Abstract
Much evidence suggests a role for inflammation in the pathogenesis of endometriosis. Although investigators in numerous case-control studies have found elevation of inflammatory markers in patients with endometriosis, results were not consistent, and no prior prospective study is known to exist. We conducted a case-control study nested within the Nurses’ Health Study II in which we examined associations between levels of plasma inflammatory markers (interleukin-1 beta, interleukin-6, soluble tumor necrosis factor α receptors 1 and 2, and high-sensitivity C-reactive protein) and the risk of laparoscopically confirmed endometriosis. From blood collections in 1996–1999 and 2007, we ascertained 350 cases patients with incident endometriosis and 694 matched controls. Women with interleukin-1 beta levels in quintiles 2–4 had a higher risk of endometriosis (for the second quintile, relative risk (RR) = 3.30, 95% confidence interval (CI): 1.06, 10.3; for the third quintile, RR = 3.36, 95% CI: 1.09, 10.4; and for the fourth quintile, RR = 4.64, 95% CI: 1.58, 13.6; P for trend = 0.62), which suggested an association beginning at 0.47 pg/mL or greater. A significant nonlinear association with levels of soluble tumor necrosis factor α receptor 2 was observed, with elevated risk of endometriosis at concentrations greater than 3,400 pg/mL. Plasma interleukin-6, soluble tumor necrosis factor α receptor 1, and high-sensitivity C-reactive protein levels were not associated with endometriosis risk. Further research in larger studies with younger age at blood collection and longer time from blood to surgical diagnosis are required to confirm these associations.
Keywords: C-reactive protein, endometriosis, inflammatory markers, interleukin-1 beta, interleukin-6, soluble tumor necrosis factor α receptor-1, soluble tumor necrosis factor α receptor-2
Endometriosis is a benign, chronic, estrogen-dependent gynecologic disorder that affects 6%–10% of women of reproductive age in the United States (1). It is defined as the presence of endometrial-like tissue outside of the uterine cavity (2). Common signs and symptoms include pelvic pain, dysmenorrhea, dyspareunia, and reduced fertility (1). The mostly widely accepted theory of endometriosis pathogenesis is that the disorder originates from retrograde menstruation of endometrial tissue sloughed through the fallopian tubes into the peritoneal cavity, which attaches to the peritoneum, invades its epithelium, and proliferates (3). However, as retrograde menstruation occurs in 76%–90% of women, the lower comparative prevalence of symptoms of endometriosis suggests that other processes must contribute to the implantation of endometrial cells and persistence of endometriotic lesions among this smaller subset of women (1, 4).
Aberrant immunologic mechanisms and inflammatory responses in the pathophysiology of endometriosis have been implicated in previous studies. The peritoneal fluid of women with endometriosis has been reported to contain increased numbers of immune cells, which appear to enhance the survival and proliferation of ectopic endometrial cells by secreting various local products such as growth factors and cytokines (5, 6). Additionally, many studies have reported that inflammatory factors, including interleukin-1 beta (IL-1β), interleukin-6 (IL-6), tumor necrosis factor α (TNF-α) and high-sensitivity C-reactive protein (hs-CRP), were elevated in the peritoneal fluid and peripheral blood of women with endometriosis compared with controls (7–10).
It is unclear whether the observed elevation of inflammatory markers results from inflammatory reactions in the disease process or whether it is among the causes of the disease. To our knowledge, no prospective studies have been conducted to elucidate this question. Moreover, although many studies have found elevated levels of IL-1β, IL-6, TNF-α or hs-CRP among women with endometriosis compared with controls (7–10), some have also observed no association for these markers (11–13). Therefore, we conducted a case-control study nested within the Nurses’ Health Study II (NHSII), to prospectively investigate the association between plasma levels of inflammation markers (IL-1β, IL-6, soluble tumor necrosis factor α receptor-1 (sTNFR-1), soluble tumor necrosis factor α receptor-2 (sTNFR-2) and hs-CRP) and the subsequent risk of laparoscopically confirmed endometriosis.
METHODS
Study population and data collection
The NHSII is a prospective cohort study of 116,430 US female nurses who were aged 25–42 years in 1989. At baseline, participants completed a detailed questionnaire regarding medical history, lifestyle, and reproductive information. Blood samples were collected between 1996 and 1999 from 29,611 cohort members who were aged 32–54 years (14). Premenopausal women who had not taken hormones and who had not breastfed or been pregnant within 6 months (n = 18,521) provided a mid-luteal sample 7–9 days before the anticipated start of their next cycle. Women who declined or who were unable to provide a timed sample (e.g., currently using oral contraceptives) (n = 11,090) provided an untimed blood sample. Samples were shipped with an ice-pack via overnight courier to our laboratory where they were processed, separated into plasma, red blood cell and white blood cell components, and stored in liquid nitrogen freezers.
All women completed a questionnaire at the time of blood draw that recorded the date and time of day of blood sample collection, current weight, smoking status, medication use, hours since last food intake, as well as the first day of the menstrual cycle during which the blood samples were drawn. Follow-up of the blood cohort was over 96% in 2005. This research was approved by the Institutional Review Board of Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health.
