Abstract
Autologous hematopoietic cell transplantation (AHCT) in multiple myeloma (MM) patients with renal insufficiency (RI) is controversial. Patients who underwent AHCT for MM between 2008-2013 were identified (N =1492) and grouped as normal/mild (≥60 ml/min), N=1240, moderate (30-59), N=185 and severe RI (<30), N=67 based on MDRD. Multivariate analysis of non-relapse mortality (NRM), relapse, progression-free survival (PFS) and overall survival (OS) was performed. Of the 67 patients with severe RI, 35 were on dialysis prior to AHCT. Patients received melphalan 200 mg/m2 (Mel200) in 92% (normal/mild), 75% (moderate) and 33% (severe) RI; remainder received 140 mg/m2 (Mel140). Thirty four of 35 patients with severe RI achieved post-AHCT dialysis independence. The 5-year PFS for normal, moderate and severe RI was 35 (95% CI, 31-38)%, 40 (31-49)% and 27 (15-40)% respectively, (p=0·42); 5-year OS for normal, mod and severe RI was 68 (65-71)%, 68 (60-76)% and 60 (46-74)% respectively, (p=0·69). With moderate RI, 5-year PFS for HDM 140 mg/m2 was 18 (6-35)% and for Mel200 was 46 (36-57)% (p=0·009). With severe RI, 5-year PFS Mel140 was 25 (11-41) % and for Mel200 was 32 (11-58)% (p=0·37). We conclude that AHCT is safe and effective in patients with MM with RI.
Keywords: renal failure, transplant, melphalan dose
Background
Renal insufficiency (RI) is a common complication of multiple myeloma (MM) present in approximately 20-50% of patients.(1) Approximately 5% of MM patients are dialysis dependent· Factors contributing to RI in patients with MM include light chain-induced proximal tubular damage, cast nephropathy, interstitial nephritis, hypercalcemia, dehydration, hyperuricemia, amyloid deposition and plasma cell infiltration.(1) Renal dysfunction at presentation is considered a risk factor for early death and has traditionally been associated with an unfavorable prognosis. This may be due to its reflection of advanced disease, lack of effective treatments previously and perhaps an arbitrary cutoff for using high dose chemotherapy due to a perception of increased morbidity and mortality.
High dose melphalan (HDM) with autologous hematopoietic cell transplantation (AHCT) is considered an effective therapy for MM, both, in the upfront and in the salvage setting.(2–4) The decision to perform an AHCT depends on the physician assessment of patients’ eligibility, which typically involves the evaluation of co-morbidities, performance status and the age of patients. Several reports have shown that both the use of aggressive induction regimens incorporating the proteasome inhibitors and the use of AHCT is safe and effective in patients with myeloma presenting with renal insufficiency.(5–13) Patients generally are treated with dose reductions in HDM although there is single institutional data that HDM at 200 mg/m2 is safe and effective in patients with RI with creatinine clearance 30 to 60 ml/min.(14) The International Myeloma Working Group recommends restricting the dose of melphalan to 100-140 mg/m2 as grade C evidence.(15)
We undertook this analysis to study RI at the time of AHCT in a contemporaneous cohort of MM patients reported to the Center for International Blood and Marrow Transplant Research (CIBMTR®) from 2008-2013 in order to analyze the impact of the severity of RI on AHCT outcomes.
Methods
Data source
The CIBMTR registry is a prospectively maintained transplant database that collects transplant data from over 450 centers worldwide. Data are submitted to the Statistical Center at the Medical College of Wisconsin in Milwaukee, where computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality· Data are collected pre-transplantation, 100 days and 6 months post-transplantation, and annually thereafter until death or last follow up. Observational studies conducted by the CIBMTR are performed with approval of the institutional review boards of the National Marrow Donor Program and the Medical College of Wisconsin.
Patients
All patients from US/Canada who underwent AHCT for MM and reported to the CIBMTR between 2008 and 2013 and had a reported creatinine at AHCT were included in this study (N =1492). Patients were grouped by GFR using the Modification of Diet in Renal Disease (MDRD) equation at AHCT as Normal/Mild (≥60 ml/min), moderate (30-60) and severe RI (<30).
