Abstract
While the liver is the primary site of metabolism and biliary excretion for many medications, data are limited on the liver’s pharmacokinetic abilities in cirrhosis. Cirrhosis develops through collagen deposition eventually culminating in end-stage liver disease that compromises hepatic drug metabolism. Consequently, the United States Food and Drug Administration (US FDA) recommends evaluating the pharmacokinetics of medications in subjects with hepatic impairment if hepatic metabolism constitutes more than 20% of their elimination or if they have a narrow therapeutic range.
A variety of noninvasive indices and radiologic procedures can be employed to assess hepatic drug metabolism and excretion. The Child-Pugh score is the most commonly used scale for assessing hepatic impairment among drugs submitted for US FDA approval. The score, originally developed to guide operative mortality in patients undergoing hepatic resection, has not been modified since its inception five decades ago. Furthermore, the score was not originally intended to be a guide for potential dose modification in patients with hepatic impairment. These reasons, in combination with the availability of a variety of new imaging modalities and an enhanced understanding of hepatic biology, should foster the development of novel methods to assess the effect of hepatic impairment on liver drug metabolism.
Keywords: Cirrhosis, impaired hepatic function, liver drug metabolism, pharmacokinetics, pharmacodynamics
Introduction
Pharmacokinetic studies in subjects with hepatic impairment are typically used to derive dosing recommendations for patients with hepatic insufficiency. The United States Food and Drug Administration (US FDA) recommends conducting pharmacokinetic studies in subjects with hepatic impairment when hepatic metabolism and excretion account for more than 20% of drug elimination or if the drug has a narrow therapeutic range 1. Currently, the US FDA recommends the use of the Child-Pugh score for classifying liver impairment in pharmacokinetic studies in subjects with hepatic impairment submitted for regulatory approval 2. Other systems to stage liver impairment include the National Cancer Institute (NCI) index, model for end-stage liver disease (MELD) score, the Mayo risk scores for primary biliary cirrhosis and primary sclerosing cholangitis, and the Maddrey-Carithers discriminant function for acute alcoholic hepatitis. Unfortunately, none of these systems has been designed to reliably estimate the relationship between hepatic impairment and the pharmacokinetics and pharmacodynamics of medications. Thus, assessment of liver function in the setting of clinical pharmacology studies remains an important priority for drug development and clinical care.
In this paper, we provide an overview of the biology of liver disease that results in chronic fibrosis, supply recommendations on methods to assess liver dysfunction, and review data on how these changes can affect liver metabolism and excretion of medications. We also provide an overview of the current state of pharmacokinetic studies in subjects with hepatic impairment. Finally, we attempt to highlight the most important areas in which further research is needed in order to enhance our understanding of how liver impairment affects drug metabolism and elimination.
Pathobiology of chronic liver diseases
Liver fibrosis is a generic wound healing response to chronic liver disease regardless of etiology 3. Progressive fibrosis can eventually result in cirrhosis, end-stage liver disease (ESLD), hepatocellular carcinoma and death. Portal hypertension, in which high pressure within the liver affects the hepatic vasculature, is associated with worse outcomes from ESLD including variceal bleeding, renal dysfunction, hepatic encephalopathy, increased susceptibility to infection, and multi-organ dysfunction. From the clinical perspective, cirrhosis can be differentiated into two broad categories, compensated and decompensated. Decompensation develops when the hepatic protein synthesis capacity is impaired resulting in the clinical abnormalities of ascites, hepatic encephalopathy, and bleeding diathesis. Recent observations have discovered that underlying these manifestations is an increased inflammatory response as illustrated by the finding that markers of bacterial translocation are increased in over 40% of cirrhotic patients 4. Several markers of systemic inflammation that correlate with portal hypertension and mortality are also elevated in advanced cirrhosis 5, 6. Increases in inflammatory cytokines have also been associated with downregulation of selected cytochrome P450 (CYP) enzymes responsible for drug metabolism 7. Inflammation can also affect drug transporter expression levels, which might also play a role in how the impaired liver handles drug metabolism and excretion.