Case ascertainment
Starting in 1993, women were asked whether they had ever had physician-diagnosed endometriosis and, if so, reported the date of diagnosis and whether it had been confirmed by laparoscopy (15). In total, 363 women were diagnosed with laparoscopically confirmed endometriosis at least 1 year after blood draw. The final data set included 350 cases of endometriosis. Cases were excluded because of inadequate sample volumes or the inability to adequately match with a control specimen.
Control selection
For each case of endometriosis, 2 controls were randomly selected from the risk set of each case within ±1 year of age, who were of the same race/ethnicity (white, Asian, African American, Hispanic, or other), and had similar infertility history and menopausal status at diagnosis. Cases and controls were also matched by sample type (luteal, untimed), luteal day (for luteal samples), month (±1 month), time of day (±2 hours), and fasting status (<2, 2–4, 5–7, 8–11, ≥12 hours) at blood draw.
Assessment of exposure
All assays were performed in the laboratory of Dr. Nadir Rifai (Boston Children’s Hospital, Boston, Massachusetts). IL-6 was measured by a quantitative sandwich enzyme immunoassay technique. The concentration of hs-CRP was determined using an immunoturbidimetric assay on the Roche P Modular system (Roche, Inc., Basel, Switzerland). IL-1β, sTNFR-1, and sTNFR-2 were measured by enzyme-linked immunosorbent assay. The soluble TNF receptors are produced by the proteolytic cleavage of TNF cell surface receptors following induction by TNF or other cytokines. They have a longer half-life than TNF and are detected with higher sensitivity. Blinded quality controls samples were included in every batch for each assay, which allowed us to calculate coefficient of variations (CV) within and across batches. In the present study, the inter-assay CV for each analyte was 70.0% for IL-1β, 8.1% for IL-6, 9.2% for sTNFR-1, 6.8% for sTNFR-2, and 4.2% for hs-CRP. Because of the large CV for the IL-1β assay, we restricted our analyses to batches in which CV was less than 20%.
Statistical analyses
Quintile cutpoints for the inflammatory markers were defined by the distribution among the controls. For C-reactive protein, we also used current recommended clinical cutoffs (low, <1.0 mg/L; average, 1.0–2.9 mg/L; and high, ≥3.0 mg/L) (16). Although the primary analysis for IL-1β was restricted to batches in which the CV was less than 20%, we conducted alternative analyses to evaluate the sensitivity of obtained results to the choice of IL-1β CV cutoff. These analyses used different CV cutoffs of less than 10%, 15%, and 30%, and also included all samples.
Conditional logistic regression models that accounted for all matching factors were used to estimate the relative risks and 95% confidence intervals that adjusted for potential confounders. Tests for linear trend were conducted with median quintile concentrations. Stepwise restricted cubic splines (17, 18) were used to examine the possible nonlinearity of the relationship between each inflammatory marker and endometriosis. A likelihood ratio test was used to assess the significance of any nonlinearity.
We examined whether the associations between inflammatory markers and endometriosis varied by factors such as age at blood draw (<40 years vs. ≥40 years), age at endometriosis diagnosis (<45 years vs. ≥45 years), body mass index (BMI; calculated as weight (kg)/height (m)2) at age 18 years (<20 vs. ≥20), BMI at blood draw (<25 vs. ≥25) and infertility at diagnosis (ever vs. never) using tertiles of markers to account for the smaller within-strata sample sizes in the stratified analyses. We assessed the statistical significance of heterogeneity using a likelihood ratio test. In addition, the following sensitivity analyses were conducted: women who were premenopausal at blood draw; women with luteal phase samples; and excluding endometriosis cases diagnosed within 2 or within 4 years after the blood collection. Analyses were performed using SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
The study population comprised a total of 350 cases and 694 controls. The mean age at blood draw was 41.7 years (range, 33–52 years) for cases and 42.1 years (range, 32–52 years) for controls. The median time between blood draw and endometriosis diagnosis was 4 years, with an interquartile range of 3–6 years. Overall, cases had earlier age at menarche, higher BMI at blood draw, higher likelihood of being nulliparous, lower likelihood to be a smoker, and a lower household income in 2001 compared with controls. Mean levels of IL-1β, sTNFR-1, and sTNFR-2 were similar between cases and controls, with cases having slightly higher IL-6 and hs-CRP levels (Table 1). Spearman correlation coefficients among markers were between 0.12 and 0.23 (data not shown).
Table 1.