Statistical Analysis
Patient-, disease- and treatment-related factors were compared using the Chi-square test for categorical and the Kruskall-Wallis test for continuous variables. Outcomes analyzed included non-relapse mortality (NRM), relapse/progression, progression-free survival (PFS) and overall survival (OS). Estimates of outcomes were reported as probabilities with 95% confidence intervals (CI). The probability of OS was calculated with the Kaplan-Meier estimator, with the variance estimated by Greenwood formula. Comparison of survival curves was done with the log-rank test. Multivariate analysis of NRM, relapse/progression, PFS and OS was performed using Cox proportional hazards models with RI as the main effect. Other variables tested in the multivariate analysis included age, gender, race, Karnofsky performance score (KPS), hematopoietic cell transplant-comorbidity index (HCT-CI) adjusted to remove renal comorbidity, myeloma immunoglobulin subtype, International Staging System (ISS) stage, high risk status, lines of chemotherapy prior to transplant, induction chemotherapy, chemosensitivity, disease status prior to transplant, year of transplant and post-transplant therapy (consolidation/maintenance). Cumulative incidence curves and probabilities for NRM were calculated by treating relapse as a competing risk. The pointwise comparison was used to analyze outcomes of different interested groups. All the tests are two-sided with a significant level of 0·05.
A retrospective power analysis was done in order to ensure we had sufficient power in this study. Since it is known that RI would be associated with lower survival probability, we considered a one-sided test. With a 5% significance level and 80% power, our analysis would be able to detect at least a 20% difference and a 10% difference in the 5-year survival probability between severe/RF (N=67) vs normal/mild (N=1240) and moderate (N=185) vs normal/mild (N=1240), respectively.
Results
Baseline characteristics
Of the cohort, 1240 patients had normal/mild, 185 patients had moderate and 67 patients had severe RI (Table 1). Thirty five patients were on dialysis prior to AHCT. Karnofsky performance status was ≥90% in 56% of patients with normal/mild RI, 57% in patients with moderate RI and 37% in patients with severe RI. Light chain MM was more frequent in severe RI (55%) compared to 30% (moderate) and 18% (normal/mild). There was no difference in high-risk versus non-high risk cytogenetic abnormalities between the three groups. Induction chemotherapies included bortezomib based treatments in 73% of normal/mild, 81% of moderate and 80% of severe RI and ≥2 lines of chemotherapy in 20% normal/mild, 22% moderate and 45% severe RI. Pre-transplant disease status was similar between the groups with ≥VGPR in 49% normal/mild, 49% moderate and 50% severe RI. The median time from diagnosis to AHCT was <6 months in 38% of normal/mild, 36% of moderate and 25% of patients with severe RI. Melphalan dose was 200mg/m2 in 92% patients with normal/mild, 75% patients with moderate and 33% patients with severe RI. Remaining patients received Melphalan 140 mg/m2
Table 1.
Characteristics of patients who underwent first auto transplant for MM in 2008-2013 and reported with CIBMTR
| Renal function at HCT1
|
|||
|---|---|---|---|
| Variable | Normal/Mild | Moderate | Severe/RI |
| Number of patients | 1240 | 185 | 67 |
| Age at transplant, years | |||
| median age (range) | 59 (28-78) | 62 (33-75) | 60 (23-74) |
| 18-39 | 39 (3) | 6 (3) | 5 (7) |
| 40-49 | 189 (15) | 20 (11) | 7 (10) |
| 50-59 | 463 (37) | 46 (25) | 22 (33) |
| 60-69 | 474 (38) | 97 (52) | 27 (40) |
| 70+ | 75 (6) | 16 (9) | 6 (9) |
| Gender, Male | 741 (60) | 97 (52) | 36 (54) |