Hepatic steatosis, or fat in the liver, is another pathologic process that can affect hepatic drug metabolism and occurs largely as a consequence of metabolic processes such as insulin resistance, diabetes, and hyperlipidemia. The same processes can also result in nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). Several studies in animal models have been conducted to evaluate the effect of NASH on changes in hepatic drug absorption, distribution, metabolism, and excretion through the evaluation of both gene and protein expression levels 8,9. Specifically, efflux transporters belonging to the adenosine triphosphate binding cassette family are induced while uptake transporters belonging to the solute carrier family are repressed. These findings suggest that changes in liver drug transporters may have a hepatoprotective effect in chronic liver diseases, such as NASH, through the facilitation of efflux transporters and repression of uptake transporters. These changes may also result in increased plasma drug concentration in NASH patients.
Portal hypertension is defined as an increase in porto-systemic pressure gradient in any portion of the portal-venous system 10. The porto-systemic gradient is assessed by measuring the wedged hepatic venous pressure (measurement of sinusoidal hepatic pressure) and subtracting from the hepatic venous pressure (systemic pressure), thereby obtaining the hepatic venous pressure gradient (HVPG). The HVPG is normally 3 to 5 mmHg and a value greater than 5 mmHg is defined as portal hypertension. Values greater than 10 mmHg define clinically significant portal hypertension and predict the development of esophageal varices 11, 12, clinical decompensation 12, and hepatocellular carcinoma 13. Esophageal varices, dilated veins in the esophagus that develop as a direct consequence of increased HVPG, are a leading cause of morbidity and mortality in cirrhotic individuals. Although portal hypertension can result from pre-, post-, and intra-hepatic noncirrhotic causes, cirrhosis is by far the most common cause.
Recent observations have identified that cirrhosis is also comprised of reversible and irreversible components 14. The process initiates when chronic liver injury and the resulting inflammatory response activate hepatic stellate cells (HSC), which subsequently transdifferentiate into a myofibroblast phenotype. HSC are thought to be the primary producers of collagen types I and IV, the primary extracellular matrix proteins in the liver. Although originally apoptosis was thought to be the only process to remove activated HSC, more recent data have demonstrated that approximately 40% of these cells revert to a quiescent phenotype following cessation of liver injury 15,16 although factors associated with reversion to the quiescent phenotype remain incompletely understood 17. Consequently, whether fibrosis is reversible or irreversible and the cell types responsible for collagen deposition are potentially important factors for elucidation of hepatic drug metabolism in cirrhotic individuals 18–21. For example, does fibrosis regress in all of those who have achieved viral eradication after treatment for hepatitis C and how quickly does regression occur? Do hepatic drug disposition activities in decompensated individuals improve after viral eradication?
Assessment of hepatic fibrosis
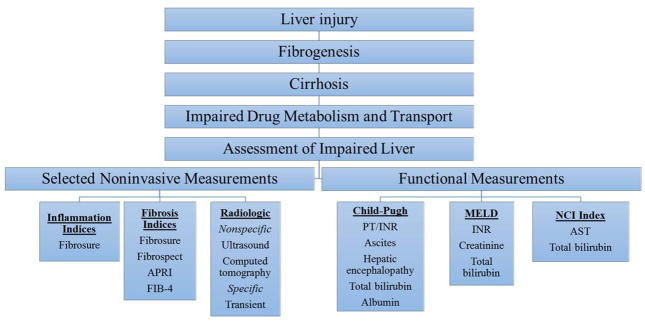
From the clinical perspective, assessment of liver function is important to understand the fibrosis stage because, if severe, it may play a role in medication dose adjustment and may affect liver drug metabolism. Although the liver biopsy has long been considered the gold standard for the staging of hepatic fibrosis, over the past decade several noninvasive serum biomarker indices have been developed, which have largely obviated the need for biopsy (Fig. 1). An in depth review of these topics are included in the article by Zeremski and Martinez in this issue of Clinical Pharmacology in Drug Development 22. Additional recent developments include the approval by the US FDA of transient elastography (TE), a modified ultrasound-based technique that assesses liver stiffness. The underlying premise of TE is that an inverse relationship exists between liver pliability and deposition of extracellular matrix proteins, mostly collagen types 1 and 4 23. TE can reliably identify cirrhosis with an area under the receiver operator characteristic curve of 90 or higher, and it provides an estimate of liver stiffness ranging from 1.5 to 75 kilopascals (kPa). Results less than 5 kPa are considered normal and values greater than 12.7 kPa indicate cirrhosis 23. TE has also been advocated for the identification of asymptomatic compensated cirrhotic patients who are at risk of development of decompensation. Specifically, patients with TE values >20–25 kPa are at risk of having endoscopic signs of portal hypertension and therefore should undergo upper endoscopy to diagnose esophageal varices 24. Other recently developed noninvasive modalities used to stage hepatic fibrosis include magnetic resonance (MR) elastography, acoustic radiation force impulse (ARFI), diffusion-weighted MR imaging, and fibro-computed tomography.
