Abstract
Background
frailty is associated with an increased risk of fragility fractures. Less is known, however, about the association between frailty and bone health.
Methods
men aged 40–79 years were recruited from population registers in eight European centres for participation in the European Male Aging Study. Subjects completed a comprehensive assessment which included quantitative ultrasound (QUS) scan of the heel (Hologic-SAHARA) and in two centres, dual-energy bone densitometry (dual-energy x-ray absorptiometry, DXA). Frailty was defined based on an adaptation of Fried's phenotype criteria and a frailty index (FI) was constructed. The association between frailty and the QUS and DXA parameters was determined using linear regression, with adjustments for age, body mass index and centre.
Results
in total, 3,231 subjects contributed data to the analysis. Using the Fried categorisation of frailty, pre-frail and frail men had significantly lower speed of sound (SOS), broadband ultrasound attenuation (BUA) and quantitative ultrasound index (QUI) compared to robust men (P< 0.05). Similar results were seen using the FI after categorisation into ‘high’, ‘medium’ and ‘low’ levels of frailty. Using the Fried categorisation, frail men had lower femoral neck bone mineral density (BMD) compared to robust men (P < 0.05), but not lower lumbar spine BMD. Using the FI categorisation, a ‘high’ level of frailty (FI > 0.35) was associated with lower lumbar spine BMD (P < 0.05) when compared to those with low (FI < 0.2), but not lower femoral neck BMD. When analysed as a continuous variable, higher FI was linked with lower SOS, BUA and QUI (P < 0.05).
Conclusions
optimisation of bone health as well as prevention of falls should be considered as strategies to reduce fractures in frail older people.
Keywords: Frailty, male health, heel ultrasound, bone mineral density, older people
Background
The aging process is characterised by a complex alteration of anatomical, physiological and psychological factors. In a significant number of individuals, these changes can result in frailty, a syndrome that has been defined as ‘an excess vulnerability to stressors, with reduced ability to maintain homoeostasis after a destabilising event’ [1]. Frailty is linked with adverse health outcomes including an increased risk of falls and institutionalisation [2]. Frailty has also been linked in prospective studies with an increased risk of future fractures, though whether this is related to the increased susceptibility to falls or whether there is in addition an associated reduction in bone strength remains uncertain [3]. Previous studies have investigated the relationship between frailty and bone mineral density (BMD) [1, 4–11], however, the results have been somewhat discrepant. Some, though not all, suggest an association between frailty and markers of bone strength, including calcaneal BMD [4] and femoral neck or lumbar spine BMD [5, 6, 8]. However, there are few data in men. Such data are important; knowledge of the factors which predispose to fracture, including bone strength, may help improve targeted preventative measures in this high risk group. If frailty is linked with reduced bone strength, then fracture prevention measures should include not just falls prevention but also measures to optimise bone strength. The aim of this study was to investigate the relationship between frailty and bone health defined using both BMD and quantitative ultrasound (QUS) measurements, in a population of community dwelling European men.
Methods
Participants
Subjects were recruited for participation in the European Male Aging Study (EMAS) from eight European centres (Florence, Italy; Leuven, Belgium; Malmö, Sweden; Manchester, UK; Santiago de Compostela, Spain; Łódź, Poland; Szeged, Hungary; Tartu, Estonia). Participants completed a postal questionnaire and attended a research centre for further assessment. Ethical approval for the study was obtained in accordance with local institutional requirements in each centre. Each participant provided written consent.
Assessments
The postal questionnaire included items concerning health and lifestyle [12]. Participants were asked also whether they were currently receiving treatment for a range of medical conditions. The interviewer assisted questionnaire included the short form (SF36), the Physical Activity Scale for the Elderly [13], Reuben's Physical Performance test [14], Beck's Depression Inventory [15] and the Tinetti balance and postural stability index [16]. A range of anthropometric measurements were performed including mid-upper arm circumference (cm) and triceps skinfold thickness (mm). Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m).
QUS of the heel
QUS of the left heel was performed in all subjects with the Sahara Clinical Sonometer (Hologic, Inc, Bedford, MA, USA) using a standardised protocol in all centres. Outputs included broadband ultrasound attenuation (BUA, dB/MHz), speed of sound (SOS, m/s) and quantitative ultrasound index (QUI) which is a parameter derived from SOS and BUA (0.41*SOS+0.41*BUA-571). Short term precision was measured by performing duplicate measurements in 20 randomly selected subjects from one centre (Leuven, Belgium). The in vivo coefficients of variation (CVs) were 2.8% and 0.3% for BUA and SOS, respectively. Repeat measurements (n = 10) were performed on a roving phantom at each of the eight centres. Standardised CVs (SCVs) for within machine variability ranged by centre: for SOS, from 1.0% to 5.6%, and BUA from 0.7% to 2.7%. SCVs for between machine variability were 4.8% for BUA and 9.7% for SOS [17].