| Race | |||
| Caucasian | 938 (76) | 145 (78) | 53 (79) |
| African American | 226 (18) | 30 (16) | 10 (15) |
| Others2 | 38 (3) | 4 (2) | 3 (4) |
| Missing | 38 (3) | 6 (3) | 1 (1) |
| Karnofsky Score, ≥ 90% | 700 (56) | 106 (57) | 25 (37) |
| Adjusted HCTCI scores (Renal comorbidity excluded) | |||
| 0 | 505 (41) | 62 (34) | 29 (43) |
| 1 | 188 (15) | 28 (15) | 11 (16) |
| 2 | 192 (15) | 26 (14) | 9 (13) |
| 3 | 175 (14) | 32 (17) | 8 (12) |
| >3 | 158 (13) | 36 (19) | 9 (13) |
| Missing | 22 (2) | 1 (<1) | 1 (1) |
| Disease-related | |||
| Immunochemical subtype | |||
| IgG | 722 (58) | 96 (52) | 21 (31) |
| IgA | 259 (21) | 27 (15) | 7 (10) |
| Light chain only | 219 (18) | 56 (30) | 37 (55) |
| Others3 | 15 (1) | 4 (2) | 2 (3) |
| Non-secretory | 25 (2) | 2 (1) | 0 |
| ISS Stage III at diagnosis | 326 (26) | 84 (45) | 43 (64) |
| Cytogenetic abnormality (FISH and/or karyotype) | |||
| High risk (HR)4 | 151 (12) | 27 (15) | 13 (19) |
| Non-HR | 876 (71) | 125 (68) | 45 (67) |
| Missing5 | 213 (17) | 33 (18) | 9 (13) |
| Serum creatinine at diagnosis | |||
| median (range) | 1 (<1-14) | 2 (<1-18) | 5 (2-17) |
| < 2 mg/dl | 926 (75) | 77 (42) | 1 (1) |
| ≥ 2 mg/dl | 94 (8) | 72 (39) | 48 (72) |
| Missing | 220 (18) | 36 (19) | 18 (27) |
| Serum creatinine prior to transplant | |||
| median (range) | 1 (<1-2) | 1 (1-2) | 3 (2-12) |
| < 2 mg/dL | 1240 | 166 (90) | 2 (3) |
| ≥ 2 mg/dL | 0 | 19 (10) | 65 (97) |
| GFR at transplant (mL/min per 1·73m2), median(range) | 97 (60-222) | 49 (31-60) | 19 (5-29) |
| Transplant-related | |||
| Lines of chemotherapy | |||
| 1 | 988 (80) | 144 (78) | 37 (55) |
| 2 | 199 (16) | 32 (17) | 22 (33) |
| >2 | 53 (4) | 9 (5) | 8 (12) |
| Chemotherapy | |||
| VTD | 84 (6) | 25 (14) | 15 (22) |
| RVD | 516 (42) | 59 (32) | 15 (22) |
| CVD | 154 (12) | 31 (17) | 12 (18) |
| VD6 | 146 (12) | 33 (18) | 12 (18) |
| RD | 230 (19) | 31 (17) | 4 (6) |
| TD | 87 (7) | 5 (2) | 4 (6) |
| VAD/similar | 23 (2) | 1 (<1) | 5 (8) |
| Mobilization strategy | |||
| GCSF | 761 (61) | 104 (56) | 50 (75) |
| GCSF+chemo | 307 (25) | 45 (24) | 16 (24) |
| GCSF+pleraxifor | 172 (14) | 36 (19) | 1 (1) |
| Disease status at transplant | |||
| sCR/CR | 228 (18) | 37 (20) | 11 (16) |
| VGPR | 385 (31) | 53 (29) | 23 (34) |
| PR | 534 (43) | 82 (44) | 29 (43) |
| SD | 62 (5) | 8 (4) | 3 (4) |
| Rel/Prog | 31 (3) | 5 (3) | 1 (1) |
| Melphalan dose, mg/m2 | |||
| 140 | 98 (8) | 47 (25) | 45 (67) |
| 200 | 1142 (92) | 138 (75) | 22 (33) |
| Time from diagnosis to transplant | |||
| < 6 months | 474 (38) | 66 (36) | 17 (25) |
| 6 - 12 months | 766 (62) | 119 (64) | 50 (75) |
| Year of transplant | |||
| 2008 | 356 (29) | 58 (31) | 26 (39) |
| 2009 | 135 (11) | 22 (12) | 10 (15) |
| 2010 | 127 (10) | 17 (9) | 6 (9) |
| 2011 | 178 (14) | 19 (10) | 6 (9) |
| 2012 | 173 (14) | 32 (17) | 5 (7) |
| 2013 | 271 (22) | 37 (20) | 14 (21) |
| Median follow-up of survivors (range), months | 48 (3-97) | 48 (4-96) | 60 (6-75) |
Evaluated by glomerular filtration rate (GFR), mL/min per 1·73m2· Abbreviated MDRD study equation is: GFR (mL/min per 1·73m2) = 186 × (SCr)−1·154 × (Age)−0·203 × (0·742 if female) × (1·210 if African-American)· SCr is serum creatinine concentration at transplant in mg/dL.
- Normal/Mild – GFR score≥60
- Moderate - GFR score (30-59)
- Severe/Renal Failure – GFR score <30
Asian (30), American Indian (12), Islander (3).
IgD (16), IgE (1), IgM (4).
High risk genomic abnormalities consist of t(4;14), t(14;16), del17p, hypodiploidy and any abnormality in chromosome 1· All other cytogenetic lesions are considered non high-risk.
Including No metaphases (9), Cytogenetics not tested (157) and Unknown (89).
Including Bortezomib +Doxil+Dexamethasone (2).
