Abstract
There has been an explosion of interest in the study of microorganisms inhabiting the gastrointestinal tract (gut microbiota) and their impact on host health and physiology. Accumulating data suggest that altered communication between gut microbiota and host systems could participate in disorders such as obesity, diabetes mellitus, and autoimmune disorders; as well as neuropsychiatric disorders including autism, anxiety, and major depressive disorders. The conceptual development of the microbiome-gut-brain axis has facilitated understanding of the complex and bidirectional networks between gastrointestinal microbiota and their host, highlighting potential mechanisms through which this environment influences central nervous system (CNS) physiology. Communication pathways between gut microbiota and the CNS could include autonomic, neuroendocrine, enteric, and immune systems; with pathology resulting in disruption to neurotransmitter balance, increases in chronic inflammation, and/or exacerbated hypothalamic-pituitary-adrenal (HPA)-axis activity. However, uncertainty remains as to the generalizability of controlled animal studies to the more multifaceted pattern of human pathophysiology, especially with regard to the therapeutic potential for neuropsychiatric health. This narrative review summarizes current understanding of gut microbial influence over physiologic function, with an emphasis on neurobehavioral and neurologic impairment based on growing understanding of the gut-brain axis. Experimental and clinical data regarding means of therapeutic manipulation of gut microbiota as a novel treatment option for mental health are described, and important knowledge gaps are identified and discussed.
Keywords: depression, gut-brain axis, gut dysbiosis, mental health, microbiota transplant, probiotics
Introduction and historical context
Gut microbiota comprise all microorganisms inhabiting the intestinal tract and their respective genomes. Tens of trillions of microorganisms populate the human intestine; and although bacteria predominate, viruses, phages, and fungi are likewise included (1). Accumulating data implicates this dynamic population in physiologic functions including nutrition/digestion, growth, inflammation/immunity, and protection against foreign pathogens (2),(3),(4). Experimental and clinical studies suggest that disruption to gut microbiota can impair physical and mental health (3),(5),(6),(7), suggesting that intestinal microbiota could underlie host susceptibility to illness (8),(9). Links between microbiota and pathophysiology triggered an explosion of interest in this field, with 85% of the over 10,000 PubMed publications on “intestinal microbiota” arising in the last 5 years, currently averaging about 5 new publications per day. Indeed, the most recent Rome Foundation guidelines on gastrointestinal (GI) disorders emphasize disruption on the gut-brain axis in functional GI disease (10). Furthermore, the Integrative Human Microbiome Project (iHMP) was deployed in 2014 to drive continuing understanding of microbiome-based influences on health and disease following the 2013 culmination of the NIH Human Microbiome Project (http://hmpdacc.org/) (11). This substantial investment of intellectual and financial capitol reflects the hopeful expectation for new ways to drive beneficial mutualism with these symbiotic microbes to foster optimal health.
Notwithstanding recent investments in discovery-based approaches, microbiome manipulation is actually an ancient concept in human medicine. The first reported application of therapeutic fecal transplantation arises from the fourth century by Chinese physician Ge Hong, describing a “yellow soup” prescribed as an oral remedy for a patient with severe diarrhea (12). In the less distant past, Elie Metchnikoff theorized that health could be enhanced and “senility” delayed by manipulating the intestinal microbiome with host-friendly bacteria (13). These progressive theories might have remained adrift amongst the fringes of biomedical research had it not been for the development of high-throughput sequencing, which revolutionized microbiology with highly efficient and cost-effective strategies to identify and investigate microbial community structure. Historically, microbiology was almost entirely culture-dependent, with members of microbial communities identified by structural characteristics such as affinity for Gram stains. This restriction to cultivable microorganisms hindered researchers’ ability to fully assess diversity in physiological niches, especially at lower taxonomic levels. With the advent of inexpensive and culture-independent next-generation high-throughput sequencing (14), analyses of DNA isolated directly from biologic sites have enabled characterization of taxonomic diversity and functional metagenomics, driving the explosion of data on human microbiomes. In this non-systematic narrative review, we summarize current understanding of intestinal microbial influences on physiologic function with an emphasis on behavioral and neurologic impairment. We also summarize available experimental and clinical data regarding therapeutic manipulation of gut microbiota as a novel GI-based treatment option for mental health. Lastly, we identify important knowledge gaps, and include discussion of potential approaches to filling such gaps.
Gut microbiota and mental health: experimental evidence
Mental illness contributes substantially to the global burden of disability, and to uncover new avenues for treatment the generally tight association of neurobehavioral and metabolic dysfunction has come under intense scrutiny (15),(16),(17). The search for underlying mechanisms common to both bowel and mental illness has revealed many humoral and neural pathways of gut-brain communication (18), and gut microbiota have emerged as a key node in this system (19),(20). Indeed, bi-directional pathways between gut and brain regulates metabolism and energy balance, and represents an ancient biological defense system to guarantee adequate energy (21). The role of gut microbiota in this system is highlighted by the well-established relationship between over-nutrition and reduced intestinal microbial diversity and disrupted pathogen/commensal balance (dysbiosis) (22),(23),(24),(25). Intestinal dysbiosis is also linked to behavioral impairment (5),(6),(7),(26),(27), stimulating extensive research into the role of gut microbiota in mental/neurologic health.
An pivotal early study on gut microbiota and neurobehavioral function revealed that that germ-free mice lacking intestinal and other microbiota display maladaptive and exaggerated responses to stress that can be normalized by probiotic-induced intestinal recolonization (28). Indeed, germ-free mice show that gut microbiota are essential for development of neuronal circuits underlying motor control, anxiety behavior, and social responses (29),(30). Fecal microbial transfer experiments likewise demonstrate the link between intestinal microbiota and behavior. For example, germ-free BALB/c mice typically display impaired sociality and exaggerated caution (31). However, microbiome transplants from NIH Swiss mice, which lack social/exploratory impairment, normalize behavior in BALB/c recipients (32). The reverse is also true, as NIH Swiss mice transplanted with BALB/c microbiota display exaggerated caution and hesitancy (33). Subsequent studies in conventionally housed mice with microbiome transplants from high-fat diet-fed donor mice revealed that high fat-shaped microbiota are sufficient to disrupt exploratory, cognitive, and stereotypical/impulsive behaviors (6). Other reports reveal that probiotics improve mood, anxiety, and cognition, as well as signaling and neural activity in animal models (34),(35),(36),(37). Finally, experimental studies have shown that probiotics prevent stress-induced decreases in hippocampal neurogenesis and enhance expression of hypothalamic genes involved in synaptic plasticity (38).