Figure 1. Assessment of Impaired Hepatic Function.
Liver injury of various etiologies (i.e. viral, metabolic, autoimmune, etc) can lead to hepatic stellate cell activation leading to deposition of collagen types 1 and 4 ultimately culminating in cirrhosis. Cirrhosis can result in hepatocyte loss, which affects the liver’s ability to metabolize and transport medications. Current methods for assessment of impaired hepatic function can be broadly divided into noninvasive methods that assess structural changes within the liver including noninvasive indices that assess inflammation (Fibrosure) and fibrosis (Fibrosure, Fibrospect, APRI, and FIB-4). Radiologic-based techniques also can assess structural changes both nonspecifically (ultrasound, computed tomography) and specifically (ultrasound or MRI-based elastography).
Liver function can be assessed by various combinatorial indices such as Child-Pugh, Model of End-Stage Liver Disease (MELD), National Cancer Institute (NCI) Index. The Child-Pugh score is comprised of ascites, hepatic encephalopathy, prothrombin time, total bilirubin, and albumin. The MELD score is comprised of INR, total bilirubin and creatinine. Recent adaptations include inclusion of sodium, although the addition of sodium has not been uniformly embraced. The NCI index includes total bilirubin and AST.
Abbreviations: Model of End-Stage Liver Disease, MELD; National Cancer Institute, NCI Index; Magnetic resonance, MR; international normalized ratio, INR; prothrombin time, PT; aspartate aminotransferase, AST; aspartate aminotransferase to platelet ratio, APRI; fibrosis-4, FIB-4;
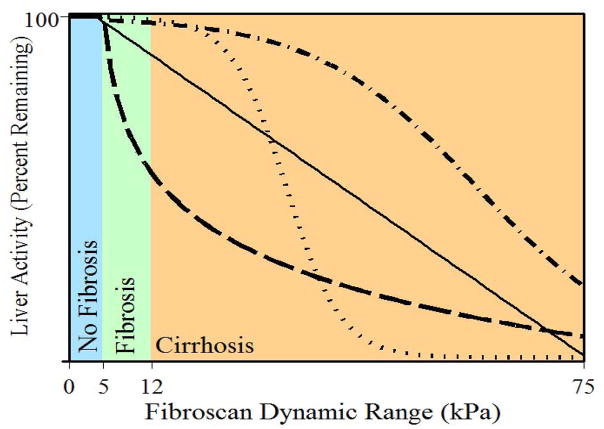
One approach to improve the accuracy of the identification of individuals with impaired liver drug clearance might be the use of TE, possibly in combination with the other available noninvasive scores (Fig 2) 25. If TE is able to identify patients at increased risk of the consequences of portal hypertension, it could be used to identify patients requiring dose modification of specific medications.
Figure 2. Vibration-controlled transient elastography (VCTE) as a possible methodology to assess changes in liver drug metabolism.
The figure illustrates the hypothesized inverse relationship between liver activity (Y-axis), as would be measured for example by hepatic drug metabolism, and liver stiffness (X-axis), as would be measured by VCTE. The lines depict the hypothesized potential nature of the relationship, i.e. sigmoidal, linear, exponential, etc. Liver stiffness as determined by Fibroscan is measured in kilopascals (kPa).
Assessment of hepatic steatosis
Although liver biopsy has been considered the gold standard for the diagnosis of hepatic steatosis, TE has recently been proposed as an alternative methodology 26, especially after the development of the controlled attenuation parameter (CAP) technology 27, 28. A recent meta-analysis of 11 studies comprising 13 cohorts reported that CAP has reasonable sensitivity and specificity for the detection of steatosis grades 1 to 3 29.