HCT-CI, hematopoietic cell transplantation-comorbidity index; ISS, International Staging System; FISH, fluorescence in situ hybridization; MM, multiple myeloma; CIBMTR, Center for International Blood and Marrow Transplantation; VTD, bortezomib, thalidomide, dexamethasone; RVD, lenalidomide, bortezomib, dexamethasone; VD, bortezomib, dexamethasone; RD, lenalidomide dexamethasone; TD, thalidomide, dexamethasone; VAD, vincristine, doxorubicin, dexamethasone; GCSF, granulocyte-colony stimulating factor; sCR, stringent complete response; CR, complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; RI, renal insufficiency
Response
The probability of PFS at 5 years for patients with normal, moderate and severe RI was 35% (95% CI, 31-38%), 40% (95% CI, 31-49) and 27% (95% CI, 15-40%) respectively, (p=0·42). The probability of OS at 5 years for patients with normal, moderate and severe RI was 68% (95% CI, 65-71%), 68% (95% CI, 60-76) and 60% (95% CI, 46-74%) respectively, (p=0·69). (Figure 1) For patients with moderate RI, probability of PFS at 5 years for patients receiving Melphalan 140 mg/m2 was 18% (95% CI, 6-35%) and for patients receiving Mel 200 mg/m2 was 46% (95% CI, 36-57) (p=0·009); probability of OS at 5 years for patients receiving Mel 140 mg/m2 was 67% (95% CI, 51-82%) and for patients receiving Mel 200 mg/m2 was 68% (95% CI, 58-78) (p=0·52). For patients with severe RI, probability of PFS at 5 years for patients receiving Mel 140 mg/m2 was 25% (95% CI,11-41%) and for patients receiving Mel 200 mg/m2 was 32% (95% CI, 11-58) (p=0·37); probability of OS at 5 years for patients receiving Mel 140 mg/m2 was 63% (95% CI, 46-80%) and for patients receiving Mel 200 mg/m2 was 55% (95% CI, 31-77) (p=0·65).
Figure 1.
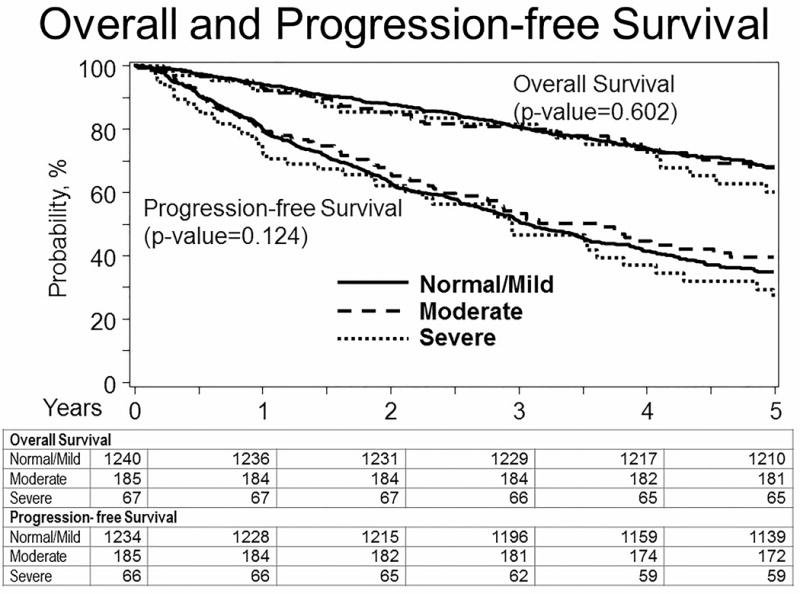
Kaplan-Meier survival curve of PFS and OS of pts with Normal/mild, moderate and severe renal insufficiency
The median (range) inpatient hospital stay was 14 days (1-90) in patients with normal/mild, 16 (3-77) in patients with moderate and 17 (4-70) in patients with severe RI. The TRM at day 100 was 0% in patients with moderate and severe RI. Time to neutrophil and platelet engraftment, NRM, day 100 responses, relapse, PFS and OS were not statistically different between the three groups (Table 2, 3).
Table 2.
