Abstract
Objectives
The integrin α4β7 is the gut-homing receptor for lymphocytes. It also is an important co-receptor for human immunodeficiency virus (HIV) via glycoprotein (gp)120 binding. Depletion of gut cluster of differentiation (CD)4 T cells is linked to chronic inflammation in patients with HIV; however, measuring CD4 cells in the gut is invasive and not routine. As such, establishing a peripheral marker for CD4 depletion of the gut is needed. We hypothesized that α4β7 CD4 T cells are depleted in the peripheral blood of treatment-naïve patients with HIV compared with healthy controls.
Methods
The study groups were treatment-naïve patients with HIV and uninfected controls. Subjects were included if they were 18 years or older with no history of opportunistic infections, active tuberculosis, or cancer. We collected peripheral blood and examined on whole blood using flow cytometry for the following cell surface markers: CD4, CD45RO, chemokine receptor type 5, C-X-C chemokine receptor type 4 (CXCR4), and the integrin β7. We collected demographic information, including age, sex, and ethnicity, as well as viral load (VL) and CD4 count. Two-sample t tests and Fisher exact tests were used to compare the differences between the two groups. Spearman correlation coefficients were calculated between CD4 count and log10− VL and percentage of CD4+/CD45RO+/β7+ and log10− VL in patients.
Results
Twenty-two subjects were enrolled in the study (12 patients with HIV and 10 controls). There were no differences in age or sex between the two groups. There were more Hispanics and fewer Asians in the group comprising patients with HIV compared with the control group (7 vs 2 and 0 vs 4, P = 0.05, respectively). Patients infected with HIV had significantly lower frequencies of CD4+/CD45RO+/β7+ cells (median 12%, range 5–18 compared with uninfected controls: median 20%, range 11–26, P = 0.0007). There was a statistically significant difference in the percentage of CD4+/CD45RO+/C-X-C chemokine receptor type 4+ cells between patients (72%, range 60%–91%) compared with controls (79%, range 72%–94%, P = 0.04). The percentage of CD4+/CD45RO+/chemokine receptor type 5+ did not differ between the group of patients with HIV and the control groups (22%, range 11%–57% vs 27%, range 14%–31%; P = 0.8, respectively). There was no correlation between percentage of CD4+/CD45RO+/β+ cells and log10− VL as measured by the Spearman correlation coefficient (r = 0.05, P = 0.88) in patients infected with HIV.
Conclusions
Memory CD4 β7+ cells are reduced significantly in the peripheral blood of untreated patients infected with HIV, which could be used as a noninvasive indicator of intestinal CD4 T cell loss and recovery. Further studies are needed to examine whether depletion of these CD4+/CD45RO+/β7+ cells in the peripheral blood parallels depletion in the gut of treatment-naïve patients with HIV and whether levels return to control levels after treatment.
Keywords: β7, gut-homing receptor, human immunodeficiency virus
The entry of human immunodeficiency virus (HIV)-1 into cluster of differentiation (CD)4+ T cells is mediated through the interaction of the viral envelope glycoproteins (gp120) with the CD4 molecule followed by binding to a chemokine receptor, usually chemokine receptor type 5 (CCR5) or C-X-C chemokine receptor type 4 (CXCR4). In mucosal tissues, CD4+ T cells express high levels of CCR5, and a subset of these cells express the integrin α4β7, the gut-homing receptor for T lymphocytes, to facilitate migration of lymphocytes from gut-inductive sites (Peyer’s patches and mesenteric lymph nodes) to the lamina propria. The integrin α4β7 is an important co-receptor for HIV via gp120 binding.1 α4β7 is increased on activated CD4+ T cells in intestinal mucosal tissues, which are important for HIV-1 pathogenesis. These cells also express high levels of CCR5.2 Evidence has shown that these CD4 T cells are highly susceptible to infection by HIV-1 in the genital mucosa, which makes them ideal targets for efficient productive infection at the point of transmission.3,4
Administration of primatized monoclonal antibody against α4β7 (α4β7 mAb) before and during repeated low-dose intravaginal simian immunodeficiency virus (SIV) challenge of rhesus macaques is protective for transmission and prevented gut-associated lymphoid tissue SIV infection.5 Byrareddy et al used α4β7 mAb in SIV infected monkeys following a 90-day course of antiretroviral therapy (ART) initiated at 5 weeks’ postinfection.6 These animals had low to undetectable viral loads for >9 months after treatment withdrawal, suggesting that a continual supply of these gut-homing cells is essential to maintain SIV replication and gut dysfunction. HIV binds the integrin α4β7 facilitating the infection of lymphocytes that home to the gut-associated lymphoid tissue (GALT). HIV infection results in an early massive and enduring depletion of intestinal CD4 T cells.7 Depletion of CD4 T cells from the gut has been linked to disease progression and systemic inflammation resulting from the translocation of microbial products.8 The degree of depletion of CD4 cells in GALT correlates with disease progression and response to therapy in patients infected with HIV9,10; however, measurement of CD4 cells from gut samples is invasive and not practical for routine monitoring of patients with HIV. As such, identification of a peripheral blood correlate of CD4 loss in intestinal tissue in humans would be valuable in the future study of the effect of HIV on CD4 depletion from the intestine.