Assessment of hepatic dysfunction
The Child-Pugh score is the most commonly used method to assess hepatic impairment among studies submitted to the US FDA. It was initially proposed by Child and Turcotte to predict operative mortality in patients undergoing portosystemic shunt surgery for esophageal variceal bleeding 30. When initially proposed, the score included ascites, hepatic encephalopathy, nutritional status, total bilirubin, and albumin. Pugh subsequently modified the score with the addition of prothrombin time and removal of nutritional status 2. A second commonly used scoring system, the Model for End-stage Liver Disease (MELD) was initially created to predict survival among patients undergoing transjugular intrahepatic portosystemic shunt (TIPS) placement 31. The MELD contains three objective variables: international normalized ratio (INR), creatinine, and total bilirubin. More recent adaptations include addition of sodium values, although this modification has not yet been uniformly adopted. Presently, the MELD is the most commonly employed system utilized to rank candidates for liver transplantation.
The MELD has also been proposed as an improvement over the Child-Pugh score since the latter contains two variables, ascites and hepatic encephalopathy, that are subjective; the premise is that intersubject variability may be introduced by the subjectivity of physician’s assessment of these two signs. Similarly, treatment of these conditions with, for example, diuretics in the case of ascites or antibiotics and lactulose in the case of encephalopathy, may change the degree of severity; unfortunately treatment-induced changes are not incorporated into the Child-Pugh calculation. A recent systematic review and meta-analysis of observational studies that compared Child-Pugh to MELD found that in most cases they provided similar prognostic significance, although each score may have improved predictive capacity in different situations 32.
The National Cancer Institute (NCI) has also recognized the need for objective measures to grade hepatic dysfunction (HD) in patients undergoing trials of cancer chemotherapy. In 2004, the NCI Organ Dysfunction Working Group (ODWG) developed criteria for hepatic dysfunction initially to guide chemotherapy dosing for NCI sponsored clinical trials independent of the Child-Pugh score 33. The criteria incorporated total bilirubin and asparate aminotransferase (AST), two objective and readily measurable laboratory values. The investigators found that total bilirubin was the most important predictor in classifying the severity of hepatic dysfunction. As assessed by the NCI-ODWG criteria, normal to mild HD corresponded to Child-Pugh group A while moderate to severe HD corresponded to Child-Pugh groups B and C. The patients in the latter NCI-ODWG group may require chemotherapy dose modification, while those in the first group do not.
Effects of HD on drug metabolism & disposition
The impact of hepatic dysfunction on drug disposition is related to the type and severity of liver disease, as well as the physiochemical and pharmacokinetic characteristics of the drug. Regarding the latter, the hepatic extraction ratio of the drug (i.e. the measure of efficiency of the liver to remove a drug from the systemic circulation) and the degree of hepatic intrinsic clearance of the drug (i.e. the enzyme-mediated biotransformation of the drug) are important determinants in how liver dysfunction affects pharmacokinetics. Cirrhosis reduces the hepatic metabolism and clearance capacities by several proposed mechanisms. Evaluation of the effects of cirrhosis-related alterations in drug disposition have primarily focused on factors that affect hepatocellularity, intrinsic hepatocellular function, hepatic vascular abnormalities, and plasma protein production 34. Collagen deposition in cirrhosis results in reduced hepatocyte mass and parenchymal cell damage thereby reducing both the quantity and quality of cells responsible for intrinsic hepatic clearance. Fibrosis-associated sinusoidal capillarization can also occur. In the healthy liver, substances freely pass from the portal circulation to the hepatocyte through fenestrations in the sinusoidal endothelium; in cirrhosis, these fenestrations can occlude and basement membranes within the sinusoids can form creating barriers between the sinusoid and hepatocytes. The loss of these fenestrations prevents larger molecules (e.g. protein-bound drug) from entering the space of Disse thereby limiting drug uptake into hepatocytes 35, 36. There is also pre-clinical evidence to suggest that capillarization of the sinusoids restricts oxygen transfer from blood to the hepatocyte resulting in the selective reduction of phase I metabolism, such as oxidative reactions 37, 38. Through its detrimental effect on plasma protein synthesis, cirrhosis can also affect drug protein binding and therefore the proportion of unbound drug concentration that is available to traverse cell membranes and undergo intracellular interactions 39.