Post-transplant characteristics
| Renal function at HCT | |||
|---|---|---|---|
| Variable | Normal/Mild | Moderate | Severe/RI |
| Number of patients | 1240 | 185 | 67 |
| Inpatient hospital stay | |||
| N evaluable (%) | 1133 (91) | 174 (94) | 65 (97) |
| median (range), days | 14 (1-90) | 16 (3-77) | 17 (4-70) |
| Day-100 response after transplant | |||
| sCR/CR | 394 (32) | 65 (35) | 23 (34) |
| VGPR | 378 (30) | 56 (30) | 16 (24) |
| PR | 287 (23) | 42 (23) | 16 (24) |
| MR/NR/SD | 111 (9) | 15 (8) | 5 (7) |
| Rel/Prog | 30 (2) | 2 (1) | 3 (4) |
| Missing | 40 (3) | 5 (3) | 4 (6) |
| Planned post-HCT therapy | |||
| Planned/completed therapy | 801 (65) | 104 (56) | 30 (45) |
| Lenalidomide + Bortezomib ± dexamethasone | 211 (17) | 17 (9) | 7 (10) |
| Lenalidomide ± dexamethasone | 492 (40) | 75 (41) | 12 (18) |
| Bortezomib ± dexamethasone | 47 (4) | 10 (5) | 7 (10) |
| Thalidomide ± dexamethasone | 21 (2) | 1 (<1) | 3 (4) |
| Others* | 30 (2) | 1 (<1) | 1 (1) |
| No post-HCT therapy | 431 (35) | 80 (43) | 37 (55) |
| Missing | 8 (<1) | 1 (<1) | 0 |
HCT, hematopoietic cell transplantation; N, number; sCR, stringent complete response; CR, complete response; PR, partial response; VGPR, very good partial response; MR, minimal response; SD, stable disease; NR, no response; RI, renal insufficiency
Table 3.
Multivariate Analysis of outcomes
| NRM | Relapse | PFS | OS | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) |
p-value | HR (95% CI) |
p-value | HR (95% CI) |
p-value | HR (95% CI) |
p-value | |
| RI | 0·22 | 0·2 | ||||||
| Normal/Mild | 1 | 1 | 1 | 1 | ||||
| Moderate | 1·3 (0·6-3·2) |
0·5 | 0·8 (0·7-1) |
0·09 | 0·8 (0·7-1) |
0·06 | 0·9 (0·7-1·2) |
0·4 |
| Severe | 2·5 (0·9-7·1) |
0·09 | 0·9 (0·6-1·2) |
0·5 | 0·8 (0·6-1·2) |
0·3 | 0·9 (0·5-1·3) |
0·5 |
| Ig subtype | NS | 0·01 | NS | <0·0001 | ||||
| IgG subtype (N=830) | 1 | 1 | ||||||
| IgA subtype (N=289) | 1·2 (1·0-1·5) |
0·03 | 1·6 (1·2-2) |
<0·0001 | ||||
| Light chain (N=311) | 0·9 (0·7-1) |
0·09 | 0·8 (0·6-1) |
0·07 | ||||
| Non-secretory/others (N=48) | 1·1 (0·8-1·6) |
0·6 | 1 (0·6-1·8) |
0·9 | ||||
| ISS at diagnosis, III vs others | NS | 1·5 (1·3-1·7) |
<0·0001 | 1·4 (1·2-1·7) |
<0·0001 | 1·9 (1·6-2·4) |
<0·0001 | |
| Lines of chemotherapy ≥2 vs 1 | NS | 1·3 (1·1-1·5) |
0·007 | 1·2 (1·1-1·5) |
0·009 | 1·4 (1·1-1·7) |
0·009 | |
| Chemo-sensitivity versus chemo-resistant | NS | 0·6 (0·5-0·8) |
<0·0001 | 0·6 (0·5-0·8) |
<0·0001 | 0·6 (0·5-0·9) |
0·007 | |
| Planned post-transplant treatment, no versus yes | NS | 1·6 (1·4-1·8) |
<0·0001 | 1·6 (1·4-1·8) |
<0·0001 | 2 (1·6-2·5) |
<0·0001 | |
NRM, non-relapse mortality; PFS, progression-free survival; OS, overall survival; CI, confidence interval; NS, not significant; Ig, immunoglobulin; ISS, International Staging System
Multivariate analysis of outcomes
On multivariate analysis, IgA or light chain subtypes, ISS Stage III, ≥2 lines of chemotherapy, lack of sensitivity to therapy and lack of planned post-transplant therapy were significantly associated with higher relapse. Renal function at AHCT was not a significant factor for predicting higher frequency of relapse. IgA subtype, ISS stage III at diagnosis, lack of planned post-transplant therapy post-AHCT, ≥2 lines of pre-AHCT therapy were associated with lower PFS and OS. (Table 3)
Patients on hemodialysis at the time of transplant (N=35)
Thirty four of 35 patients with severe RI who were on dialysis at the time of transplant achieved post-transplant dialysis independence. All patients on dialysis at time of transplant received Melphalan 140 mg/m2. The TRM at day 100 was 0%. Median CD34+ cell transfused was 4·59×106/kg CD34 dose (2·59-11·91). Median time (range) to platelet engraftment was 18 days (11-37) and time to neutrophil engraftment was 11·5 days (11–15). Average length of hospital stay was 18 days (0-63). The probability of PFS at 1 year was 70% (95% CI, 53-84) and at 5 years was 26% (95% CI, 11-44%). The probability of OS at 1 year was 88% (95% CI, 76-97) and at 5 years was 49% (95% CI, 29-68%).