Age-Standardized Characteristics of Endometriosis Case Patients and Matched Controls at Blood Draw,a Nurses’ Health Study II,b 1996–1999 and 2007
| Patient Characteristicc | Cases (n = 350) | Controls (n = 694) | ||
|---|---|---|---|---|
| % | Mean (SD) | % | Mean (SD) | |
| Age at blood draw, yearsd | 41.7 (4.6) | 42.1 (4.5) | ||
| White race | 98 | 96 | ||
| Age at menarche, years | ||||
| <12 | 26 | 20 | ||
| 12 | 33 | 31 | ||
| >12 | 41 | 49 | ||
| BMIe at age 18 years | ||||
| <18.5 | 15 | 16 | ||
| 18.5–22.4 | 61 | 60 | ||
| 22.5–24.9 | 13 | 15 | ||
| ≥25.0 | 10 | 9 | ||
| BMIe at blood draw | ||||
| <22.5 | 32 | 37 | ||
| 22.5–24.9 | 20 | 22 | ||
| 25.0–29.9 | 26 | 23 | ||
| ≥30.0 | 22 | 18 | ||
| No. of pregnanciesf | ||||
| Nulliparous | 30 | 21 | ||
| 1–2 | 49 | 50 | ||
| 3 | 22 | 29 | ||
| Months of breast feeding | ||||
| Nulliparous | 30 | 21 | ||
| 0 or <1 | 18 | 14 | ||
| 1–11 | 33 | 33 | ||
| 12–23 | 28 | 27 | ||
| ≥24 | 21 | 26 | ||
| History of infertility | 27 | 30 | ||
| Ever use of oral contraceptives | 83 | 85 | ||
| Never smoker | 70 | 65 | ||
| Alternative Health Eating Index 2010 score | 49.2 (10.4) | 50.1 (10.9) | ||
| Alcohol intake, g/day | 1.8 (0.7) | 1.8 (0.8) | ||
| Physical activity, METs/week | 18.8 (24.8) | 18.0 (20.6) | ||
| Hypertension | 10 | 8 | ||
| Hypercholesterolemia | 18 | 19 | ||
| Household income in 2001, US$ | ||||
| <49‚000 | 20 | 15 | ||
| 49‚000–99‚000 | 52 | 53 | ||
| ≥100‚000 | 29 | 32 | ||
| IL1-β, pg/mL | 1.0 (1.0) | 0.9 (1.0) | ||
| IL-6, pg/mL | 1.9 (3.7) | 1.5 (2.7) | ||
| sTNFR-1, pg/mL | 1,289 (330) | 1,281 (320) | ||
| sTNFR-2, pg/mL | 2,240 (575) | 2,214 (538) | ||
| hs-CRP, pg/mL | 2.7 (5.7) | 2.2 (4.9) | ||
Abbreviations: BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; IL-1β, interleukin-1 beta; IL-6, interleukin-6; MET, metabolic equivalent of task; sTNFR-1, soluble tumor necrosis factor α receptor-1; sTNFR-2, soluble tumor necrosis factor α receptor-2; SD, standard deviation.
a Patients with laparoscopically confirmed cases of endometriosis were matched in a 1:2 ratio with controls of comparable age, race/ethnicity, infertility history, and menopausal status at diagnosis and by month, time of day, and fasting status at blood draw.
b All values are standardized to the age distribution of the study population.
c Values of polytomous variables may not sum to 100% because of rounding.
d Value is not age adjusted.
e Weight (kg)/height (m)2.
f Pregnancies lasting longer than 6 months.
Compared with the first quintile, women in quintiles 2–4 of IL-1β plasma levels had a greater risk of endometriosis (second quintile relative risk (RR) = 3.30, 95% confidence interval (CI): 1.06, 10.3; third quintile RR = 3.36, 95% CI: 1.09, 10.4; fourth quintile RR = 4.64, 95% CI: 1.58, 13.6; fifth quintile RR = 2.16, 95% CI: 0.69, 6.74). This relationship was not linear (P for trend = 0.62), and suggests an association with a threshold IL-1B level beginning at 0.47 pg/mL or greater (Table 2). We also compared the combination of quintiles 2–5 with the first quintile (RR = 3.28, 95% CI: 1.25, 8.62). These IL-1β results remained largely unchanged when we applied alternative cutoffs of CV to define inclusion (<10%, <15%, and <30%), but were attenuated when all data with CV >70% were included (data not shown).
Table 2.
Relative Risk of Laparoscopically Confirmed Endometriosis by Quintile of Plasma Inflammatory Biomarkers, Nurses’ Health Study II, 1996–1999 and 2007
| Marker and Quintile | Cutpointsa | No. of Cases | No. of Controls | Model 1b | Model 2c | ||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | ||||
| IL-1β | |||||||
| 1 | <0.47 | 11 | 42 | 1.00 | Referent | 1.00 | Referent |
| 2 | 0.47–0.60 | 21 | 42 | 1.93 | 0.79, 4.72 | 3.30 | 1.06, 10.3 |