Pharmacokinetic studies in patients with hepatic impairment
A survey of drug product labeling of new molecular entities approved by the US FDA between 2000 and 2014 (n=341) showed that ~70% (n=240) of these labels contain language regarding drug administration in patients with hepatic impairment. Seventy seven percent (n=185) of these labels were informed by dedicated pharmacokinetic studies in subjects with hepatic impairment. Other sources of label information include empirical prediction based on elimination properties of drugs, population pharmacokinetic analysis, and clinical experience with the drug pre-approval or the drug class. Ninety five percent of dedicated pharmacokinetic studies utilized Child-Pugh classification to stage liver impairment while the NCI criterion was used in 2% of the cases. None of these studies utilized the MELD score to stage liver function. Most of these studies enrolled subjects with Child-Pugh A and B liver impairment (~90%) while 42% of the studies enrolled subjects with Child-Pugh C liver impairment. From this survey, no consistent observations emerged regarding the changes in exposure as a function of the degree of hepatic impairment. While some studies produced significant changes in exposure with worsening liver impairment, others did not. It is unclear whether these observations are driven by the properties of drugs or the sensitivity and appropriateness of the scales used to assess liver function. To address these issues, a more rigorous understanding of changes in enzymes and transporters as a function of liver impairment is needed. If the lack of consistency between drug exposure and worsening hepatic impairment is related to the poor performance characteristics of the method used to classify liver impairment, then more sensitive classification systems are needed that can reliably differentiate exposure changes in patients with hepatic impairment from those without.
Effect of hepatic impairment on pharmacokinetics of medications used to treat substance use disorders
The development of appropriate systems to assess hepatic impairment is particularly relevant to those individuals with substance use disorders given the high prevalence of co-occurring viral hepatitis and metabolic liver disease in the substance using population. Presently methadone and buprenorphine are two major pharmacologic therapies used to treat opioid addiction. The extent to which liver dysfunction affects methadone pharmacokinetics remains largely undefined and has not been studied prospectively in patients with hepatic insufficiency 40. In an early study, patients with severe chronic alcoholic liver disease were found to have decreased methadone clearance 41 although limited evidence exists to guide optimal methadone dose initiation and titration strategies in patients with liver impairment; such strategies could reduce withdrawal and toxic effects and improve overall treatment adherence.
With regard to buprenorphine, the route of administration greatly influences its potential to result in hepatotoxicity. Specifically, overdoses and intravenous administration, as opposed to sublingual, can result in hepatic necrosis and microvesicular steatosis 42,43. HCV seropositive subjects have been shown to have higher buprenorphine exposure compared to seronegative subjects 44. In patients with moderate hepatic impairment, buprenorphine and naloxone exposures are increased up to three-fold, while in patients with more severe hepatic impairment, naloxone concentrations increase up to ten-fold compared to healthy individuals 45. Therefore, treatment with buprenorphine is not recommended in patients with severe hepatic impairment and may be inappropriate for patients with moderate hepatic impairment 45.
Conclusions
While several noninvasive indices and radiologic techniques can accurately assess fibrosis stage, their use in the assessment of in vivo liver drug clearance has not been studied. Since there are multiple mechanisms that can potentially lead to liver insufficiency, it is likely that a variety of different non-invasive tests may be required to accurately stage hepatic insufficiency. Additional investigation will also be required to determine the influence of hepatic insufficiency on the absorption, distribution, metabolism, and excretion of particular drugs. This knowledge may result in improved medication dosing, thereby optimizing patient care by maintaining the pharmacologic therapeutic window for specific drugs. In case of drugs used to treat opioid addiction, such knowledge could lead to avoidance of toxic and withdrawal symptoms that would also likely improve patient adherence.
Acknowledgments
Funding Sources: Supported in part by funding from the Troup Fund of the Kaleida Health Foundation, NIH grants #2UM1AI069511, #5UM1AI106701-03 and #5K23AI108355-02
Footnotes
Financial Disclosure: AHT has received Grant/Research Support from Merck, Gilead, Tibotec, Abbott, AbbVie, Intercept, and Tobira; has been a consultant/advisor for Merck, Abbott Diagnostics, AbbVie, and Chronic Liver Disease Foundation; and is a member of the speakers’ bureaus for Chronic Liver Disease Foundation and Empire Liver Disease Foundation.