Impact of post-transplant therapies
The probability of PFS at 5 years for patients who received maintenance therapy (n=935) was 40% (95% CI, 36-44%) and for those who did not receive maintenance therapy (n=548) was 26% (95% CI, 22-31%) (p<0·001). The probability of OS at 5 years for patients who received maintenance therapy was 74% (95% CI, 69-78%) and for those who did not receive maintenance therapy was 58% (95% CI, 53-63%) (p<0·001).
Cause of death
In this cohort, 386 deaths were seen during follow up. Causes of death appeared similar between the 3 groups with relapse as the cause of death in 255 (81%), 36 (73%) and 16 (73%) in the normal/mild, moderate and severe RI respectively. Other pertinent causes of death included infection in 8 (3%) of normal/mild, 2 (4%) of moderate, 0 of severe, second malignancy in 5 (1%) of normal/mild, 1 (2%) of moderate and 1 (5%) of severe, organ failure in 9 (3%) of normal/mild (including 2 renal), 3 (6%) of moderate and 2 (9%) of severe (including 1 renal). (Table 4)
Table 4.
Cause of Death
| Renal function at HCT | |||
|---|---|---|---|
| Normal/Mild | Moderate | Severe/RI | |
| Number of patients | 1240 | 185 | 67 |
| Number of death | 315 | 49 | 22 |
| Cause of death | |||
| Primary disease | 255 (81) | 36 (73) | 16 (73) |
| Infection | 8 (3) | 2 (4) | 0 |
| Organ failure1 | 9 (3) | 3 (6) | 2 (9) |
| Secondary malignancy | 5 (2) | 1 (2) | 1 (5) |
| Other2 | 8 (3) | 2 (4) | 0 |
| Unknown | 30 (9) | 5 (10) | 3 (14) |
Organ failure: Normal/Mild: ARDS (2), Cardiac (2), Renal (2), TTP/HUS (1), Not specified (2); Moderate: ARDS (2), Cardiac (1); Severe/RF: Pulmonary Edema (1), Renal (1)·
Intracranial (1), subarachnoid hemorrhage (1), accidental death (1), thromboembolic (1), prior malignancy (1), hypoxemia, sepsis, hypotension, neutropenia (1), perforated viscus (1), respiratory failure (1), septic shock (1), severe metabolic acidosis (1).
(severe metabolic acidosis: Moderate renal function at HCT)
HCT, hematopoietic cell transplantation; RI, renal insufficiency
Discussion
In this large database study, we make the following observations: 1) High dose melphalan and AHCT is safe in patients with moderate and severe RI at the time of transplant, 2) Dose of melphalan matters and patients with moderate RI who received Mel 200 mg/m2 had improved outcomes, 3) adjusted analysis failed to show independent impact of RI at transplant on 5-year outcomes and 4) a significant portion of patients on dialysis pre-transplant were reported to achieve dialysis independence subsequently
Renal impairment is a frequent problem at diagnosis in patients with myeloma and is associated with adverse prognostic consequences. Renal recovery is associated with improved outcomes in patients with myeloma. Improvement in supportive care practices has permitted AHCT to be used in patients traditionally considered to be high risk.
Transplant related mortality has varied widely after single transplant in dialysis-dependent patients, with reports of 15% by St Bernard et al., 2·6% by Badros et al. and 12% by Lee et al.(7, 10, 12) In contrast, there was no TRM at day 100 in our analysis in patients with moderate or severe RI, including patients who were dialysis-dependent.