| 3 | 0.61–0.72 | 21 | 43 | 1.83 | 0.76, 4.38 | 3.36 | 1.09, 10.4 |
| 4 | 0.73–1.09 | 36 | 43 | 3.16 | 1.37, 7.25 | 4.64 | 1.58, 13.6 |
| 5 | ≥1.10 | 20 | 43 | 1.71 | 0.67, 4.37 | 2.16 | 0.69, 6.74 |
| P for trendd | 0.44 | 0.62 | |||||
| IL-6 | |||||||
| 1 | <0.63 | 65 | 138 | 1.00 | Referent | 1.00 | Referent |
| 2 | 0.63–0.85 | 66 | 139 | 1.02 | 0.67, 1.55 | 0.95 | 0.61, 1.48 |
| 3 | 0.86–1.16 | 67 | 139 | 1.03 | 0.67, 1.59 | 0.95 | 0.60, 1.50 |
| 4 | 1.17–1.75 | 69 | 139 | 1.05 | 0.69, 1.60 | 0.86 | 0.54, 1.38 |
| 5 | ≥1.76 | 81 | 138 | 1.24 | 0.82, 1.87 | 1.03 | 0.64, 1.67 |
| P for trendd | 0.23 | 0.76 | |||||
| sTNFR-1 | |||||||
| 1 | <1,032 | 75 | 138 | 1.00 | Referent | 1.00 | Referent |
| 2 | 1,032–1,160 | 57 | 139 | 0.75 | 0.49, 1.16 | 0.73 | 0.46, 1.15 |
| 3 | 1,161–1,305 | 68 | 139 | 0.91 | 0.60, 1.39 | 0.86 | 0.55, 1.35 |
| 4 | 1,306–1,514 | 77 | 139 | 1.01 | 0.67, 1.53 | 0.89 | 0.57, 1.39 |
| 5 | ≥1,515 | 73 | 138 | 0.98 | 0.65, 1.50 | 0.78 | 0.47, 1.28 |
| P for trendd | 0.64 | 0.52 | |||||
| sTNFR-2 | |||||||
| 1 | <1,795 | 75 | 138 | 1.00 | Referent | 1.00 | Referent |
| 2 | 1,795–2,017 | 54 | 139 | 0.71 | 0.46, 1.10 | 0.70 | 0.45, 1.10 |
| 3 | 2,018–2,279 | 92 | 139 | 1.21 | 0.81, 1.80 | 1.17 | 0.76, 1.78 |
| 4 | 2,280–2,583 | 64 | 139 | 0.85 | 0.56, 1.29 | 0.76 | 0.49, 1.20 |
| 5 | ≥2,583 | 65 | 138 | 0.86 | 0.56, 1.31 | 0.68 | 0.43, 1.09 |
| P for trendd | 0.70 | 0.18 | |||||
| hs-CRP | |||||||
| 1 | <0.26 | 74 | 138 | 1.00 | Referent | 1.00 | Referent |
| 2 | 0.26–0.54 | 70 | 139 | 0.94 | 0.63, 1.41 | 0.79 | 0.51, 1.21 |
| 3 | 0.55–1.10 | 61 | 139 | 0.83 | 0.55, 1.23 | 0.73 | 0.47, 1.12 |
| 4 | 1.11–2.66 | 63 | 140 | 0.85 | 0.57, 1.27 | 0.69 | 0.43, 1.11 |
| 5 | ≥2.67 | 82 | 137 | 1.12 | 0.75, 1.66 | 0.82 | 0.49, 1.38 |
| P for trende | 0.31 | 0.96 | |||||
Abbreviations: CI, confidence interval; hs-CRP, high sensitive C-reactive protein; IL-1β, interleukin-1 beta; IL-6, interleukin-6; MET, metabolic equivalent of task; RR, relative risk; sTNFR-1, soluble tumor necrosis factor receptor-1; sTNFR-2, soluble tumor necrosis factor receptor-2.
a Values for IL-1β, IL-6, sTNFR-1, sTNFR-2 are expressed in pictograms per milliliter; values for hs-CRP are expressed as milligrams per liter.
b Relative risks and 95% confidence intervals were estimated using conditional logistic regression conditioned on the matching factors, including year of birth, current and past infertility status (yes vs. no), menopausal status at time of diagnosis (for timed samples; premenopausal, postmenopausal, or dubious/missing/unknown), month and year at blood draw, time of day at blood draw (2-hour blocks), fasting status at blood draw (fasting vs. nonfasting), luteal day, and race/ethnicity (African-American, Asian, Hispanic, white, or other).
c Matching factor conditioned models were further adjusted for age at menarche (<12, 12, or >12 years), body mass index at age 18 years (weight (kg)/height (m)2; <18.5, 18.5–22.5, 22.5–24.9, ≥25.0), BMI at blood draw (<22.5, 22.5–25.0, 25.0–29.9, or ≥30.0), number of pregnancies lasting more than 6 months (0, 1–2, >3 births), total months of breast feeding (nulliparous, 0 or <1, 1–12, 12–23, or ≥24 months), smoking history (never, past, or current), past oral contraceptive use (yes vs. no), Alternative Health Eating Index 2010 score (<45, 45–54, or ≥55), alcohol consumption (0, 0–5, or >5 g/day), physical activity level (<3, 3–9, 10–18, >18 metabolic equivalents of task/week), history of hypertension and hypercholesterolemia, and pre-tax household income in 2001 (<$49‚000, $49,000–$99,000, or ≥$100,000).
dP values were calculated using the Wald test of a score variable represented by the median values of quintiles.