Disclaimer
IY is affiliated with the United States Food and Drug Administration, Silver Spring, Maryland. However, contributions to the article were made in his private capacity. No official support or endorsement by the Food and Drug Administration is intended or should be inferred.
References
- 1.US FDA Center for Drug Evaluation and Research. Guidance for industry: Pharmacokinetics in patients with impaired hepatic function. [Accessed July 18, 2016];Study design, data analysis, and impact on dosing and labeling. Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064982.htm.
- 2.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 3.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 4.Albillos A, de la Hera A, Gonzalez M, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37(1):208–217. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 5.Mookerjee RP, Sen S, Davies NA, Hodges SJ, Williams R, Jalan R. Tumour necrosis factor alpha is an important mediator of portal and systemic haemodynamic derangements in alcoholic hepatitis. Gut. 2003;52(8):1182–1187. doi: 10.1136/gut.52.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortensen C, Andersen O, Krag A, Bendtsen F, Moller S. High-sensitivity C-reactive protein levels predict survival and are related to haemodynamics in alcoholic cirrhosis. Eur J Gastroenterol Hepatol. 2012;24(6):619–626. doi: 10.1097/MEG.0b013e328351db6e. [DOI] [PubMed] [Google Scholar]
- 7.Morgan ET. Impact of infectious and inflammatory disease on cytochrome P450-mediated drug metabolism and pharmacokinetics. Clin Pharmacol Ther. 2009;85(4):434–438. doi: 10.1038/clpt.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canet MJ, Hardwick RN, Lake AD, Dzierlenga AL, Clarke JD, Cherrington NJ. Modeling human nonalcoholic steatohepatitis-associated changes in drug transporter expression using experimental rodent models. Drug Metab Dispos. 2014;42(4):586–595. doi: 10.1124/dmd.113.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lake AD, Novak P, Fisher CD, et al. Analysis of global and absorption, distribution, metabolism, and elimination gene expression in the progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2011;39(10):1954–1960. doi: 10.1124/dmd.111.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bari K, Garcia-Tsao G. Treatment of portal hypertension. World J Gastroenterol. 2012;18(11):1166–1175. doi: 10.3748/wjg.v18.i11.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353(21):2254–2261. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 12.Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133(2):481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Ripoll C, Groszmann RJ, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50(5):923–928. doi: 10.1016/j.jhep.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallowfield JA. Future mechanistic strategies for tackling fibrosis--an unmet need in liver disease. Clin Med (Lond) 2015;15(Suppl 6):s83–87. doi: 10.7861/clinmedicine.15-6-s83. [DOI] [PubMed] [Google Scholar]
- 15.Kisseleva T, Cong M, Paik Y, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109(24):9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troeger JS, Mederacke I, Gwak GY, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143(4):1073–1083. e1022. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015;22(7):512–518. doi: 10.1002/jhbp.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramachandran P, Pellicoro A, Vernon MA, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109(46):E3186–3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Issa R, Zhou X, Constandinou CM, et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126(7):1795–1808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Desmet VJ, Roskams T. Cirrhosis reversal: a duel between dogma and myth. J Hepatol. 2004;40(5):860–867. doi: 10.1016/j.jhep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Zeremski M, Martinez A. Liver disease and fibrosis assessment in substance use related disorders. Clinical Pharmacology in Drug Development. doi: 10.1002/cpdd.312. In Press. [DOI] [PubMed] [Google Scholar]