Wang et al compared β7(HIGH) integrin expression on CD4+ T cells in blood with loss of CD4+ T cells in the intestine of macaques during SIV infection.11 The loss of β7(HIGH) CD4+ T cells in blood paralleled the loss of intestinal CD4+ T cells and proved to be a more reliable marker of intestinal CD4+ T-cell loss than monitoring CCR5+ memory CD4+ T cells. Similar studies have not been conducted in humans infected with HIV, however.
Because we hypothesize that treatment-naïve patients infected with HIV have fewer α4β7+ CD4+ cells than controls, the primary objective was to compare the levels of β7 CD4 T cells from treatment-naïve patients infected with HIV with uninfected controls. The secondary objectives were to examine CD4+ T cells for HIV coreceptors CCR5 and CXCR4 and to determine the correlation between the frequencies of α4β7 and the degree of viremia in patients with HIV.
Methods
Patients with HIV were recruited from the Thomas Street Health Center in Houston, Texas, a freestanding facility dedicated to outpatient care of patients with HIV. This center is part of the Harris County Health System. The study protocol was approved by the University of Texas Health Science Center institutional review board and the Harris Health System. We collected demographic information, including age, sex, and ethnicity. We also collected information from the patient’s electronic medical records, including comorbid conditions, most recent viral load and CD4 count, and time of HIV diagnosis. Patients were included in the study if they were 18 years or older and HIV positive as evidenced by positive HIV serology and confirmatory Western blot. We excluded the following patients from the study: patients with an active opportunistic infection; co-infection with hepatitis C as evidenced by a positive hepatitis C immunoglobulin G or positive hepatitis C polymerase chain reaction; active tuberculosis; and presence of malignancy, including lymphoma.
After obtaining informed consent, we performed phlebotomy to obtain 10 mL blood in ethylenediaminetetraacetic acid (Vacutainer, Becton Dickinson, Franklin Lakes, NJ). We performed the following measurements on whole blood using flow cytometry: CD4, a helper T-cell marker; CD45RO, a memory T-cell marker; CCR5 and CXCR4, chemokine receptors used by HIV to enter the cell; and the integrin β7.
For flow cytometry staining, 100 μL whole blood was stained with optimally titered mAbs (0.1–0.5 μg/mL) for 30 minutes at room temperature. Antibodies include CD4-AlexaFluor700, integrin β7-fluorescein isothiocyanate, CCR-AlexaFluor647, CXCR4-PerCPCy5.5 (BioLegend, San Diego, CA) and CD45RO-PE (BD Biosciences, San Jose, CA). Stained samples were then lysed and fixed with a single-platform Immunoprep system (TQ-prep instrument, Beckman Coulter, Indianapolis, IN). Flow cytometer voltage and compensation settings were established based on unstained, isotype, single-color, and fluorescence −1 controls. Data were acquired with a Gallios flow cytometer and analyzed with Kaluza 1.2 software (Beckman Coulter, Indianapolis, IN); 50,000 light scatter-gated lymphocytes were recorded for analysis. For some samples, excessive autofluorescent background was removed from analysis by a 45° angle and Boolean gating. For absolute cell counts, calibrated counting beads were added to each stained tube (Spherotech AccuCount, Spherotech, Lake Forest, IL).