The intestinal ecosystem is thought to be established at or soon after birth, facilitated by vertical transmission and exposure/ingestion of environmental flora (39),(40),(41). Thus maternal influences on the offspring’s microbiome are significant, potentially altering the risk for mental impairment. Indeed, it has been proposed that both the development of the brain and risks of future illness can be viewed in the context of the developing microbiome (42). For example, data suggest that obesity and diabetes during pregnancy increases risk for neuropsychiatric disorders in offspring (43), and that maternal diet-induced intestinal dysbiosis can impair offspring behavior in a sex-specific manner (44). Maternal stress and immune activation can also program maladaptive offspring behavior via microbiome alterations. For example, maternal immune activation causes behavioral impairment in offspring that is prevented by probiotics (45). Furthermore, prenatal stress-induced changes to the vaginal microbiome alter vertical transmission of microbiota, seemingly shaping an intestinal niche that increases disease risk (46),(47). These collective data suggest a significant and essential link between maternal microbiota and offspring programming, raising the possibility that maternal microbiota could link unhealthy modern diets to the increased prevalence of neurodevelopmental and childhood disorders (48),(49),(50). While currently under intensive study (reviewed in (51), (52)), the potential pathways whereby maternal microbiota affect offspring neurologic function remain unknown (Fig. 1).
Figure 1. Schematic diagram showing the four major pathways of potential information transfer from the maternal gut microbiome to the brain and behavior of offspring.
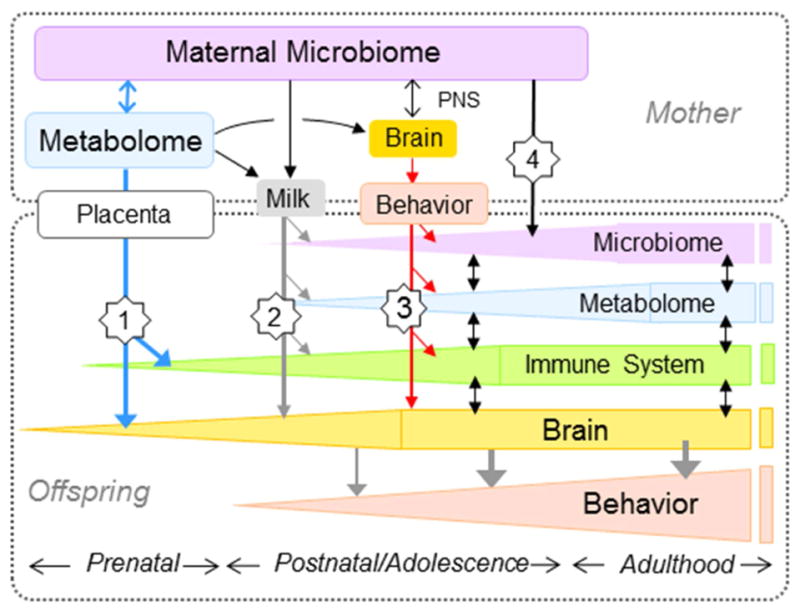
(1) Metabolites shaped by the maternal microbiome may enter the fetal circulation and affect early neurodevelopment of the fetus during gestation. (2) Metabolites shaped by the maternal microbiome contained in the milk may be ingested by offspring during lactation. (3) Maternal microbiota could affect mothering behavior via the mother’s brain during the early neonatal period. (4) Maternal microbiota could be directly vertically transferred to offspring at birth, and reinforced through coprophagia during the neonatal period. In addition, there are complex interactions between the microbiome, metabolome, immune system, brain, and behavior within offspring as shown in Fig. 2.
Potential mechanisms whereby intestinal microbiota influence host health
Numerous pathways between the gut and brain have been delineated, and data indicate that gut-brain communication is bidirectional and mediated by neural and humoral mechanisms. Specific descending pathways include autonomic and enteric pathways and the hypothalamic-pituitary-adrenal (HPA) axis. Ascending pathways include sensory vagal and dorsal root ganglion pathways, cytokines and immune mediators, and secreted microbial/intestinal metabolites. With neural pathways acting in a rapid and somatotopic manner combined with the slower and somatically less specific humoral route, these complementary pathways facilitate the effects of gut microbiota on brain and behavior (Fig. 2).
Figure 2. Signaling pathways between maternal gut microbiota and behavior.
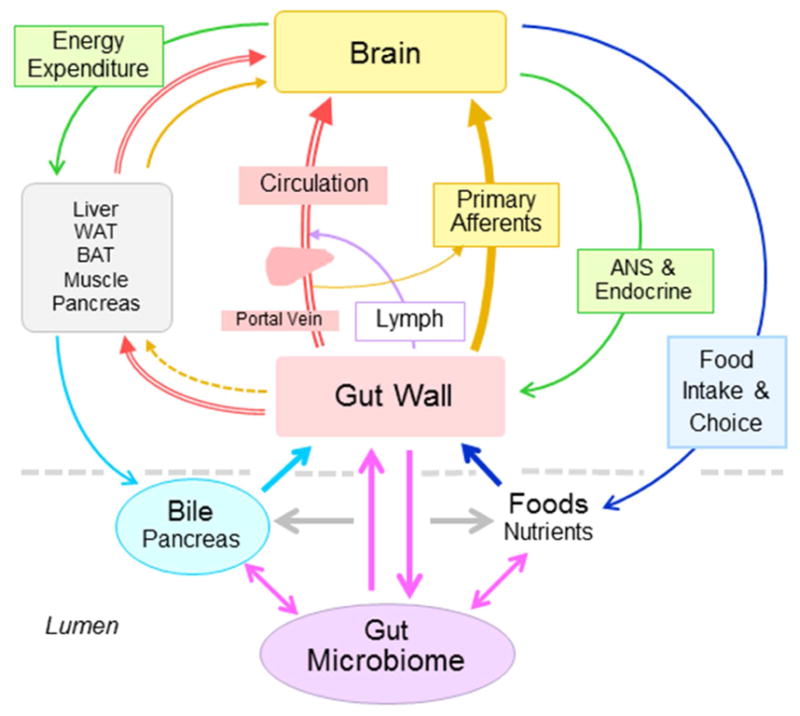
Schematic diagram showing potential signaling pathways through which the gut microbiome can affect metabolic and mental health. In the intestinal lumen, microbiota, food, bile acids, and mucosal factors interact with each other. The intestinal mucosa then propagates information to other organs including the brain through the blood circulation and sensory nerves. In turn, the brain can affect the mucosa and eventually gut microbiota directly or indirectly (via other organs) through changes in autonomic nervous system (ANS) and endocrine outflow and through changes in food intake quantity and choice.
Direct activation of neuronal pathways
Vagal primary afferents provide pervasive sensory innervation of the GI tract, with an estimated 60,000 fibers in humans and 20,000 fibers in the mouse, comprising the only set of fibers that directly connect the GI mucosa to the brain (53). Numerous studies have demonstrated activation of GI tract vagal afferents by gut hormones, cytokines, microbial signals, and mechanical stimuli (reviewed in (54)), and vagal afferents have been implicated in probiotic-induced neurobehavioral changes in the mouse (35,55). For example, the probiotic Lactobacillus rhamnosus can directly increase single- and multiunit firing rate of the mesenteric nerve bundle and can decrease stress-induced corticosterone and anxiety/depression in mice (35),(56). Moreover, these behavioral effects of L. rhamnosus are abolished by vagotomy (35). However, selective vagal deafferentation has not yet been used to conclusively prove a role for vagal afferents in microbiota-brain communication. This is important because the non-selective vagotomy technique used in these studies eliminates vagal motor innervation of the entire GI tract which is crucial for its homeostasis. For example, stimulation of vagal motor outflow attenuates macrophage activation and dampens intestinal inflammation via α-7 nicotinic cholinergic receptors (57),(58). Relatively selective (surgical) vagal deafferentation in a rat model (59) resulted in reduced innate anxiety-like behaviors, increased expression of auditory fear conditioning, and associated neurochemical changes in limbic system components (60). Therefore, this surgical model or next-generation genetic-based selective vagal deafferentation (61) should be used in future studies to tease out the exact role of vagal afferents in microbiome-brain interactions.