- 23.Castera L. Non-invasive tests for liver fibrosis progression and regression. J Hepatol. 2016;64(1):232–233. doi: 10.1016/j.jhep.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 24.de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Castera L, Sebastiani G, Le Bail B, de Ledinghen V, Couzigou P, Alberti A. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J Hepatol. 2010;52(2):191–198. doi: 10.1016/j.jhep.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51(2):454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 27.Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36(11):1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Shi KQ, Tang JZ, Zhu XL, et al. Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: a meta-analysis of diagnostic accuracy. J Gastroenterol Hepatol. 2014;29(6):1149–1158. doi: 10.1111/jgh.12519. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Fan Q, Wang T, Wen J, Wang H, Zhang T. Controlled attenuation parameter for assessment of hepatic steatosis grades: a diagnostic meta-analysis. Int J Clin Exp Med. 2015;8(10):17654–17663. [PMC free article] [PubMed] [Google Scholar]
- 30.Child CG, Turcotte JG. Surgery and portal hypertension. Major problems in clinical surgery. 1964;1:1–85. [PubMed] [Google Scholar]
- 31.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 32.Peng Y, Qi X, Guo X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine (Baltimore) 2016;95(8):e2877. doi: 10.1097/MD.0000000000002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel H, Egorin MJ, Remick SC, et al. Comparison of Child-Pugh (CP) criteria and NCI organ dysfunction working group (NCI-ODWG) criteria for hepatic dysfunction (HD): Implications for chemotherapy dosing. J Clin Oncol. 2004;22(14):531s–531s. [Google Scholar]
- 34.Palatini P, De Martin S. Pharmacokinetic drug interactions in liver disease: An update. World J Gastroenterol. 2016;22(3):1260–1278. doi: 10.3748/wjg.v22.i3.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaffner F, Poper H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963;44:239–242. [PubMed] [Google Scholar]
- 36.Van Beers BE, Materne R, Annet L, et al. Capillarization of the sinusoids in liver fibrosis: noninvasive assessment with contrast-enhanced MRI in the rabbit. Magn Reson Med. 2003;49(4):692–699. doi: 10.1002/mrm.10420. [DOI] [PubMed] [Google Scholar]
- 37.Angus PW, Morgan DJ, Smallwood RA. Review article: hypoxia and hepatic drug metabolism--clinical implications. Aliment Pharmacol Ther. 1990;4(3):213–225. doi: 10.1111/j.1365-2036.1990.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 38.Hickey PL, Angus PW, McLean AJ, Morgan DJ. Oxygen supplementation restores theophylline clearance to normal in cirrhotic rats. Gastroenterology. 1995;108(5):1504–1509. doi: 10.1016/0016-5085(95)90700-9. [DOI] [PubMed] [Google Scholar]
- 39.Chu X, Korzekwa K, Elsby R, et al. Intracellular drug concentrations and transporters: measurement, modeling, and implications for the liver. Clin Pharmacol Ther. 2013;94(1):126–141. doi: 10.1038/clpt.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandok N, Watt KDS. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85(5):451–458. doi: 10.4065/mcp.2009.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novick DM, Kreek MJ, Arns PA, Lau LL, Yancovitz SR, Gelb AM. Effect of severe alcoholic liver disease on the disposition of methadone in maintenance patients. Alcohol Clin Exp Res. 1985;9(4):349–354. doi: 10.1111/j.1530-0277.1985.tb05558.x. [DOI] [PubMed] [Google Scholar]
- 42.Berson A, Gervais A, Cazals D, et al. Hepatitis after intravenous buprenorphine misuse in heroin addicts. J Hepatol. 2001;34(2):346–350. doi: 10.1016/s0168-8278(00)00049-0. [DOI] [PubMed] [Google Scholar]
- 43.Berson A, Fau D, Fornacciari R, Degove-Goddard P, Sutton A, Descatoire V, et al. Mechanisms for experimental buprenorphine hepatotoxicity: major role of mitochondrial dysfunction versus metabolic activation. J Hepatol. 2001;34:261–269. doi: 10.1016/s0168-8278(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 44.Masson CL, Rainey PM, Moody DE, McCance-Katz EF. Effects of HCV seropositive status on buprenorphine pharmacokinetics in opioid-dependent individuals. Am J Addict. 2014;23(1):34–40. doi: 10.1111/j.1521-0391.2013.12052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasser AF, Heidbreder C, Liu Y, Fudala PJ. Pharmacokinetics of Sublingual Buprenorphine and Naloxone in Subjects with Mild to Severe Hepatic Impairment (Child-Pugh Classes A, B, and C), in Hepatitis C Virus-Seropositive Subjects, and in Healthy Volunteers. Clin Pharmacokinet. 2015;54(8):837–849. doi: 10.1007/s40262-015-0238-6. [DOI] [PubMed] [Google Scholar]