [Google Scholar]
- 42.Jiao Y., Hu F., Zhang Z., et al. Efficacy and safety of loading-dose rosuvastatin therapy in elderly patients with acute coronary syndromes undergoing elective percutaneous coronary intervention. Clinical Drug Investigation. 2015;35(12):777–784. doi: 10.1007/s40261-015-0335-1. [DOI] [PubMed] [Google Scholar]
- 43.Wu W., Zhao L., Yang P., et al. Inflammatory Stress Sensitizes the Liver to Atorvastatin-Induced Injury in ApoE-/- Mice. PLoS ONE. 2016;11(7):p. e0159512. doi: 10.1371/journal.pone.0159512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salmela P. I., Sotaniemi E. A., Niemi M., Maentausta O. Liver function tests in diabetic patients. Diabetes Care. 1984;7(3):248–253. doi: 10.2337/diacare.7.3.248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S. Figure 1: statin therapy and incidence of liver injury; sensitivity analysis excluding one study at a time S. Figure 2: meta-analysis of the association between statin therapy and incidence of liver injury excluding Frank's study. S. Figure 3: meta-analysis of the association between statin therapy and incidence of liver injury excluding Tonkin's study. S. Figure 4: meta-analysis of the association between statin therapy and incidence of liver injury using the fixed-effect model according to the Mantel-Haenszel (M-H) model.