There is conflicting data as to whether melphalan dose reduction is required prior to AHCT in patients with renal failure including those requiring dialysis. Knudsen et al. reported a TRM of 50% in dialysis dependent myeloma patients who received Melphalan 200 mg/m2 (9) Badros et al. reported on 81 myeloma patients in renal failure, including 38 on dialysis.(7) Melphalan dose was reduced to 140 mg/m2 from 200 mg/m2 in the last 21 patients due to excessive toxicity. In a matched pair analysis by Raab et al., toxicity, TRM and survival were similar in dialysis dependent myeloma patients who received melphalan 100 mg/m2 compared to patients without renal failure who received melphalan 200 mg/m2 (11) In the study by Fakih et al analyzing outcome of 24 patients who were dialysis dependent, the TRM was 0% at 12-months regardless of melphalan dose used – 14 patients received Mel 200, 7 patients received Mel 140 and 3 patients received Mel 180. Dialysis independence was seen in 3 patients (13%).(8) In a large scale pharmacodynamic analysis of high dose melphlan, melphalan exposure above the median (12·84 mg l(−1) h) was associated with improved survival, with an acceptable increase in transplant toxicity. (16) In our study, improved PFS was noted among patients with moderate RI receiving Mel 200 mg/m2 compared to Mel 140 mg/m2. This difference was not noted in patients with severe RI. All patients who were dialysis-dependent received Mel 140 mg/m2 and dialysis independence was seen in the majority of patients in our study. Additionally, patients on dialysis had adequate hematopoietic cell collection and the engraftment kinetics were similar to patients who were not on dialysis. If performance status permits, our analysis leads us to recommend a possible dose of Mel 200 mg/m2 in patients with moderate renal insufficiency and a dose of Mel 140 mg/m2 in patients with severe RI, including patients on dialysis dependence. Perhaps a PK-guided dose may help better determine optimum dose of melphalan in an individual patient in the future.(17)
Our data did not show an effect of RI on 5-year outcomes on multivariate analysis. Factors well reported to affect long term outcomes such as IgA subtype, ISS III at diagnosis, number of pre-transplant chemotherapies, disease status prior to transplant and use of post-transplant treatment were significant. Maintenance treatment has been shown to improve PFS and OS in patients with normal renal function.(18, 19) Raab et al. compared the data of 10 patients with RI who did not receive maintenance treatment with those who either received thalidomide (n = 10), IFN (n = 3) or bortezomib (n = 4) and found a significantly longer event-free survival (median, 29·9 vs 47 months, p = 0·006) in favor of patients on maintenance therapy, whereas OS was not found to be significantly different (median, 28·7 vs 37 months, P = 0·16).(11) In our analysis, post-transplant maintenance treatment was associated with improved outcomes. However, our study was limited by the absence of detailed data on consolidation/maintenance post-transplant therapies and the duration of use. Of note, 55% of patients with severe RI at AHCT did not receive post-AHCT maintenance treatment. Our study also demonstrates that relapse/progressive disease remains the primary cause of death for MM patients, regardless of the degree of RI. Thus, measures to sustain post-AHCT responses are important.
The retrospective nature and reliance on center-reported data reported to the CIBMTR for our analysis is a limitation of our study. In particular, our data would have been further strengthened by having more detailed information on the 35 patients reported to be on dialysis at transplant. Without having confirmed the data on each of these individuals from the individual center, we hesitate to unequivocally conclude that AHCT was the reason for dialysis dependence. The improved outcomes of patients with RI in our analysis compared to historical data may be reflective of the improved outcomes seen in patients with MM in general in recent years, with improvement in supportive care during AHCT and the use of novel agents as part of induction and maintenance. Reporting bias however cannot be excluded. Lastly, additional comorbidities can also factor into decisions for adjusting the dose of high dose melphalan. Our study however outlines that in the real world setting, AHCT can be performed safely in patients with severe RI including those on dialysis with good outcomes.
In conclusion, our analysis indicates that high dose melphalan with AHCT is safe and effective in patients with MM with RI at the time of transplant with 0 TRM at 100 days in patients with moderate and severe RI. Patients with moderate RI appear to benefit from Mel 200 mg/m2. Post-transplant therapies improve outcomes, and a high proportion of patients are reported to achieve dialysis independence.
Acknowledgments
Funding Sources: This publication is funded in part by the Research and Education Program Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin and by KL2TR001438 from the Clinical and Translational Science Award program of the National Center for Advancing Translational Sciences (D’Souza, A). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; AstraZeneca; Be the Match Foundation; *Bluebird Bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cellular Dynamics International, Inc.; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; Mesoblast; MesoScale Diagnostics, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; *Sanofi US; *Seattle Genetics; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government
*Corporate Members
Footnotes
This work was presented in part as an oral presentation at the 59th Annual Meeting of the American Society of Hematology, San Diego, December 2016.
The authors have no conflicts of interests to report
Author contributions:
Conception and design: Anuj Mahindra, Parameswaran Hari, Raphael Fraser, Anita D’Souza.