Besides the vagus nerve, spinal pathways also serve as high-speed links between gut and brain. On the sensory side, dorsal root ganglion (DRG) afferents relay pain and other supra-physiological stimuli from the gut to the brain (62). While a role for DRG afferents in the effects of microbiome manipulation on brain function is plausible, it has not yet been tested likely owing to difficulties in selective ablation/stimulation of these diffuse nerve fibers. Sympathetic innervation is another avenue for the brain to influence gut function and its organization is slightly more accessible for selective manipulation. Much of the sympathetic innervation to the gut is relayed from preganglionic to postganglionic neurons within the celiac and supramesenteric ganglia and activity of postganglionic neurons can be blocked relatively selectively with noradrenergic antagonists.
Microbial metabolism of nutrients and production of circulating mediators
Intestinal microbial elements can influence host physiology with products of their own metabolism, including the neuroactive molecules 5-hydroxytryptophan (5-HT (serotonin)) and γ-aminobutyric acid (GABA) (63),(64),(65). Specific examples include generation of GABA by members of the Lactobacilli and Bifidobacteria families, dopamine and noradrenalin by members of the Bacillus family, and noradrenalin and 5-HT by the Escherichia family (66),(66,67). Furthermore, male germ-free mice have increased hippocampal 5-HT, increased plasma tryptophan, decreased hippocampal BDNF, and altered anxiety (68), suggesting that microbiota alter the development of CNS neurotransmitter systems. Overall, metabolism of plant-based carbohydrates by intestinal bacteria produces a variety of neuroactive compounds, which potentially could affect both local autonomic/enteric neurons as well as central neural elements.
Short chain fatty acids (SCFAs) including butyrate, propionate and acetate are produced by microbial metabolism of otherwise indigestible dietary fibers also significantly impact host physiology (69). SCFAs are the preferential energy source for colonocytes (70) and also provide a significant fraction of the daily caloric requirement in humans (71). Furthermore, butyrate increases mitochondrial oxidation, attenuates NFkB activation and metabolic endotoxemia, and activates gluconeogenesis (72). With regard to mental health, SCFA directly stimulate sympathetic and autonomic nervous system via activation of G protein-coupled receptors 41 and 43 (73),(74). While SCFA are blood-brain permeable, the degree to which endogenous SCFAs cross into the brain to affect neurologic function remains unclear, although they have been shown to maintain the integrity of the blood brain barrier (75),(76). Application of exogenous SCFAs at pharmacological concentrations, however, has pronounced effects on the brain. For example, application of the combined propionate-, butyrate-, and acetate-sodium salts reverses defects in microglia differentiation and function observed in germ-free mice (77). In addition, sodium butyrate can reduce disease progression in animal models of Parkinson’s and Huntington’s disease (78),(79). However, other studies suggest that SCFA increase microglial signaling and motor dysfunction in animal models of Parkinson’s disease (80), and propionic acid has specifically been implicated in autism (81), based on studies of behavioral, electrographic, neuroinflammatory function in rodents (82),(83). While it is clear that SCFA are powerful signaling molecules, further studies must link changes in their production to altered gut microbial populations and to specific disease states.
Bile acids have been coined “the new gut hormones” as understanding of their function has expanded from simple emulsifiers to inter-organ communicators (84). Synthesized in the liver, conjugated primary and secondary bile acids are de-conjugated by gut microbiota, and activate bile acid receptors such as the nuclear receptor farnesoid receptor and the G protein-coupled bile acid receptor-1 (85). While frequently tied to weight regulation (86), altered bile acid profiles are associated with a range of systemic diseases (reviewed in (87). For example, tauroursodeoxycholic acid (TUDCA) the taurine conjugate of ursodeoxycholic acid, has neuroprotective and anti-inflammatory properties and protects against both Alzheimer’s and Parkinson’s pathology in animal models (88),(89). Further, circulating bile acids are linked to circadian rhythmicity (90), while brain levels are altered in animal and human models of Alzheimer’s disease (91). While these reports are compelling, experimental studies that directly link bile acids to neurologic or physiologic disease are needed to confirm any potential roles of these signaling moieties in CNS disease. Importantly, whether and when bile acids enter the general circulation in sufficient amounts to affect CNS function and/or enter the brain needs to be very clearly addressed.
Immune activation and circulating inflammatory mediators
Inflammation typifies both intestinal dysbiosis and neurologic/psychiatric disorders (92),(93), (94), highlighting the potential role of inflammatory mediators linking gut to brain. Intestinal microbiota influence the generation, maturation, and function of numerous immune cells; which in turn modify the balance and metabolic activity of intestinal microbes (95). This important reciprocal relationship likely participates in inflammation and autoimmunity (reviewed in (96) (97)). For example, intestinal microbiota modify autoimmune processes driven by myelin-specific CD4+ T cells in mice (98). With regard to diet-induced intestinal inflammation, dietary fats, particularly trans and saturated fats, have been shown to transiently increase intestinal inflammation even in healthy subjects (99),(100), which in turn alters gut microbial populations by decreasing commensal (i.e., Bacteroidetes) and increasing pathogenic (i.e, Proteobacteria, Enterobacteriaceae) taxa (101),(102),(103). Importantly, hexacylated endotoxin produced by pathogenic gram-negative strains is a more powerful agonist of toll receptors and more effectively degrades mucosal barriers relative to pentacylated endotoxin produced by commensals like Bacteroidetes (104),(105),(106),(107). Indeed, increases in intestinal Proteobacteria are associated with intestinal permeability and circulating endotoxin (24),(108),(109),(110),(111), which are in turn linked to brain inflammation and neurobehavioral dysfunction (6). Gut dysbiosis and intestinal permeability are also seen in genetic mouse models of autism (45), though cause and effect relationships are unknown. Indeed, the effect of host genetics on gut microbiota is not fully understood (112), although available data strongly support innate immune effects on diet-independent variations in gut microbiota (113),(114),(115). Collectively, these data indicate that intestinal dysbiosis and increased intestinal inflammation can synergistically drive a cascade of local-to-systemic inflammation.