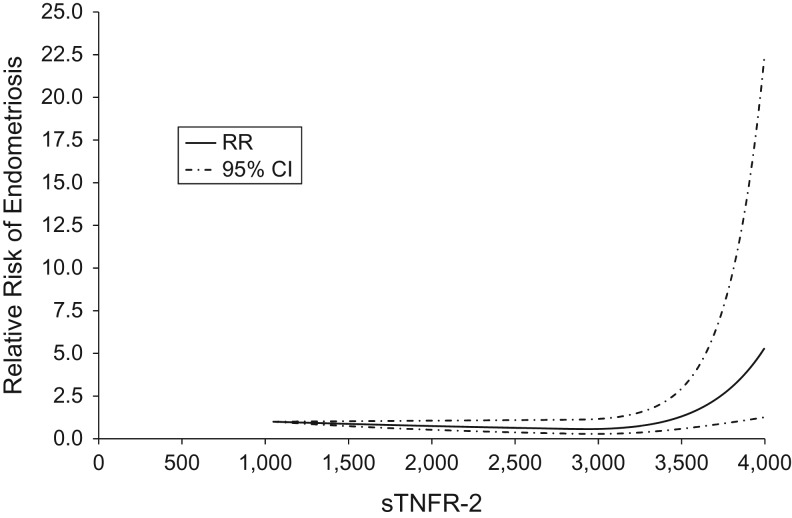
When evaluated within quintile pair-wise comparisons, these data showed no evidence of linear or threshold relationships between plasma IL-6, sTNFR-1, sTNFR-2, and hs-CRP levels and the risk of endometriosis (top vs. bottom quintile: IL-6 RR = 1.03, 95% CI: 0.64, 1.67, P for trend = 0.76; sTNFR-1 RR = 0.78, 95% CI: 0.47, 1.28, P for trend = 0.52; sTNFR-2 RR = 0.68, 95% CI: 0.43, 1.09, P for trend = 0.18; hs-CRP RR = 0.82, 95% CI: 0.49, 1.38, P for trend = 0.96) (Table 2). In addition, when applying current recommended clinical cutoffs for CRP, the results were similar (high vs. low RR = 1.14, 95% CI: 0.84, 1.55) (data not shown). However, in cubic regression spline analyses, sTNFR-2 was observed to have a significant nonlinear relationship with the risk of endometriosis (P for nonlinearity = 0.001), which suggested a threshold sTNFR-2 level beginning at greater than 3,400 pg/mL (Figure 1). No other inflammatory markers had a significant nonlinear relationship with the risk of endometriosis.
Figure 1.
Soluble tumor necrosis factor α receptor-2 (sTNFR-2) concentration in relation to risk of laparoscopically confirmed endometriosis, Nurses’ Health Study II, 1996–1999 and 2007. The dotted line indicates where the relative risk (RR) is equal to 1.0. CI, confidence interval.
We examined effect modification by age at blood draw, age at diagnosis of endometriosis, BMI at 18 years, BMI at blood draw, and infertility history. sTNFR-1 was associated with endometriosis risk among women aged less than 40 years at blood draw (sTNFR-1 third tertile RR = 2.1, 95% CI: 1.1, 3.8), but not among women age 40 years and older at blood draw (P for heterogeneity = 0.002). Similar patterns were observed for sTNFR-2 and hs-CRP compared with the first tertile (sTNFR-2 third tertile RR = 1.6, 95% CI: 0.9, 2.9; hs-CRP third tertile RR = 1.4, 95% CI: 0.8, 2.6; P for heterogeneity ≤ 0.05).
All results remained largely unchanged when the sample was restricted to women who were premenopausal at blood draw and those with luteal samples (data not shown). The positive association between IL-1β and risk of endometriosis was similar when endometriosis cases that were diagnosed within 2 or within 4 years after blood draw were excluded (data not shown). There remained no associations between IL-6, sTNFR-1, sTNFR-2, and hs-CRP with the risk of endometriosis even when these incident cases were excluded (data not shown).
DISCUSSION
In the present nested case-control study, plasma IL-1β levels were positively associated with the increased risk of laparoscopically confirmed endometriosis. However, we found no overall associations between plasma IL-6, TNF-α, or CRP levels and the risk of endometriosis. Our data also suggested that higher TNF-α levels were associated with increased endometriosis risk among women who were younger than 40 years at blood draw but not among women who were 40 years and older.
Cytokines such as IL-1β, IL-6, and TNF-α have been found in experimental studies to be involved in adhesion of endometrial cells to the peritoneum, and in angiogenesis and proliferation of endometriotic lesions (6, 19). In vitro studies have shown that the adhesion of human endometrial cells to mouse peritoneum and human peritoneal mesothelial cells was increased by treatment with interleukin IL-1β, IL-6 or TNF-α (20, 21). IL-6 and vascular endothelial growth factor were shown to have angiogenic activity in vitro and in vivo (22, 23). IL-1β was shown to play an important role in angiogenesis within human endometriotic lesions by stimulating the induction of vascular endothelial growth factor and IL-6 in endometriotic stromal cells but not in normal endometrial stromal cells (24). Finally, the cell-free fraction of peritoneal samples from patients with endometriosis was found to stimulate the proliferation of human normal endometrial cells (25), and TNF-α was shown to induce the proliferation of human endometriotic stromal cells (26).
Previous case-control studies of the associations between levels of IL-1β, IL-6, TNF-α, and hs-CRP and endometriosis risk have produced inconsistent results. Elevated IL-1β levels were reported in patients with endometriosis compared with controls (7, 27–29), although Bedaiwy et al. (8) found no elevation. Increased concentrations of IL-6 were observed in women with endometriosis compared with controls (12, 27, 29, 30); however, Somigliana et al. (11) found no association. Many studies (8, 9, 13, 27) have found an association between raised levels of TNF-α and endometriosis, whereas Othman et al. (12) found no association. Similarly, Abrao et al. (10) found that CRP appeared to be increased in women with endometriosis, whereas in a more recent study, Xavier et al. (13) did not find an increase. These inconsistent findings may be due to sampling from different case populations, different assay sensitivities, low statistical power, inappropriate control selection, and insufficient adjustment for confounders. The sample size of those studies was generally small (most studies <50 cases), which make the results more susceptible to random error than in the present study (n = 350 cases). Moreover, these case-control studies either selected controls from women without pathology who underwent tubal ligation (proven to be fertile) or from women who had other pathologies as indication for laparoscopy but had no evidence of endometriosis upon laparoscopy. In these study designs, the results may have been driven by the risk factor distribution for the underlying causes of infertility or other pathology rather than by endometriosis, and may therefore be biased in unpredictable directions.
