Abstract
Although some genomic rearrangements are caused by replication or transcription, the etiology of others is unclear. Reporting in Cell, Canela et al. (2017) reveal that type II topoisomerase-mediated release of torsional strain at chromosomal loop anchors generates DNA double-strand breaks that drive multiple oncogenic translocations in a transcription-independent manner.
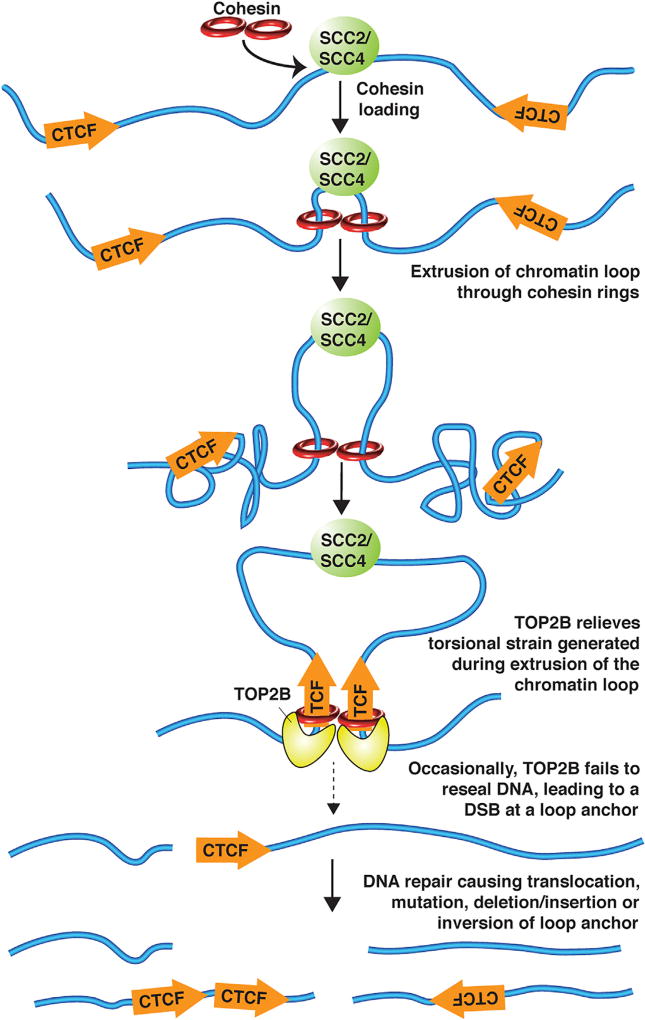
It is becoming increasingly clear that the higher-order organization of chromosomes into spatially restricted chromatin domains plays a critical role in the regulation of transcriptional programs (Dixon et al., 2016). Long-range chromatin loops occur between promoters and enhancers to initiate transcription. Other chromatin loops, called “insulated neighborhoods,” isolate enhancers from interacting with inappropriate gene promoters outside of the loop (Hnisz et al., 2016a) (Figure 1). While it is clear that chromatin loops are mediated by the ring-shaped protein complex cohesin and are often anchored by the insulator protein CTCF binding to its DNA recognition sequences, how chromatin loops form is a long-standing question (Dixon et al., 2016). Recent ground-breaking work spearheaded by Andre Nussenzweig’s group (Canela et al., 2017) reveals that a type II topoisomerase (TOP2) functions at chromatin loop anchors to dissipate the torsional strain generated by chromatin loop formation, providing compelling evidence for an “extrusion” model of chromatin loop formation (Dixon et al., 2016) (Figure 1). Furthermore, the DNA double-strand breaks (DSBs) induced by TOP2 at chromatin loop anchors can trigger genome instability that drives carcinogenic translocations.
Figure 1. Model for How DNA Damage Occurs at Chromatin Loop Anchors.