Therapeutic manipulation of gut microbiota
Diet quality
Studies show that the effects of diet on the human gut microbiome are rapid (116),(117),(118) and driven by quality and quantity of dietary fat and carbohydrates (119). Interestingly, the diversity of gut microbiota is increased in rural/indigenous populations that consume high fiber plant-based diets compared to industrialized urban populations with highly processed/low fiber foods (120),(121),(122) and indeed, high fat/low fiber diets are known to reduce intestinal microbial diversity (reviewed in (123)). More importantly, recent experimental data indicate that chronic consumption of high fat/low fiber diets across generations in mice progressively decreases intestinal microbiome diversity, and that this pattern becomes irreversible even when high fiber is reintroduced (124). The typical Western diet may thus permanently reduce the capacity of the gut to support diversity, a serious concern given the association of reduced microbial diversity with disease (125),(126). For example, highly processed, lower quality foods that decrease intestinal microbial diversity and disrupt pathogen/commensal balance are linked to increased risk for mental disorders (127), and clinical studies show significant inverse relationships between symptoms of mental illness and metrics of diet quality (128),(129),(130),(131). However, the data on this association are mixed (132),(133), indicating the need for more controlled trials. Nonetheless, a diet rich in diverse and seasonal plant-based products is in keeping with most general dietary guidelines and would likely foster a more diverse and resilient microbiome. Finally, while the components of a healthy diet are generally well known, it is important to note that numerous societal factors - poverty, crime, food deserts, and irregular work schedules - can undercut an individual’s ability to maintain a healthy diet.
Probiotics and prebiotics
A potential tactic to counterbalance the effects of Western diets is via probiotics and/or prebiotics. The term “psychobiotic” was originally coined to describe probiotics (134), but has been extended to include prebiotics (135) that share the potential to relieve neuropsychiatric symptoms. Probiotics are living microorganisms, generally gram-positive taxa from the Lactobacillus and/or Bifidobacterium genus(135),(136,137,138), and are frequently prescribed for GI conditions like constipation and irritable bowel syndrome (IBS). Interestingly, gut dysbiosis in IBS correlates not just with GI symptoms but and also with regional brain volumes and early life trauma (139), and B. longum reduces depression and increases quality of life in IBS patients (140). Other randomized trials confirm beneficial effects of probiotics on mood (138),(137),(112); and a recent placebo-controlled trial showed that L. casei reduces physiologic responses to stress while increasing intestinal microbial diversity (141). However, other studies report no benefit of probiotic over placebo on mood, anxiety, stress or sleep quality in healthy volunteers (142). Finally, while the Generally Recognized as Safe (GRAS) designation has been applied to many probiotics (143), systematic studies are needed to define the prevalence/severity of adverse events, particularly in vulnerable populations (144). Overall, while evidence supporting probiotics in mental health is compelling, additional trials in diverse populations are needed to define efficacy and address key limitations in the field, including questions on beneficial strains, dosing and administration, and duration of treatment and benefits (145).
Prebiotics are nondigestible plant-based carbohydrates (oligosaccharides and resistant starches) that cultivate beneficial microbiota. Prebiotics can attenuate stress behaviors in rodents (146),(147), and a randomized human trial revealed decreased stress responses and improved emotional affect following galactooligosaccharide supplementation in healthy volunteers (148). While the exact mechanisms of the beneficial effects of prebiotics are not fully understood, fermentation of prebiotics affects both the mass and diversity of cecal and colonic microbiota, and also produces the SCFA butyrate. In summary, while regular consumption of probiotics and/or prebiotics may improve mental health and quality of life by supporting gut microbial diversity and composition, additional well-controlled and well-powered studies are needed.
Fecal microbiota transplantation
Although not as widely used as probiotics, fecal microbiota transplantation (FMT) is a procedure in which healthy donor fecal microbiota are introduced orally or through enemas or colonoscopy into recipient patients. FMT has been progressively refined over the past several years, and has shown success in treating refractory Clostridium difficile infection (149), even in profoundly ill patients or those with C. difficile in the context of Inflammatory Bowel Disease (150). Other applications for FMT may include diseases associated with dysbiosis such as inflammatory bowel syndromes (151), although the efficacy of FMT in treatment of ulcerative colitis and Crohn’s disease is inconsistent (152). A double-blind clinical trail of FMT for obesity/metabolic syndrome compared auto-transplants (patient’s own feces) to transplants from thin donors (experimental group), showing significant improvements in insulin sensitivity in the experimental group (153). Regarding neuropsychiatric disorders, Parkinson’s disease, depression, and autism are all frequently comorbid with GI dysfunction (154),(155), and fecal transplants from affected humans to mice can impair behavioral and motor function (156),(80). Nonetheless, the clinical efficacy of FMT for neurobehavioral disorders remains generally untested with the exception of a recent open-label trial of FMT in children with autism (157), showing improved GI and behavioral function following FMT. There was also a significant negative correlation between metrics of GI distress and parent-reported behavioral improvement (157). However, this trial was not placebo-controlled, blinded, nor randomized; and further trials are needed to confirm the therapeutic benefit and long-term safety of FMT for autism. The relative contribution of FMT-adjunctive medications (e.g., antibiotics, proton pump inhibitors), as well as practical issues related to donor selection/screening, sample handling and storage, and transplant administration also require resolution before this approach could be widely deployed.
Open Questions and Future Directions
These provocative data strongly suggest that intestinal microbiota could be harnessed to fight disease, and this prospect has garnered significant attention. New companies offer personalized analyses of fecal microbial content, promising consumers inroads into personalized health, while untested and potentially risky DIY instructions for microbiome “cleansing” are proliferating online. Headlines such as ‘We Are Our Bacteria’, from the The New York Times and the @microbiome Twitter handle also attest to the growing enthusiasm for this field. And logically so, as gut microbiota can be manipulated directly and efficiently, as opposed to traditional neurologic therapies that must unfortunately expose the entire body to the high drug levels needed to breach the blood-brain barrier. Overall, while this topic has raised considerable hope (and hype) in popular literature, key data are needed to confirm legitimacy of the microbiome as a valid therapeutic target for mental illness (see Table 1).
Table 1.
Conceptual and technical knowledge gaps, and potential experimental resolution, in the development of microbiome-based therapies for mental health.