We observed significantly increased risk of endometriosis in the second to fourth quintiles of IL-1β levels, but not in the fifth quintile (although the magnitude of effect was more than 2-fold). Although we did not observe any associations between IL-6, sTNFR-1, sTNFR-2, and hs-CRP levels and risk of endometriosis by quintiles, we observed that very high levels of sTNFR-2 (>3,400 pg/mL) were associated with increased endometriosis risk. We observed slightly higher mean levels of IL-6 and hs-CRP among cases compared with controls, but found null results with quintiles. This discrepancy may be due to the right skew distribution of IL-6 and hs-CRP. Both basic science and epidemiologic evidence has been pointing to the important role of inflammation in endometriosis. However, it has been unclear whether the inflammatory reactions are involved in the pathogenesis of endometriosis or whether they are merely an epiphenomenon of the disease process. Based on our findings, it appears that previously observed elevation of IL-6 and hs-CRP levels in peritoneal fluid or peripheral blood may be the result of endometriosis itself rather than a causal factor of the disease. However, it is possible that IL-1β, and possibly very high levels of TNF-α, are initiating pathogenic factors of endometriosis through an unknown mechanism.
It is also possible that IL-1β and TNF-α are merely the earliest among the 4 inflammatory markers to be elevated and detectable to indicate the existence of endometriosis. A recent multicenter study in 10 countries observed an average delay of 7 years from symptom onset to surgical diagnosis of endometriosis (31). Our validation study determined that the average diagnostic delay was 4 years in the NHSII. When excluding endometriosis cases diagnosed within 2 and within 4 years after blood collection, our results remained largely unchanged. However, because the pathophysiologic history remains unknown and endometriosis may develop long before symptoms emerge, we cannot rule out the possibility that IL-1β and TNF-α are early disease markers rather than pathogenic causes of endometriosis.
We observed that higher TNF-α levels were positively associated with endometriosis risk among women younger than 40 years of age but not among women older than 40 years at blood draw. This is consistent with our earlier findings that endometriosis was associated with higher risk of coronary heart disease, potentially mediated through an inflammatory milieu; the associations were strongest among women younger than 40 years of age (32). Together, these results suggest that the inflammatory milieu associated with endometriosis may be etiologically relevant among younger women. It is possible that the inflammatory milieu changes caused by disease progression are distinct between younger and older women. Alternatively, endometriosis that is clinically evident at younger ages may have a different pathogenesis and risk profile (e.g., inflammation) compared with endometriosis that first becomes clinically evident at older ages.
The present study has several limitations. The IL-1β assay was discovered to yield large variation within and among assay batches (overall CV >70%). A strength of the present study, however, is that we spaced quality control samples so that at least 2 fall into each assay batch, with the laboratory blinded to their location. This allowed us to identify the large assay variation as well as to determine which batches had a more acceptable level of variation. Had these data not been available, we would have attributed the null association that we observed among all data to reflect evidence for no association between IL-1β and endometriosis risk rather than to laboratory error. Restriction of analysis to batches with CV less than 20% allowed the valid assay relationship to be observed. In addition, levels of some inflammatory markers such as CRP have been shown to vary over the menstrual cycle (33); however, results were similar when analysis was restricted to luteal phase samples, which suggested that sampling timing had little influence on the results.
The mean age at blood draw within the NHSII was 42 years. In order to rigorously evaluate the potential etiologic relation between inflammatory markers and endometriosis risk (i.e., to “cause” disease, the marker elevation must be present before disease diagnosis) and preserve the consequently required prospective design of the present study, only those endometriosis cases diagnosed after blood draw were eligible for study inclusion. Therefore, the mean age at diagnosis of endometriosis within this nested case-control population was 46 years, approximately 10 years older than the mean age of diagnosis in the whole cohort. This is an inalterable reality of this sample population. Although these results are internally valid within this segment of the endometriosis spectrum, it may not be generalizable to all women with endometriosis if the role of inflammation in the pathophysiology of endometriosis differs according to age at diagnosis. As we observed elevated risks of endometriosis with increased levels of TNF-α in women younger than 40 years at blood draw and a similar pattern for hs-CRP, a stronger relation between inflammation and endometriosis risk may have been observed if the study population was younger at the time of blood collection.
Another limitation of the present study is the inability to identify the true time of onset of endometriosis at a molecular or cellular level, which is true for all studies of endometriosis focused on etiologic discovery regardless of design or study population. Although we defined endometriosis incidence by the date of diagnosis and conducted subanalyses requiring that time from blood collection to diagnosis be 4 years or more, today’s diagnostic technology is unable to determine exactly how far before diagnosis (or symptom onset) endometriosis is present. In addition, we do not have information on the stage of endometriosis for most cases. However, disease stage does not have a clear association with symptom severity (34), and is thus unlikely to influence our results.