After recruitment by the cohesin loader, cohesin slides along chromatin, extruding a chromatin loop and inducing torsional strain in the chromosome, until its movement is stopped by encountering CTCF. Topoisomerase TOP2B is recruited to the chromatin loop anchor to relieve the torsional strain by breaking and resealing the DNA, but occasionally it does not reseal the breaks, leading to oncogenic chromosome rearrangements.
DNA-based processes, such as replication, repair, and transcription, as well as packaging the genome into chromatin and chromosome condensation, all generate torsional strain within the genome. This is relieved by transient breaking and rejoining of either one strand of DNA by type I topoisomerase or of both DNA strands by TOP2. Failure of TOP2 to accurately reseal the DSBs it generates causes susceptibility to mutation and genome rearrangements, apparent from therapy-related acute myeloid leukemia (t-AML) caused when the TOP2 inhibitor etoposide, which inhibits DNA sealing but not DNA cutting, is used as a chemotherapy drug (Leone et al., 1999). To test the hypothesis that oncogenic chromosomal translocations are due to failure to accurately rejoin TOP2-induced DSBs, Canela and colleagues (2017) used their innovative END-seq method in which biotinylated hairpin adapters are ligated onto DNA DSBs (Canela et al., 2016) to map DSBs in primary B cells in the presence of etoposide (Canela et al., 2017). They discovered that TOP2-induced DSBs occurred in the same DNA regions that typically undergo translocation within the MLL gene and its translocation partners ENL, AF9, and AF4. Intriguingly, in some cases the same DSBs occurred without etoposide treatment, albeit at approximately 10-fold lower frequency, indicating that DSBs at certain translocation breakpoints reflect the normal function of TOP2. This finding explains why de novo AML and t-AML are caused by translocations between identical breakpoints.
Strikingly, the function of TOP2 in causing DSBs at translocation breakpoints was not limited to the specific cell type in which the translocation causes human disease. For example, TOP2-induced DSBs occurred in B cells at the TMPRSS2 gene that mediates translocations in 50% of prostate cancer in humans, even though this gene is not expressed in B cells (Canela et al., 2017). Similarly, TOP2-induced DSBs within the MLL gene and its translocation fusion partners occurred in human breast cancer cells. As such, the DSBs that cause these oncogenic translocations result from normal TOP2 function in numerous different cell types. Therefore, oncogenic translocations are probably more promiscuous than previously documented, while their ability to drive carcinogenesis likely involves selection processes that occur only in specific cell types.
A key question is what biological process generates the torsional strain that has to be relieved by TOP2 at oncogenic translocation breakpoints? This study shows that of the two mammalian TOP2 isoforms, TOP2B was clearly responsible for the DSBs at the translocation breakpoints because they did not occur in mouse embryonic fibroblasts null for TOP2B (Canela et al., 2017). In agreement, the DSBs colocalized with TOP2B protein on the genome. Although TOP2B is known to induce DSBs within specific genes during transcription (Calderwood, 2016), transcription was convincingly shown to be dispensable for generation of most DSBs by TOP2B (Canela et al., 2017). Intriguingly, TOP2B-induced DSBs and TOP2B protein colocalized on the genome with the insulator protein CTCF and the RAD21 subunit of cohesin (Canela et al., 2017), indicating that TOP2B functions at anchors of chromatin loops. Although transcription was not essential for most TOP2B-induced DSBs (Canela et al., 2017), they may themselves promote efficient transcription through chromatin loops. For example, almost one-third of all TOP2B-induced DSBs occurred within 1 kb of transcription start sites. This provides a logical explanation for the marked preference of translocations at transcription start sites (Chiarle et al., 2011). The TOP2B-induced DSBs tended to be enriched at both ends of promoter-enhancer loops of active genes, along with TOP2B, CTCF, and cohesin (Canela et al., 2017). These data are consistent with TOB2B functioning to relieve torsional strain to further stimulate the promoter-enhancer looping that initiates transcription (Figure 1). This idea could be tested by determining whether MEFs lacking TOP2B have reduced promoter- enhancer looping and/or reduced transcriptional activation of corresponding genes.