Statistical Analysis
To detect the difference (mean percentage) of β7+ CD4 T cells between two independent groups (patients with HIV and controls), the two-sided, two-sample t test calculated the sample size under various scenarios. The significance level alpha was set at 0.05. Group sample sizes of 12 achieve 80% power to detect a mean difference of 12% between the two groups, assuming a common standard deviation of 10.0 with a significance level (alpha) of 0.05 using a two-sided, two-sample t test. PASS 2011 (NCSS Statistical Software, Kaysville, UT) was used for the calculations.
The descriptive statistics were calculated for all of the variables for the case and control groups, respectively. The viral load values were transformed by base 10 logarithm and the transformed values were used in the analysis. Two sample t tests were used to compare the means of continuous variables between two groups. Fisher exact tests compared the distribution in race and sex between two groups. The Spearman correlation coefficients were calculated between CD4 count and log10− VL and percentage of CD4+/CD45RO+/β7+ and log10− VL in patients.
Results
Twenty-two subjects were enrolled in the study (12 patients with HIV and 10 controls). The Table summarizes the demographics. There were no differences in age (P = 0.07) or sex (P = 1.0) between the patients with HIV and control groups. There were more Hispanics and fewer Asians in the patients with HIV group compared with controls (7 vs 2 and 0 vs 4, P = 0.05, respectively). There also was no statistically significant difference in CD4 cell count between the two groups; the median CD4 for patients with HIV was 384 cells per square millimeter (range 60–1058) and the median CD4 for controls was 502 cells per square millimeter (range 308–1479, P = 0.17).
Table.
Demographics and basic characteristics of patients with HIV and controls
| Variable | Patients, n = 12 | Controls, n = 10 | P |
|---|---|---|---|
| Median age, y (range) | 40 (21–52) | 35 (31–52) | 0.7 |
| Race | |||
| Asian | 0 | 4 | 0.05 |
| Black | 4 | 2 | |
| White | 1 | 2 | |
| Hispanic | 7 | 2 | |
| Sex | |||
| Female | 2 | 2 | 1.0000 |
| Male | 10 | 8 | |
| Median CD4 (range) | 384 (60–1058) | 502 (308–1479) | 0.17 |
| Median VL (range) | 72,050 (1120–1,500,000) | 0 | N/A |
| Median log10− VL (range) | 4.86 (3.05–6.18) | 0 | N/A |
| Median % CD4/CD45RO/B7 (range) | 12 (5–18) | 20 (11–25) | 0.0007 |
| Median % CD4/CD45RO/R5 (range) | 22 (11–57) | 27 (14–31) | 0.8 |
| Median % CD4/CD45RO/X4 (range) | 72 (60–91) | 79 (72–94) | 0.04 |
HIV, human immunodeficiency virus; N/A, not applicable; VL, viral load.
Patients with HIV had significantly lower frequencies of CD4+/CD45RO+/β7+ cells (median 12%, range 5–18) compared with uninfected controls (median 20%, range 11–25, P = 0.0007). The Figure illustrates the expression of β7+ in CD45RO+CD4+ cells of a patient (a) and a control (b).
Fig.
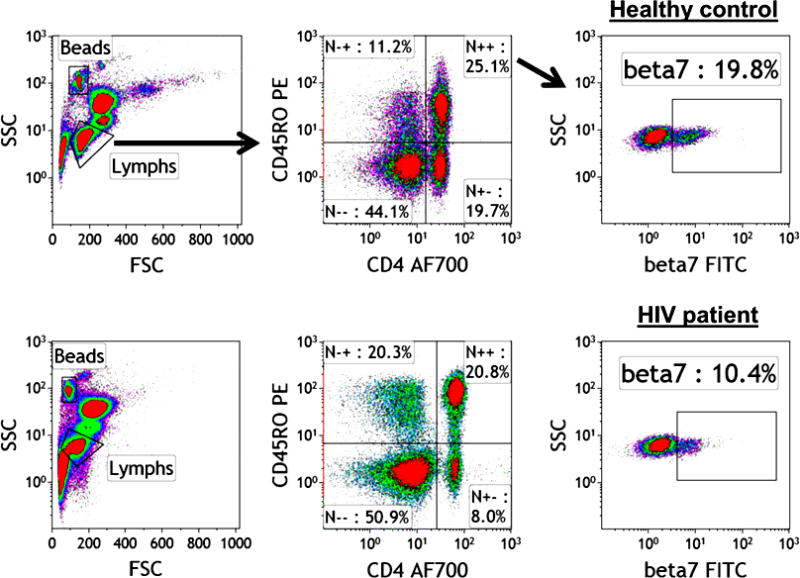
Frequency of CD4+/CD45RO+/β7+ cells in the peripheral blood of an uninfected control and a treatment-naïve patient with HIV. FITC, fluorescein isothiocyanate; FSC, forward-scattered light; HIV, human immunodeficiency virus; SSC, side-scattered light.