Finally, asymptomatic endometriosis may be present among some of our control women, which would drive results of the present study toward the null. However, as mentioned above, 2 alternative methods of defining controls are present in the literature, both with their own limitations. First, if controls were limited to women who had undergone tubal ligation, intractable bias could be introduced if levels of key inflammatory markers are influenced by multiparity or infertility independent of endometriosis. In addition, although the goal of a tubal ligation is not to discover endometriosis, it is not certain that women who have undergone this procedure are definitively endometriosis-free. Second, if controls are limited to women who have undergone a diagnostic laparoscopy who are not observed to have endometriosis, these women may not reflect a “healthy” comparison population but rather a nonendometriosis pathology that underlies their indication for surgery (pain or infertility). This pathology may also be associated with inflammation and would therefore bias our results to the null. Although minimizing the likelihood of endometriosis among the controls is a concern, given the low prevalence of undiagnosed endometriosis in the general population (<2% suggested by Zondervan et al. (35)), the number of undiagnosed cases in the control group is likely to be small and would only marginally dilute the true association.
Despite these limitations, this is one of the largest studies of inflammatory markers and endometriosis to date and the only study with a prospective design. The key strength of this study is that blood samples were collected on average 4 years prior to diagnosis of endometriosis. The prospective study design uniquely allows evaluation of the temporality between inflammatory levels and risk of endometriosis, or at least within a time window earlier in the disease process than analyses conducted at or after the time of surgical diagnosis. All matched nested case-control triplets were shipped and assayed blinded to case-control status, and also included systematically spaced blinded quality control samples. Moreover, our control selection from within the large NHSII cohort minimizes the potential for selection bias that may have been inevitable in previous case-control studies that selected controls from women proven to be fertile or undergoing laparoscopy because of other pathologies. Finally, infertility history at diagnosis was matched between cases and controls, which minimizes the possibility that our results are explained by underlying conditions that caused infertility (i.e., oversampling of infertility among cases of women with endometriosis).
In summary, in this prospectively matched, nested case-control study, higher plasma levels of IL-1β and sTNFR-2 were associated with increased risk of subsequent laparoscopically confirmed endometriosis. Further research in larger studies with younger age at blood collection and longer time from blood to surgical diagnosis will be required to confirm these associations and further hypothesize regarding these results.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Fan Mu, Janet W. Rich-Edwards, Susan E. Hankinson, Eric B. Rimm, Donna Spiegelman, Stacey A. Missmer); Program in Epidemiology, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington (Holly R. Harris); Connors Center for Women’s Health and Gender Biology, Brigham and Women’s Hospital, Boston, Massachusetts (Janet W. Rich-Edwards); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (Susan E. Hankinson, Eric B. Rimm); Division of Biostatistics and Epidemiology, School of Public Health and Health Sciences, University of Massachusetts, Amherst, Massachusetts (Susan E. Hankinson); Department of Nutrition, Harvard T. H. Chan School of Public Health, Boston, Massachusetts (Eric B. Rimm, Donna Spiegelman); Department of Biostatistics, Harvard T. H. Chan School of Public Health, Boston, Massachusetts (Donna Spiegelman); Division of Adolescent and Young Adult Medicine, Department of Pediatrics, Boston Children’s Hospital and Harvard Medical School, Boston, Massachusetts (Stacey A. Missmer); and Department of Obstetrics, Gynecology, and Reproductive Biology, College of Human Medicine, Michigan State University, Grand Rapids, Michigan (Stacey A. Missmer).
F.M. and H.R.H. contributed equally to this work.
This work was supported by the National Institute of Child Health and Human Development (grant HD57210 to S.A.M.) and the National Cancer Institute (grants CA50385, CA67262, and K22 CA193860 to H.R.H.).