| Key Open Questions | Promising Experimental Approaches |
|---|---|
| 1. Does intestinal dysbiosis impair mental health, and/or do mental health conditions disrupt intestinal microbiota? |
Animal Studies: rigorously designed studies (following NIH guidelines with regard to controls, statistical power, and biological variables) in which microbiota are transplanted from diseased mice to healthy mice to determine if the phenotype is reproduced and from healthy mice to diseased mice to determine if phenotype is reversed. Human Studies: RCT of microbiome-based therapies in affected patients to test for efficacy (see Question 5). |
| 2. Which microbes have the capacity to drive disease, and which are merely ancillary? | Animal and Human Studies: Systematic, unbiased approach to identify taxa in which enrichment/depletion is associated with a specific phenotype across models (depends in part on resolution of Question #4). |
| 3. What are the mechanisms whereby altered microbiota disrupt brain function? | Animal Studies: rigorously designed studies using genetic, surgical, and/or pharmacologic means to interrupt key signaling pathways (eg, selective vagotomy, bile acid receptors, toll-like receptors) between gut and brain (depends on resolution of Question #1). |
| 4. Can fecal samples adequately reflect intestinal microbiota across proximal-distal and luminal-mucosal axes? | Animal Studies: systematic sampling of luminal and mucosal microbiota along GI tract under both control and disease conditions to determine fidelity of fecal sampling. |
| 5. Under what conditions are microbiome-based therapeutics (FMT, probiotics, and/or prebiotics) beneficial for mental health? |
Animal Studies: rigorously designed studies to inform human trials – important issues include identification of the most beneficial therapy (transplant versus probiotic (strain?) versus prebiotic (type?)), dose-response relationships, duration of benefits, and adverse effects. Animal Studies: RCT of microbiome-based therapies for specific conditions (eg, anxiety, depression, autism). |
Cause and effect relationships need to be established, as many studies do not meet this criterion. Even if gut dysbiois is capable of facilitating disease, the reciprocal effects of impaired host physiology on intestinal populations remain unclear. For example, a recent report documented decreased microbial diversity in elderly subjects in residential facilities compared to community dwellers, and a correlation between frailty metrics and distinct microbiome signatures in elderly subjects (158). While a causal relationship of diet/residence in long-term care facilities on microbiome diversity is reasonable; the reverse relationship - i.e., does frailty/poor health disrupt the gut microbiome - was not explored. Another example relates to obesity, as a recent study sought to assess causality in this system by transplanting microbiota from discordant twins (one obese, one not) into mice. Mice previously colonized with an ‘obese’ microbiome lost weight when supplied with a ‘lean microbiome’, but only when also fed a low-fat diet as neither transplant nor diet were effective alone (159). This well-controlled and thorough experiment underscores the myriad of factors that complicate delineation of gut microbiota-induced pathophysiology. Understanding when dysbiosis causes disease rather than accompanying it would advance this important field.
In a related note, identification of the specific bacterial populations that drive maladaptive physiology would be valuable. For example, even if distinct microbiome signatures are causally linked to disease, whether this effect is mediated collectively by groups of microbes or by individual microbes with other taxa acting as mere passengers of the particular phenotype remains unknown. Indeed, the exact changes in microbiota that reflect pathology are unknown even in animal systems, and this limits the diagnostic utility of microbiome analyses and contributes to the unacceptable signal/noise ratio in this field. An unbiased systemic approach to identify the specific taxa where enrichment or depletion is linked to loss of homeostasis could resolve this issue, particularly if adequately powered and validated across multiple models. Modern technology can now also allow for finer distinctions and the study of multiple genes in parallel, an ability that may enable the identification of ‘metabolic networks’ revealing the biochemical reactions that a microbiome can perform. This kind of analysis could identify gene combinations, potentially from multiple species across a microbial community that affect health for good or ill.
An additional issue relates to the validity of fecal sampling as a reliable tool to assess intestinal health along both vertical and horizontal axes. Much of the work on gut microbiota is based on stool samples using 16S metagenomics analyses (161). However, intestinal bacteria affect specific and discrete gut segments and niches of the luminal/mucous layers (162),(163),(164). While some experimental studies have investigated site-specific microbiota-host interactions (165),(166),(167),(168), understanding of these discrete regional interactions between microbiota, diet components, bile acids, digestive enzymes, and the host mucosa are likely necessary to yield the promise of microbiome-based therapeutics. While longitudinal biopsy samples from throughout the GI tract are understandably contraindicated in human studies, animal studies could be designed to test if specific fecal signatures can be identified that reflect health/disease in more proximal GI locations.
Once a direct relationship between gut dysbiosis and a pathophysiological phenotype is established, microbiome-based therapeutic strategies could be generated. However, it is possible that realization of microbiome-based benefits may require elucidation of mechanisms. Indeed, determining if dysbiosis triggers disease via activation of vagal afferents, impaired regulation of serum tryptophan, and/or intestinal transfer of endotoxin could direct which therapeutic avenue to pursue. However, still remaining to be resolved are the numerous limitations of current microbiome based therapies (discussed individually above) across different cohorts. These limitations are likely based in part on the remarkable degree of inter-personal variability in human microbiota (160). Thus, it seems clear that collective resolution of cause and effect, mechanisms, participating taxa, and fidelity of fecal samples to overall intestinal and systemic physiology will be necessary before the full potential of personalized medicine offered by microbiome-based therapeutics can be realized.
Acknowledgments
The authors’ work is supported by grant from the National Institutes of Health (NIH), including MH110117; and by Genomics and Animal Behavior/Phenotyping core facilities supported by 3P30-GM118430, NIH 2P30 DK072476, and NIH P50AT002776-11.
Footnotes
Financial Disclosure
The authors declare no competing biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8(5) doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robles Alonso V, Guarner F. Linking the gut microbiota to human health. Br J Nutr. 2013;109(Suppl 2):S21–6. doi: 10.1017/S0007114512005235. [DOI] [PubMed] [Google Scholar]
- 3.Collins SM, Kassam Z, Bercik P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: experimental evidence and clinical implications. Curr Opin Microbiol. 2013;16:240–5. doi: 10.1016/j.mib.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Jarchum I, Pamer EG. Regulation of innate and adaptive immunity by the commensal microbiota. Curr Opin Immunol. 2011;23:353–60. doi: 10.1016/j.coi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167:374–379. doi: 10.1001/jamapediatrics.2013.497. [DOI] [PubMed] [Google Scholar]
- 6.Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard Et, Taylor CM, Welsh DA, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77:607–15. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aziz Q, Doré J, Emmanuel A, Guarner F, Quigley EM. Gut microbiota and gastrointestinal health: current concepts and future directions. Neurogastroenterol Motil. 2013;25:4–15. doi: 10.1111/nmo.12046. [DOI] [PubMed] [Google Scholar]