There also was a statistically significant difference in the percentage of CD4+/CD45RO+/CXCR4+ cells between patients (72%, range 60–91 as compared with controls, 79%, range 72–94, P = 0.04), but the levels of CD4+/CD45RO+/CCR5+ did not differ between patients and controls (22%, range 11–57 vs 27%, range 14–31, P = 0.8, respectively).
We did not find a correlation between CD4 count and log10− VL as measured by the Spearman correlation coefficients (r = −0.38, P = 0.23) in patients with HIV. Similarly, we did not find any correlation between the percentage of CD4+/CD45RO+/β7+ cells and log10− VL as measured by the Spearman correlation coefficient (r = 0.04895, P = 0.88) in patients with HIV.
Discussion
Treatment-naïve patients with HIV have significantly lower levels of circulating CD4+/CD45RO+/β7+ cells than uninfected controls, as measured by flow cytometry. This suggests that α4β7 cells are reduced in the peripheral blood in association with untreated HIV infection similar to the previous results in the SIV infection model.11 Our results suggest that monitoring CD4+/CD45RO+/β7+ cells in the peripheral blood using whole blood may be a useful, inexpensive, and noninvasive marker for levels of depletion, which may reflect the degree of CD4 depletion in GALT and reflect levels of reconstitution. Mavigner et al examined the frequencies of CD4+CCR9+β7(HIGH) T cells in peripheral blood and gut samples from patients with HIV on prolonged ART compared with those of healthy controls.12 They found that CCR9+β7(HIGH)CD4+ T cells in the peripheral blood and small intestine mucosa were inversely correlated in individuals with HIV and uninfected controls. There were more CCR9+β7(HIGH)CD4+ T cells in the peripheral blood and fewer cells in the small intestine mucosa of patients with HIV than in controls. These results suggest that although ART may reconstitute α4β7 CD4 in the blood of patients with HIV, these cells continue to be depleted in the gut even with prolonged ART, which the authors interpreted as a failure of the cells to home to the gut. The SIV antibody treatment study suggests that such homing actually fuels the pathogenesis, however. The finding of a greater frequency of α4β7 cells in the peripheral blood of patients with HIV differs in our study; however, an important distinction is that we examined treatment-naïve patients with HIV, in whom immune reconstitution has not yet occurred. As such, it is not surprising that α4β7 cells were depleted as previously shown during untreated SIV infection. Longitudinal observational studies that follow α4β7 levels in the peripheral blood of patients with HIV from the time of diagnosis until after initiation of highly active ART and reconstitution of CD4 cells will clarify the difference in our results.
Depletion of α4β7 cells in the gut of patients with HIV has several implications. α4β7(HIGH) memory CD4 cells are a preferential target for HIV: the integrin α4β7 is approximately three times the size of the CD4 receptor on the cell surface (CD4 receptor is approximately 7 nm and α4β7 is 22 nm), making it an ideal target for virus capture.13
The interaction between gp120 and α4β7 may have implications in the early events following the sexual transmission and dissemination of HIV. Kader et al examined mucosal samples obtained from SIV-infected rhesus macaques during the early phase of infection and found that CD4 cells that had high expression of α4β7 harbored more SIV at day 10 postinfection.14 In addition, downregulation of α4β7 on the surface of SIV-infected cells may be important in CD4 depletion of the mucosal-associated lymphoid compartments and susceptibility to superinfection and/or immune evasion.15 Depletion of CD4 from the gut is immune dysregulation in patients with HIV because of microbial translocation leading to chronic immune activation and an increase in proinflammatory cytokines. This state of immune activation persists even after treatment with antiretrovirals.
Our study found that there was a lower frequency of CXCR4 CD4 cells from untreated patients with HIV than healthy controls, but expression of the CCR5 receptor did not differ between the two groups. CXCR4 could result from higher levels of stromal cell–derived factor-1 in patients with HIV compared with controls; this has not been examined.