Conflict of interest: none declared.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CRP
C-reactive protein
- CV
coefficient of variation
- hs-CRP
high-sensitivity C-reactive protein
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- NHSII
Nurses’ Health Study II
- RR
relative risk
- sTNFR-1
soluble tumor necrosis factor α receptor-1
- sTNFR-2
soluble tumor necrosis factor α receptor-2
- TNF-α
tumor necrosis factor α
REFERENCES
- 1. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. [DOI] [PubMed] [Google Scholar]
- 2. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 3. Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14(4):422–469. [Google Scholar]
- 4. Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64(2):151–154. [PubMed] [Google Scholar]
- 5. Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001;76(1):1–10. [DOI] [PubMed] [Google Scholar]
- 6. Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10. [DOI] [PubMed] [Google Scholar]
- 7. Akoum A, Al-Akoum M, Lemay A, et al. Imbalance in the peritoneal levels of interleukin 1 and its decoy inhibitory receptor type II in endometriosis women with infertility and pelvic pain. Fertil Steril. 2008;89(6):1618–1624. [DOI] [PubMed] [Google Scholar]
- 8. Bedaiwy MA, Falcone T, Sharma RK, et al. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod. 2002;17(2):426–431. [DOI] [PubMed] [Google Scholar]
- 9. Pizzo A, Salmeri FM, Ardita FV, et al. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol Obstet Invest. 2002;54(2):82–87. [DOI] [PubMed] [Google Scholar]
- 10. Abrao MS, Podgaec S, Filho BM, et al. The use of biochemical markers in the diagnosis of pelvic endometriosis. Hum Reprod. 1997;12(11):2523–2527. [DOI] [PubMed] [Google Scholar]
- 11. Somigliana E, Vigano P, Tirelli AS, et al. Use of the concomitant serum dosage of CA 125, CA 19-9 and interleukin-6 to detect the presence of endometriosis. Results from a series of reproductive age women undergoing laparoscopic surgery for benign gynaecological conditions. Hum Reprod. 2004;19(8):1871–1876. [DOI] [PubMed] [Google Scholar]
- 12. Othman Eel D, Hornung D, Salem HT, et al. Serum cytokines as biomarkers for nonsurgical prediction of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2008;137(2):240–246. [DOI] [PubMed] [Google Scholar]
- 13. Xavier P, Belo L, Beires J, et al. Serum levels of VEGF and TNF-alpha and their association with C-reactive protein in patients with endometriosis. Arch Gynecol Obstet. 2006;273(4):227–231. [DOI] [PubMed] [Google Scholar]
- 14. Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006;66(4):2476–2482. [DOI] [PubMed] [Google Scholar]
- 15. Missmer SA, Hankinson SE, Spiegelman D, et al. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784–796. [DOI] [PubMed] [Google Scholar]
- 16. Buckley DI, Fu R, Freeman M, et al. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the US Preventive Services Task Force. Ann Intern Med. 2009;151(7):483–495. [DOI] [PubMed] [Google Scholar]
- 17. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. [DOI] [PubMed] [Google Scholar]
- 18. Govindarajulu US, Spiegelman D, Thurston SW, et al. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med. 2007;26(20):3735–3752. [DOI] [PubMed] [Google Scholar]
- 19. Seli E, Arici A. Endometriosis: interaction of immune and endocrine systems. Semin Reprod Med. 2003;21(2):135–144. [DOI] [PubMed] [Google Scholar]
- 20. Beliard A, Noel A, Goffin F, et al. Adhesion of endometrial cells labeled with 111Indium-tropolonate to peritoneum: a novel in vitro model to study endometriosis. Fertil Steril. 2003;79(suppl 1):724–729. [DOI] [PubMed] [Google Scholar]
- 21. Zhang RJ, Wild RA, Ojago JM. Effect of tumor necrosis factor-alpha on adhesion of human endometrial stromal cells to peritoneal mesothelial cells: an in vitro system. Fertil Steril. 1993;59(6):1196–1201. [DOI] [PubMed] [Google Scholar]
- 22. Motro B, Itin A, Sachs L, et al. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci USA. 1990;87(8):3092–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann NY Acad Sci. 2002;955:89–100. [DOI] [PubMed] [Google Scholar]
- 24. Lebovic DI, Bentzien F, Chao VA, et al. Induction of an angiogenic phenotype in endometriotic stromal cell cultures by interleukin-1beta. Mol Hum Reprod. 2000;6(3):269–275. [DOI] [PubMed] [Google Scholar]
- 25. Surrey ES, Halme J. Effect of peritoneal fluid from endometriosis patients on endometrial stromal cell proliferation in vitro. Obstet Gynecol. 1990;76(5 pt 1):792–797. [DOI] [PubMed] [Google Scholar]
- 26. Iwabe T, Harada T, Tsudo T, et al. Tumor necrosis factor-alpha promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J Clin Endocrinol Metab. 2000;85(2):824–829. [DOI] [PubMed] [Google Scholar]
- 27. Ho HN, Wu MY, Yang YS. Peritoneal cellular immunity and endometriosis. Am J Reprod Immunol. 1997;38(6):400–412. [DOI] [PubMed] [Google Scholar]
- 28. Mori H, Sawairi M, Nakagawa M, et al. Peritoneal-fluid interleukin-1-beta and tumor-necrosis-factor in patients with benign gynecologic disease. Am J Reprod Immunol. 1991;26(2):62–67. [DOI] [PubMed] [Google Scholar]
- 29. Koumantakis E, Matalliotakis I, Neonaki M, et al. Soluble serum interleukin-2 receptor, interleukin-6 and interleukin-1a in patients with endometriosis and in controls. Arch Gynecol Obstet. 1994;255(3):107–112. [DOI] [PubMed] [Google Scholar]
- 30. Martinez S, Garrido N, Coperias JL, et al. Serum interleukin-6 levels are elevated in women with minimal-mild endometriosis. Hum Reprod. 2007;22(3):836–842. [DOI] [PubMed] [Google Scholar]
- 31. Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366.e8–373.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mu F, Rich-Edwards J, Rimm EB, et al. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes. 2016;9(3):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Porpora MG, Koninckx PR, Piazze J, et al. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6(4):429–434. [DOI] [PubMed] [Google Scholar]
- 35. Zondervan KT, Cardon LR, Kennedy SH. What makes a good case-control study? Design issues for complex traits such as endometriosis. Hum Reprod. 2002;17(6):1415–1423. [DOI] [PubMed] [Google Scholar]