- 9.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–12. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;160:1257–61. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 11.Group JCHMPDGW. Evaluation of 16S rDNA-based community profiling for human microbiome research. PLoS One. 2012;7:e39315. doi: 10.1371/journal.pone.0039315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1755. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 13.Metchnikoff E. In: The Prolongation of Life. Optimistic Studies. MPC, editor. New York: G P Putnam’s Sons; 1908. [Google Scholar]
- 14.Consortium EP. Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruce-Keller AJ, Keller JN, Morrison CD. Obesity and vulnerability of the CNS. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbadis.2008.10.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sestan-Pesa M, Horvath TL. Metabolism and Mental Illness. Trends Mol Med. 2016;22:174–83. doi: 10.1016/j.molmed.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Subero M, Anderson G, Kanchanatawan B, Berk M, Maes M. Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut-brain pathways. CNS Spectr. 2016;21:184–98. doi: 10.1017/S1092852915000449. [DOI] [PubMed] [Google Scholar]
- 19.Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: stress, health and disease. Mammalian Genome. 2014;25:49–74. doi: 10.1007/s00335-013-9488-5. [DOI] [PubMed] [Google Scholar]
- 20.Flowers SA, Ellingrod VL. The Microbiome in Mental Health: Potential Contribution of Gut Microbiota in Disease and Pharmacotherapy Management. Pharmacotherapy. 2015;35:910–6. doi: 10.1002/phar.1640. [DOI] [PubMed] [Google Scholar]
- 21.Bauer PV, Hamr SC, Duca FA. Regulation of energy balance by a gut-brain axis and involvement of the gut microbiota. Cell Mol Life Sci. 2016;73:737–55. doi: 10.1007/s00018-015-2083-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106:2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7(10):e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 26.Tillisch K. The effects of gut microbiota on CNS function in humans. Gut Microbes. 2014;5:404–10. doi: 10.4161/gmic.29232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinan TG, Quigley EM. Probiotics in the treatment of depression: science or science fiction? Psychiatry. 2011;45:1023–5. doi: 10.3109/00048674.2011.613766. [DOI] [PubMed] [Google Scholar]
- 28.Sudo N. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–27. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Molecular Psychiatry. 2013 doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacome LF, Burket JA, Herndon AL, Deutsch SI. Genetically inbred Balb/c mice differ from outbred Swiss Webster mice on discrete measures of sociability: relevance to a genetic mouse model of autism spectrum disorders. Autism Res. 2011;4:393–400. doi: 10.1002/aur.218. [DOI] [PubMed] [Google Scholar]
- 32.Brinks V, van der Mark M, de Kloet R, Oitzl M. Emotion and cognition in high and low stress sensitive mouse strains: a combined neuroendocrine and behavioral study in BALB/c and C57BL/6J mice. Front Behav Neurosci. 2007;1:8. doi: 10.3389/neuro.08.008.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 34.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–75. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–5. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–88. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Smith CJ, Emge JR, Berzins K, Lung L, Khamishon R, Shah P, et al. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am J Physiol Gastrointest Liver Physiol. 2014;307:G793–802. doi: 10.1152/ajpgi.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ait-Belgnaoui A, Colom A, Braniste V, Ramalho L, Marrot A, Cartier C, et al. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol Motil. 2014;26:510–520. doi: 10.1111/nmo.12295. [DOI] [PubMed] [Google Scholar]
- 39.Funkhouser LJ, Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11:e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asnicar F, Manara S, Zolfo M, Truong DT, Scholz M, Armanini F, et al. Studying Vertical Microbiome Transmission from Mothers to Infants by Strain-Level Metagenomic Profiling. mSystems. 2017;2:e00164–16. doi: 10.1128/mSystems.00164-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aagaard K, Stewart CJ, Chu D. Una destinatio, viae diversae: Does exposure to the vaginal microbiota confer health benefits to the infant, and does lack of exposure confer disease risk? EMBO Rep. 2016;17:1679–1684. doi: 10.15252/embr.201643483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goyal MS, Venkatesh S, Milbrandt J, Gordon JI, Raichle ME. Feeding the brain and nurturing the mind: Linking nutrition and the gut microbiota to brain development. Proc Natl Acad Sci U S A. 2015;112:14105–12. doi: 10.1073/pnas.1511465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivera HM, Christiansen KJ, Sullivan EL. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci. 2015;9:194. doi: 10.3389/fnins.2015.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruce-Keller AJ, Fernandez-Kim SO, Townsend RL, Kruger C, Carmouche R, Newman S, et al. Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS One. 2017;12:e0175577. doi: 10.1371/journal.pone.0175577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–63. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jašarević E, Howerton CL, Howard CD, Bale TL. Alterations in the Vaginal Microbiome by Maternal Stress Are Associated With Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology. 2015;156:3265–76. doi: 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jašarević E, Howard CD, Misic AM, Beiting DP, Bale TL. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci Rep. 2017;7:44182. doi: 10.1038/srep44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyall K, Munger KL, O’Reilly ÉJ, Santangelo SL, Ascherio A. Maternal dietary fat intake in association with autism spectrum disorders. Am J Epidemiol. 2013;178:209–20. doi: 10.1093/aje/kws433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan EL, Nousen EK, Chamlou KA. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol Behav. 2014;123:236–42. doi: 10.1016/j.physbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell Host Microbe. 2016;165:1762–75. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stinson LF, Payne MS, Keelan JA. Planting the seed: Origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit Rev Microbiol. 2017;43:352–369. doi: 10.1080/1040841X.2016.1211088. [DOI] [PubMed] [Google Scholar]
- 52.Garcia-Mantrana I, Collado MC. Obesity and overweight: Impact on maternal and milk microbiome and their role for infant health and nutrition. Mol Nutr Food Res. 2016;60:1865–75. doi: 10.1002/mnfr.201501018. [DOI] [PubMed] [Google Scholar]
- 53.Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191:203–12. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- 54.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 55.Patterson E, Cryan JF, Fitzgerald GF, Ross RP, Dinan TG, Stanton C. Gut microbiota, the pharmabiotics they produce and host health. Proc Nutr Soc. 2014;73:477–89. doi: 10.1017/S0029665114001426. [DOI] [PubMed] [Google Scholar]
- 56.Perez-Burgos A, Wang B, Mao YK, Mistry B, McVey Neufeld KA, Bienenstock J, et al. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2013;304:G211–20. doi: 10.1152/ajpgi.00128.2012. [DOI] [PubMed] [Google Scholar]
- 57.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 58.Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62:1214–22. doi: 10.1136/gutjnl-2012-302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz GJ, Salorio CF, Skoglund C, Moran TH. Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am J Physiol. 1999;276:R1623–9. doi: 10.1152/ajpregu.1999.276.6.R1623. [DOI] [PubMed] [Google Scholar]
- 60.Klarer M, Arnold M, Gunther L, Winter C, Langhans W, Meyer U. Gut vagal afferents differentially modulate innate anxiety and learned fear. Journal of Neuroscience. 2014;34:7067–76. doi: 10.1523/JNEUROSCI.0252-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Lartigue G, Ronveaux CC, Raybould HE. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol Metab. 2014;3:595–607. doi: 10.1016/j.molmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 63.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 64.Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16:495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol. 2014;817:221–3. doi: 10.1007/978-1-4939-0897-4_10. [DOI] [PubMed] [Google Scholar]
- 66.Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 67.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33:574–81. doi: 10.1002/bies.201100024. [DOI] [PubMed] [Google Scholar]