Our study has limitations. The sample size of populations was likely a limiting factor in not finding a correlation between the degree of CD4+/CD45RO+/β7+ depletion and viral load. A larger study will help locate this correlation. Our study focused on measuring CD4+/CD45RO+/β7+ in peripheral blood and did not correlate the level with expression of CD4+/CD45RO+/β7+ with gut samples. Future studies will use the same protocol on peripheral blood and correlate that with levels of CD4+/CD45RO+/β7+ in gut samples of untreated patients with HIV.
Conclusions
CD4+/CD45RO+/β7+ cells are depleted in untreated HIV infection. These may be a useful and practical marker of gut CD4 depletion that can be performed on whole blood and may indicate such depletion in the gut. Future studies are needed to examine whether the degree of depletion of α4β7cells in the peripheral blood parallels depletion in the gut samples of treatment-naïve patients with HIV and whether levels return to control levels after treatment and immune reconstitution.
Key Points.
β7 memory cluster of differentiation4 cells are depleted in untreated human immunodeficiency (HIV) infection.
The low expression of C-X-C chemokine receptor type 4 could be the result of higher levels of stromal cell–derived factor-1 in patients with HIV compared with controls, which could account for their apparent reduction in detection.
The interaction between glycoproteins 120 and α4β7 may have implications in the early events following sexual transmission and dissemination of HIV.
Acknowledgments
The authors thank the staff at the Division of Infectious Diseases, University of Texas, and the clinic staff at the Harris Health System for their support of this study. The authors thank Melissa Beck for help in editing the manuscript.
The research was funded by Baylor College of Medicine/University of Texas Medical School at Houston Center for AIDS Research grant no. 5P30AI36211 and by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases grant no. AI R37 36682 (to N.F.).
Footnotes
X.Y. and D.E.L. have received compensation from the NIH. R.A. has received compensation from the NIH. The remaining authors did not report any financial relationships or conflicts of interest.
References
- 1.Arthos J, Cicala C, Martinelli E, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 2.Cicala C, Arthos J, Fauci AS. HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV. J Transl Med. 2011;9(Suppl 1):S2. doi: 10.1186/1479-5876-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKinnon LR, Nyanga B, Chege D, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187:6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 4.Nawaz F, Cicala C, Van Ryk D, et al. The genotype of early-transmitting HIV gp120s promotes α (4) β(7)-reactivity, revealing α (4) β(7) +/CD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 2011;7:e1001301. doi: 10.1371/journal.ppat.1001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrareddy SN, Kallam B, Arthos J, et al. Targeting α4β7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat Med. 2014;20:1397–1400. doi: 10.1038/nm.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrareddy SN, Arthos J, Cicala C, et al. Sustained virologic control in SIV+ macaques after antiretroviral and α4β7 antibody therapy. Science. 2016;354:197–202. doi: 10.1126/science.aag1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krzysiek R, Rudent A, Bouchet-Delbos L, et al. Preferential and persistent depletion of CCR5+ T-helper lymphocytes with nonlymphoid homing potential despite early treatment of primary HIV infection. Blood. 2001;98:3169–3171. doi: 10.1182/blood.v98.10.3169. [DOI] [PubMed] [Google Scholar]
- 8.Ibarrondo FJ, Wilson SB, Hultin LE, et al. Preferential depletion of gut CD4-expressing iNKT cells contributes to systemic immune activation in HIV-1 infection. Mucosal Immunol. 2013;6:591–600. doi: 10.1038/mi.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sousa AE, Carneiro J, Meier-Schellersheim M, et al. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol. 2002;169:3400–3406. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 10.Paiardini M, Muller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. 2013;254:78–101. doi: 10.1111/imr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Xu H, Gill AF, et al. Monitoring alpha4beta7 integrin expression on circulating CD4+ T cells as a surrogate marker for tracking intestinal CD4+ T-cell loss in SIV infection. Mucosal Immunol. 2009;2:518–526. doi: 10.1038/mi.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mavigner M, Cazabat M, Dubois M, et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J Clin Invest. 2012;122:62–69. doi: 10.1172/JCI59011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicala C, Martinelli E, McNally JP, et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci USA. 2009;106:20877–20882. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kader M, Wang X, Piatak M, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009;2:439–449. doi: 10.1038/mi.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budde ML, Lhost JJ, Dudley DM, et al. Integrin alpha4beta7 is downregulated on the surfaces of simian immunodeficiency virus SIVmac239-infected cells. J Virol. 2010;84:6344–6351. doi: 10.1128/JVI.00430-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