Dual-energy x-ray absorptiometry
Areal bone mineral density (BMDa) scans were carried out in the Manchester and Leuven subsets of EMAS (N = 735). Both sites used dual-energy x-ray absorptiometry (DXA) QDR 4500A devices from the same manufacturer (Hologic, Inc, Waltham, MA, USA). BMDa was measured at the lumbar spine (L1–L4) and proximal femur (total region). The precision errors in Leuven were 0.57% and 1.28% at the lumbar spine and total femur region, respectively. In Manchester, these precision errors were 0.97% and 2.04% at the lumbar spine (L1–L4) and proximal femur (total region), respectively. Both devices were cross-calibrated with the European Spine Phantom [18].
Frailty
Frailty status was determined using a phenotypic definition adapted from Fried and colleagues based on five criteria: sarcopenia, exhaustion, slowness, weakness and low activity. Details of the EMAS frailty phenotype (FP) criteria are reported elsewhere [19]. Briefly, ‘sarcopenia’ was based on mid-upper arm muscle circumference (mid-upper arm circumference – 3.14 × triceps skinfold thickness), the threshold being the lowest 10% from men over 65 years. ‘Exhaustion’ was defined using BDI-II energy and fatigue items, responses being ‘I don't have enough energy to do very much/do anything’ or ‘I am too tired or fatigued to do a lot of/most of the things I used to’, respectively. ‘Slowness’ from the PPT 50 foot walk test, the threshold being the slowest 20% stratified by height for men 65 years and older. ‘Weakness’ from the Tinetti 5 chair stand test, the threshold being the slowest 10% for those 65 years and over, or who were unable to complete the test, and ‘low activity’ from the PASE score, the threshold being the lowest 20% for 65+ years. The Fried frailty category variable was constructed as follows: 0 criteria = robust (not frail), 1 or 2 criteria as pre-frail and those with 3 or more criteria as frail. Men with missing data for one or more components of the Fried criteria were not included in the analysis. We also calculated a frailty index (FI). An FI represents the number of defined health deficits present in an individual divided by the number of health deficits considered [20, 21]. In EMAS, 39 potential deficits were evaluated and included in the FI. These represent symptoms, signs or functional impairments that accumulate with age and are individually related to adverse outcomes. Details of the EMAS FI are reported elsewhere [22]. We analysed the FI both as a continuous variable and as a categorical variable using the threshold levels suggested by Kulminski and colleagues: low (robust), FI ≤ 0.2; medium (pre-frail), 0.2 < FI ≤ 0.35; and high (frail), FI > 0.35 [23]. Men with missing data for eight components or more (20%) of the FI were not included in the analysis.
Statistical analysis
Descriptive statistics were used to summarise subject characteristics. Linear regression analysis was used to determine the association between frailty category (using the phenotype and FI definitions), and ultrasound and DXA bone parameters, adjusted for age, centre and BMI with the results expressed as β-coefficients and 95% confidence intervals (CIs). In these analyses the β-coefficients represent the absolute difference in bone parameter among a particular frailty group compared to the referent value (either the low or robust category). From the adjusted linear regression models, post estimation of the marginal mean values of bone parameters for each frailty category was performed. We looked also at the relationship between the component features which make up the FP criteria and bone parameters. We looked also at the association between the FI expressed as a continuous variable and the bone parameters; in this analysis the β-coefficients represent the change in FI for each unit change in bone parameter. All statistical analyses were performed using STATA version 11.2 (http://www.stata.com).
Results
Subject characteristics
A total of 3,369 men were recruited to EMAS. Of these participants, 29 men were excluded because they were taking bone active therapies (calcium, vitamin D, bisphosphonates, glucocorticoids). A further 109 participants who did not have QUS of the heel measured at baseline were also excluded, leaving 3,231 men for analysis. Mean (SD) age of these subjects was 59.9 (11.0) years and mean (SD) BMI was 27.6 (4.0) kg/m2. In total, 735 men from the Manchester and Leuven centres had BMD measurements performed. Subject characteristics are shown in Table 1. Based on the Fried definition of frailty, 783 (26.4%) of the 2,965 men in whom it was possible to characterise frailty were defined as pre-frail and 72 (2.4%) as frail. The proportion of men who were frail increased with age from 0.1% at age 40–49 years to 6.9% at age 70–79 years. The proportion of men satisfying each of the component criteria varied from 5.7% (sarcopenia) to 10.3% (low physical activity). The median FI was 0.09 (IQR 0.04, 0.15). Of the 735 who also underwent BMD measurement, 151 (20.5%) were pre-frail and 11 (1.5%) frail using the Fried FP.
Table 1.