- 68.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–73. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 69.Chakraborti CK. New-found link between microbiota and obesity. World J Gastrointest Pathophysiol. 2015;6:110–9. doi: 10.4291/wjgp.v6.i4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160–6. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Respondek F, Gerard P, Bossis M, Boschat L, Bruneau A, Rabot S, et al. Short-chain fructo-oligosaccharides modulate intestinal microbiota and metabolic parameters of humanized gnotobiotic diet induced obesity mice. PLoS ONE. 2013;8:e71026. doi: 10.1371/journal.pone.0071026. [DOI] [PMC free article] [PubMed] [Google Scholar]; Keenan MJ, Janes M, Robert J, Martin RJ, Raggio AM, McCutcheon KL, et al. Resistant starch from high amylose maize (HAM-RS2) reduces body fat and increases gut bacteria in ovariectomized (OVX) rats. Obesity (Silver Spring) 2013;21:981–4. doi: 10.1002/oby.20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424–429. [PubMed] [Google Scholar]
- 71.Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 72.Sivaprakasam S, Prasad PD, Singh N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol Ther. 2016;164:144–51. doi: 10.1016/j.pharmthera.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci USA. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 75.Al-Asmakh M, Hedin L. Microbiota and the control of blood-tissue barriers. Tissue Barriers. 2015;3:e1039691. doi: 10.1080/21688370.2015.1039691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;263:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–77. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laurent RS, O’Brien LM, Ahmad ST. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience. 2013;246:382–390. doi: 10.1016/j.neuroscience.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, et al. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sampson T, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167:1469–1480. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.MacFabe DF. Enteric short-chain fatty acids: microbial messengers of metabolism, mitochondria, and mind: implications in autism spectrum disorders. Microb Ecol Health Dis. 2015;26:28177. doi: 10.3402/mehd.v26.28177. [DOI] [PMC free article] [PubMed] [Google Scholar]; Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35:S6–16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- 82.Foley KA, Ossenkopp KP, Kavaliers M, Macfabe DF. Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS One. 2014;9:e87072. doi: 10.1371/journal.pone.0087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shultz SR, Aziz NA, Yang L, Sun M, MacFabe DF, O’Brien TJ. Intracerebroventricular injection of propionic acid, an enteric metabolite implicated in autism, induces social abnormalities that do not differ between seizure-prone (FAST) and seizure-resistant (SLOW) rats. Behav Brain Res. 2015;278:542–8. doi: 10.1016/j.bbr.2014.10.050. [DOI] [PubMed] [Google Scholar]; Foley KA, MacFabe DF, Kavaliers M, Ossenkopp KP. Sexually dimorphic effects of prenatal exposure to lipopolysaccharide, and prenatal and postnatal exposure to propionic acid, on acoustic startle response and prepulse inhibition in adolescent rats: relevance to autism spectrum disorders. Behav Brain Res. 2015;278:244–56. doi: 10.1016/j.bbr.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 84.Pournaras DJ, le Roux CW. Are bile acids the new gut hormones? Lessons from weight loss surgery models. Endocrinology. 2013;154:2255–6. doi: 10.1210/en.2013-1383. [DOI] [PubMed] [Google Scholar]
- 85.Wahlstrom A, Sayin SI, Marschall HU, Backhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F, et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7421–6. doi: 10.1073/pnas.1323599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joyce SA, Gahan CG. Disease-Associated Changes in Bile Acid Profiles and Links to Altered Gut Microbiota. Dig Dis. 2017;35:169–177. doi: 10.1159/000450907. [DOI] [PubMed] [Google Scholar]
- 88.Dionísio PA, Amaral JD, Ribeiro MF, Lo AC, D’Hooge R, Rodrigues CM. Amyloid-β pathology is attenuated by tauroursodeoxycholic acid treatment in APP/PS1 mice after disease onset. Neurobiol Aging. 2015;36:228–40. doi: 10.1016/j.neurobiolaging.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 89.Moreira S, Fonseca I, Nunes MJ, Rosa A, Lemos L, Rodrigues E, et al. Nrf2 activation by tauroursodeoxycholic acid in experimental models of Parkinson’s disease. Exp Neurol. 2017 doi: 10.1016/j.expneurol.2017.05.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 90.Govindarajan K, MacSharry J, Casey PG, Shanahan F, Joyce SA, Gahan CG. Unconjugated Bile Acids Influence Expression of Circadian Genes: A Potential Mechanism for Microbe-Host Crosstalk. PLoS One. 2016;11:e0167319. doi: 10.1371/journal.pone.0167319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pan X, Elliott CT, McGuinness B, Passmore P, Kehoe PG, Hölscher C, et al. Metabolomic Profiling of Bile Acids in Clinical and Experimental Samples of Alzheimer’s Disease. Metabolites. 2017;7 doi: 10.3390/metabo7020028. pii: E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–75. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lyman M, Lloyd DG, Ji X, Vizcaychipi MP. Neuroinflammation: The role and consequences. Neurosci Res. 2014;79C:1–12. doi: 10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 94.Mielcarz DW, Kasper LH. The gut microbiome in multiple sclerosis. Curr Treat Options Neurol. 2015;17:344. doi: 10.1007/s11940-015-0344-7. [DOI] [PubMed] [Google Scholar]
- 95.Tomkovich S, Jobin C. Microbiota and host immune responses: a love-hate relationship. Immunology. 2016;147:1–10. doi: 10.1111/imm.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 98.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–41. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 99.Okada Y, Tsuzuki Y, Sato H, Narimatsu K, Hokari R, Kurihara C, et al. Trans fatty acids exacerbate dextran sodium sulphate-induced colitis by promoting the up-regulation of macrophage-derived proinflammatory cytokines involved in T helper 17 cell polarization. Clin Exp Immunol. 2013;174:459–71. doi: 10.1111/cei.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deopurkar R, Ghanim H, Friedman J, Abuaysheh S, Sia CL, Mohanty P, et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care. 2010;33:991–7. doi: 10.2337/dc09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–29. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 102.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–89. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pédron T, Sansonetti P. Commensals, bacterial pathogens and intestinal inflammation: an intriguing ménage à trois. Cell Host Microbe. 2008;3:344–7. doi: 10.1016/j.chom.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 104.Munford RS, Varley AW. Shield as signal: lipopolysaccharides and the evolution of immunity to gram-negative bacteria. PLoS Pathog. 2006;2:e67. doi: 10.1371/journal.ppat.0020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–8. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 106.Moreira AP, Texeira TF, Ferreira AB, Peluzio C, Alfenas R. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108:801–9. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- 107.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–8. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cani PD, Bibiloni B, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes Care. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 109.Etxeberria U, Arias N, Boqué N, Macarulla MT, Portillo MP, Milagro FI, et al. Shifts in microbiota species and fermentation products in a dietary model enriched in fat and sucrose. Benef Microbes. 2014 doi: 10.3920/BM2013.0097. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 110.Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, et al. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut. 2014;63:116–24. doi: 10.1136/gutjnl-2012-304119. [DOI] [PubMed] [Google Scholar]
- 111.Piya MK, Harte AL, McTernan PG. Metabolic endotoxaemia: is it more than just a gut feeling? Curr Opin Lipidol. 2013;24:78–85. doi: 10.1097/MOL.0b013e32835b4431. [DOI] [PubMed] [Google Scholar]
- 112.Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9:279–90. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- 113.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fava F, Gitau R, Griffin BA, Gibson GR, Tuohy KM, Lovegrove JA. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int J Obes (Lond) 2013;37:216–23. doi: 10.1038/ijo.2012.33. [DOI] [PubMed] [Google Scholar]