TOP2B-induced DSBs at translocation breakpoints reflect the 3D chromosome architecture and provide unparalleled insight into the mechanism of chromatin loop formation (Canela et al., 2017). Hi-C chromosome mapping of loop anchor positions showed that the location of DSBs identified via END-seq overlapped with the location of loop anchors, in addition to cohesin and CTCF (Canela et al., 2017). Furthermore, chromatin loops are the cause of TOP2B-induced DSBs at loop anchors. This was apparent from the fact that single-nucleotide polymorphisms between two different mice species that reduced CTCF and RAD21 occupancy also resulted in loss of TOP2B-induced DSBs (Canela et al., 2017). That chromatin loops induce topoisomerase-mediated DNA breaks indicates that the mechanism by which chromatin loops form, which was previously unclear, induces torsional strain. This finding is consistent with a loop extrusion model, whereby cohesin forms progressively larger loops but stalls at loop anchors by interaction with boundary proteins like CTCF (Figure 1). Consistent with the loop extrusion model, as opposed to CTCF recruiting cohesin to chromatin, the probability of a DSB at any given loop anchor was directly proportional to the amount of the cohesin loader SCC2/SCC4 within the chromatin loop (Canela et al., 2017).
Taken together, this pioneering study (Canela et al., 2017) has revealed that TOP2B relieves torsional strain that is generated as chromatin fibers are extruded through cohesin during chromatin loop formation and that the TOP2B-induced DSBs at loop anchors can lead to oncogenic genome rearrangements. The TOP2B-induced DSBs may additionally provide a mechanism to account for the insertions/deletions, tandem duplications, and mutations that inactivate loop anchors in other human diseases, including many cancers in which aberrant activation of oncogenes occurs via hijacking of an enhancer normally insulated in an adjacent chromatin loop (Ji et al., 2016; Hnisz et al., 2016b; Weischenfeldt et al., 2017). As such, the mechanism used to generate the 3D chromatin architecture has been adopted to drive cancer and is also likely to have played an important role in evolution.
References
- Calderwood SK. Transcription. 2016;7:75–83. doi: 10.1080/21541264.2016.1181142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canela A, Sridharan S, Sciascia N, Tubbs A, Meltzer P, Sleckman BP, Nussenzweig A. Mol. Cell. 2016;63:898–911. doi: 10.1016/j.molcel.2016.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canela A, Maman Y, Jung S, Wong N, Callen E, Day A, Kieffer-Kwon KR, Pekowska A, Zhang H, Rao SSP, et al. Cell. 2017;170:507–521. doi: 10.1016/j.cell.2017.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarle R, Zhang Y, Frock RL, Lewis SM, Molinie B, Ho YJ, Myers DR, Choi VW, Compagno M, Malkin DJ, et al. Cell. 2011;147:107–119. doi: 10.1016/j.cell.2011.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JR, Gorkin DU, Ren B. Mol. Cell. 2016;62:668–680. doi: 10.1016/j.molcel.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D, Day DS, Young RA. Cell. 2016a;167:1188–1200. doi: 10.1016/j.cell.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D, Weintraub AS, Day DS, Valton AL, Bak RO, Li CH, Goldmann J, Lajoie BR, Fan ZP, Sigova AA, et al. Science. 2016b;351:1454–1458. doi: 10.1126/science.aad9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Dadon DB, Powell BE, Fan ZP, Borges-Rivera D, Shachar S, Weintraub AS, Hnisz D, Pegoraro G, Lee TI, et al. Cell Stem Cell. 2016;18:262–275. doi: 10.1016/j.stem.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone G, Mele L, Pulsoni A, Equitani F, Pagano L. Haematologica. 1999;84:937–945. [PubMed] [Google Scholar]
- Weischenfeldt J, Dubash T, Drainas AP, Mardin BR, Chen Y, Stütz AM, Waszak SM, Bosco G, Halvorsen AR, Raeder B, et al. Nat. Genet. 2017;49:65–74. doi: 10.1038/ng.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]