Collection and assembly of data: Anuj Mahindra, Parameswaran Hari, Raphael Fraser, Mingwei Fei, Anita D’Souza
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval: All authors
References
- 1.Dimopoulos MA, Sonneveld P, Leung N, Merlini G, Ludwig H, Kastritis E, et al. International Myeloma Working Group Recommendations for the Diagnosis and Management of Myeloma-Related Renal Impairment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(13):1544–57. doi: 10.1200/JCO.2015.65.0044. [DOI] [PubMed] [Google Scholar]
- 2.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. The New England journal of medicine. 1996;335(2):91–7. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 3.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. The New England journal of medicine. 2003;348(19):1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 4.Cook G, Ashcroft AJ, Cairns DA, Williams CD, Brown JM, Cavenagh JD, et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. The Lancet Haematology. 2016;3(7):e340–51. doi: 10.1016/S2352-3026(16)30049-7. [DOI] [PubMed] [Google Scholar]
- 5.Dimopoulos MA, Roussou M, Gavriatopoulou M, Psimenou E, Eleutherakis-Papaiakovou E, Migkou M, et al. Bortezomib-based triplets are associated with a high probability of dialysis independence and rapid renal recovery in newly diagnosed myeloma patients with severe renal failure or those requiring dialysis. American journal of hematology. 2016;91(5):499–502. doi: 10.1002/ajh.24335. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Roussou M, Gkotzamanidou M, Nikitas N, Psimenou E, Mparmparoussi D, et al. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia. 2013;27(2):423–9. doi: 10.1038/leu.2012.182. [DOI] [PubMed] [Google Scholar]
- 7.Badros A, Barlogie B, Siegel E, Roberts J, Langmaid C, Zangari M, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. British journal of haematology. 2001;114(4):822–9. doi: 10.1046/j.1365-2141.2001.03033.x. [DOI] [PubMed] [Google Scholar]
- 8.El Fakih R, Fox P, Popat U, Nieto Y, Shah N, Parmar S, et al. Autologous Hematopoietic Stem Cell Transplantation in Dialysis-Dependent Myeloma Patients. Clinical lymphoma, myeloma & leukemia. 2015;15(8):472–6. doi: 10.1016/j.clml.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knudsen LM, Nielsen B, Gimsing P, Geisler C. Autologous stem cell transplantation in multiple myeloma: outcome in patients with renal failure. European journal of haematology. 2005;75(1):27–33. doi: 10.1111/j.1600-0609.2005.00446.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee CK, Zangari M, Barlogie B, Fassas A, van Rhee F, Thertulien R, et al. Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative therapy and autotransplant. Bone marrow transplantation. 2004;33(8):823–8. doi: 10.1038/sj.bmt.1704440. [DOI] [PubMed] [Google Scholar]
- 11.Raab MS, Breitkreutz I, Hundemer M, Benner A, Klaus J, Hegenbart U, et al. The outcome of autologous stem cell transplantation in patients with plasma cell disorders and dialysis-dependent renal failure. Haematologica. 2006;91(11):1555–8. [PubMed] [Google Scholar]
- 12.St Bernard R, Chodirker L, Masih-Khan E, Jiang H, Franke N, Kukreti V, et al. Efficacy, toxicity and mortality of autologous SCT in multiple myeloma patients with dialysis-dependent renal failure. Bone marrow transplantation. 2015;50(1):95–9. doi: 10.1038/bmt.2014.226. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein R, Kershaw G, Bailey J, Greene M, Chhibber V, Vauthrin M, et al. Safety and efficacy of autologous hemopoietic progenitor cell collection in tandem with hemodialysis in multiple myeloma with myeloma cast nephropathy. Journal of clinical apheresis. 2014;29(2):83–9. doi: 10.1002/jca.21295. [DOI] [PubMed] [Google Scholar]
- 14.Sweiss K, Patel S, Culos K, Oh A, Rondelli D, Patel P. Melphalan 200 mg/m2 in patients with renal impairment is associated with increased short-term toxicity but improved response and longer treatment-free survival. Bone marrow transplantation. 2016;51(10):1337–41. doi: 10.1038/bmt.2016.136. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos MA, Sonnevel P, Leung N, et al. International Myeloma Working Group Recommendations for the Diagnosis and Management of Myeloma-Related Renal Impairment. J Clin Oncol. 2016;34(13):1544–57. doi: 10.1200/JCO.2015.65.0044. [DOI] [PubMed] [Google Scholar]
- 16.Nath CE, Trotman J, Tiley C, Presgrave P, Joshua D, Kerridge I, et al. High melphalan exposure is associated with improved overall survival in myeloma patients receiving high dose melphalan and autologous transplantation. Br J Clin Pharmacol. 2016;82(1):149–59. doi: 10.1111/bcp.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw PJ, Nath CE, Lazarus HM. Not too little, not too much-just right! (Better ways to give high dose melphalan) Bone Marrow Transplant. 2014;49(12):1457–65. doi: 10.1038/bmt.2014.186. [DOI] [PubMed] [Google Scholar]
- 18.Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. The New England journal of medicine. 2012;366(19):1782–91. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. The New England journal of medicine. 2012;366(19):1770–81. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]