- 120.Schnorr SL. The diverse microbiome of the hunter-gatherer. Nature. 2015;518:S14–5. doi: 10.1038/518S14a. [DOI] [PubMed] [Google Scholar]
- 121.Martinez I, Stegen JC, Maldonado-Gomez MX, Eren AM, Siba PM, Greenhill AR, et al. The gut microbiota of rural papua new guineans: composition, diversity patterns, and ecological processes. Cell Rep. 2015;11:527–38. doi: 10.1016/j.celrep.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 122.Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B, et al. The microbiome of uncontacted Amerindians. Sci Adv. 2015:1. doi: 10.1126/sciadv.1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. 2015;42:158–79. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–5. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 126.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–8. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 127.Dawson SL, Dash SR, Jacka FN. The Importance of Diet and Gut Health to the Treatment and Prevention of Mental Disorders. Int Rev Neurobiol. 2016;131:325–346. doi: 10.1016/bs.irn.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 128.Rienks J, Dobson AJ, Mishra GD. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: results from a large community-based prospective study. Eur J Clin Nutr. 2013;67:75–82. doi: 10.1038/ejcn.2012.193. [DOI] [PubMed] [Google Scholar]
- 129.Flórez KR, Dubowitz T, Ghosh-Dastidar MB, Beckman R, Collins RL. Associations between depressive symptomatology, diet, and body mass index among participants in the supplemental nutrition assistance program. J Acad Nutr Diet. 2015;115:1102–8. doi: 10.1016/j.jand.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huddy RL, Torres SJ, Milte CM, McNaughton SA, Teychenne M, Campbell KJ. Higher Adherence to the Australian Dietary Guidelines Is Associated with Better Mental Health Status among Australian Adult First-Time Mothers. J Acad Nutr Diet. 2016;116:1406–12. doi: 10.1016/j.jand.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 131.Mihrshahi S, Dobson AJ, Mishra GD. Fruit and vegetable consumption and prevalence and incidence of depressive symptoms in mid-age women: results from the Australian longitudinal study on women’s health. Eur J Clin Nutr. 2015;69:585–91. doi: 10.1038/ejcn.2014.222. [DOI] [PubMed] [Google Scholar]
- 132.Lai JS, Oldmeadow C, Hure AJ, McEvoy M, Byles J, Attia J. Longitudinal diet quality is not associated with depressive symptoms in a cohort of middle-aged Australian women. Br J Nutr. 2016;115:842–50. doi: 10.1017/S000711451500519X. [DOI] [PubMed] [Google Scholar]
- 133.Martínez-González MA, Sánchez-Villegas A. Food patterns and the prevention of depression. Proc Nutr Soc. 2016;75:139–46. doi: 10.1017/S0029665116000045. [DOI] [PubMed] [Google Scholar]
- 134.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–6. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 135.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PW. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–401. 1401 e1–4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Messaoudi M. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 138.Steenbergen L. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 139.Labus JS, Hollister EB, Jacobs J, Kirbach K, Oezguen N, Gupta A, et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome. 2017;5:49. doi: 10.1186/s40168-017-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: a Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.05.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 141.Kato-Kataoka A. Fermented milk containing Lactobacillus casei strain Shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl Environ Microbiol. 2016;82:3649–3658. doi: 10.1128/AEM.04134-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kelly JR, Allen AP, Temko A, Hutch W, Kennedy PJ, Farid N, et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun. 2017;61:50–59. doi: 10.1016/j.bbi.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 143.Mattia A, Merker R. Regulation of probiotic substances as ingredients in foods: premarket approval or “generally recognized as safe” notification. Clin Infect Dis. 2008;46(Suppl 2):S115–8. doi: 10.1086/523329. [DOI] [PubMed] [Google Scholar]
- 144.Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(Suppl 2):S129–34. doi: 10.1093/cid/civ085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry. 2017;16:14. doi: 10.1186/s12991-017-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, et al. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2016.12.031. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 147.Mika A, Day HE, Martinez A, Rumian NL, Greenwood BN, Chichlowski M, et al. Early life diets with prebiotics and bioactive milk fractions attenuate the impact of stress on learned helplessness behaviours and alter gene expression within neural circuits important for stress resistance. Eur J Neurosci. 2017;45:342–357. doi: 10.1111/ejn.13444. [DOI] [PubMed] [Google Scholar]
- 148.Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PW. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl) 2015;232:1793–801. doi: 10.1007/s00213-014-3810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 150.Borody TJ, Peattie D, Mitchell SW. Fecal Microbiota Transplantation: Expanding Horizons for Clostridium difficile Infections and Beyond. Antibiotics (Basel) 2015;4:254–66. doi: 10.3390/antibiotics4030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vindigni SM, Surawicz CM. Fecal microbiota transplantation. Gastroenterol Clin North Am. 2017;46:171–185. doi: 10.1016/j.gtc.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 152.Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503–516. doi: 10.1111/j.1365-2036.2012.05220.x. [DOI] [PubMed] [Google Scholar]
- 153.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 154.Su A, Gandhy R, Barlow C, Triadafilopoulos G. A practical review of gastrointestinal manifestations in Parkinson’s disease. Parkinsonism Relat Disord. 2017 doi: 10.1016/j.parkreldis.2017.02.029. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 155.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kelly JR, Borre Y, O’Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–18. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 157.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–84. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 159.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Raoult D, Henrissat B. Are stool samples suitable for studying the link between gut microbiota and obesity? European Journal of Epidemiology. 2014;29:307–9. doi: 10.1007/s10654-014-9905-4. [DOI] [PubMed] [Google Scholar]
- 162.Rozee KR, Cooper D, Lam K, Costerton JW. Microbial flora of the mouse ileum mucous layer and epithelial surface. Applied and Environmental Microbiology. 1982;43:1451–63. doi: 10.1128/aem.43.6.1451-1463.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Faderl M, Noti M, Corazza N, Mueller C. Keeping bugs in check: The mucus layer as a critical component in maintaining intestinal homeostasis. IUBMB Life. 2015;67:275–85. doi: 10.1002/iub.1374. [DOI] [PubMed] [Google Scholar]
- 164.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4659–65. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gu S, Chen D, Zhang JN, Lv X, Wang K, Duan LP, et al. Bacterial community mapping of the mouse gastrointestinal tract. PLoS ONE. 2013;8:e74957. doi: 10.1371/journal.pone.0074957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Claus SP, Tsang TM, Wang Y, Cloarec O, Skordi E, Martin FP, et al. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol Syst Biol. 2008;4:219. doi: 10.1038/msb.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wirth R, Bodi N, Maroti G, Bagyanszki M, Talapka P, Fekete E, et al. Regionally distinct alterations in the composition of the gut microbiota in rats with streptozotocin-induced diabetes. PLoS ONE. 2014;9:e110440. doi: 10.1371/journal.pone.0110440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lu HP, Lai YC, Huang SW, Chen HC, Hsieh CH, Yu HT. Spatial heterogeneity of gut microbiota reveals multiple bacterial communities with distinct characteristics. Sci Rep. 2014;4:6185. doi: 10.1038/srep06185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675–85. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