Subject characteristics
| Variable | Statistic | |
|---|---|---|
| Heel quantitative ultrasound | N = 3231 | Mean (SD) |
| BUA (dB/MHz) | 80.3 (18.9) | |
| SOS (m/s) | 1550.9 (34.1) | |
| QUI | 97.8 (21.2) | |
| Areal bone mineral density | N = 735 | Mean (SD) |
| Lumbar spine (g/cm2) | 1.055 (0.175) | |
| Femoral neck (g/cm2) | 0.807 (0.127) | |
| Frailty index | N = 2450 | n (%) |
| Low | 2016 (82.3) | |
| Medium | 342 (14.0) | |
| High | 92 (3.8) | |
| Fried frailty phenotype | N = 2965 | n (%) |
| Robust | 2110 (71.2) | |
| Pre-frail | 783 (26.4) | |
| Frail | 72 (2.4) | |
| Components of Fried frailty phenotype | N = 2965 | n (%) |
| Low physical activity | 305 (10.3) | |
| Exhaustion | 239 (8.1) | |
| Slowness | 291 (9.8) | |
| Weakness | 198 (6.7) | |
| Sarcopenia | 168 (5.7) |
FP and bone health
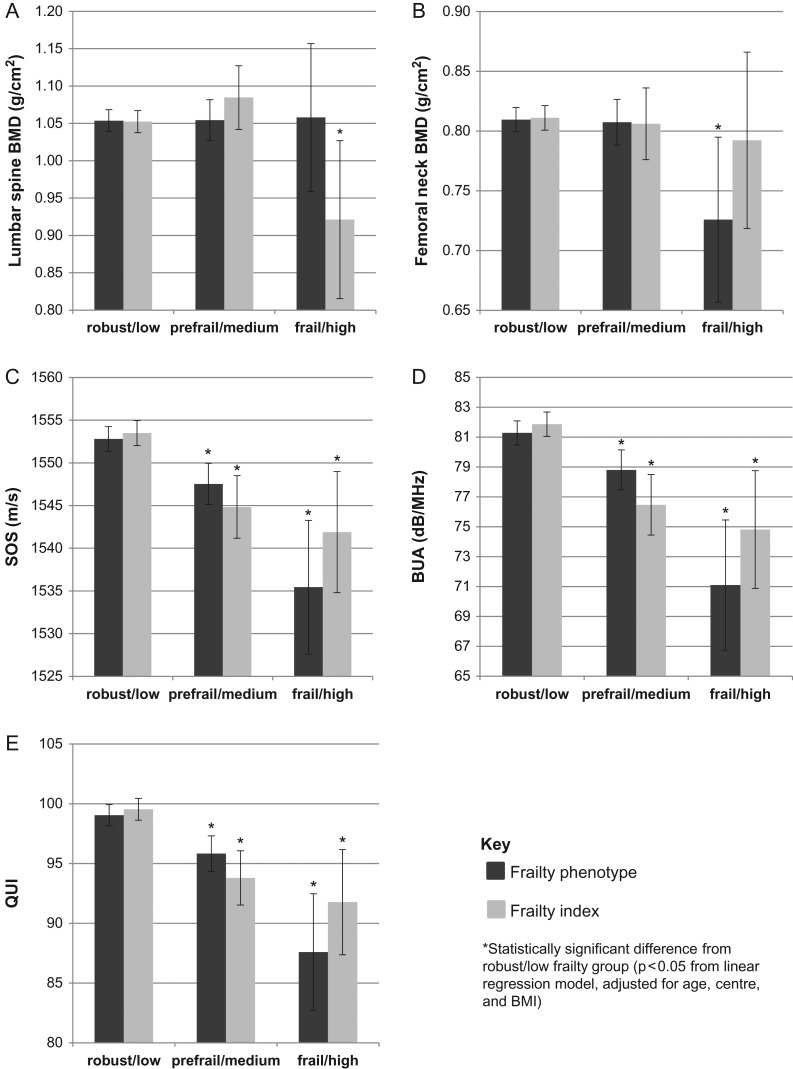
After adjustment, and compared to those who were robust, frailty defined using the phenotype approach, was associated with a reduced SOS (β coefficient −17.4; 95% CI −25.4, −9.4), BUA (β coefficient −10.2; 95% CI −14.6,-5.7) and QUI (β coefficient −11.4; 95% CI −16.4, −6.5), see Table 2 and also Figure 1. Pre-frailty was linked also significantly with reduced heel ultrasound parameters though the β coefficients were smaller. There was no association between frailty and lumbar spine BMD, though frailty was associated with reduced femoral neck BMD (β coefficient −0.084; 95% CI −0.15, −0.014). Each individual component of the FP was linked with a lower SOS, and this was statistically significant in the adjusted model for low physical activity, exhaustion, slow walking speed and weakness, see Supplementary Table S1, available at Age and Ageing online. The results were broadly similar for BUA, apart from low physical activity (QUI and BUA) and weakness (BUA) which were not statistically significant. For femoral neck BMD, low physical activity, exhaustion, weakness and sarcopenia were linked with lower BMD though this was statistically significant for exhaustion only, see Supplementary Table S1, available at Age and Ageing online.
Table 2.
Frailty and bone health parameters
| Variable | Heel quantitative ultrasound | Areal bone mineral density | |||
|---|---|---|---|---|---|
| Adjusted β coefficient (95% CI)a | Adjusted β coefficient (95% CI)a | ||||
| SOS (m/s) | BUA (dB/MHz) | QUI | Lumbar spine | Femoral neck | |
| Fried frailty category | n = 2961 | n = 2961 | n = 2961 | n = 673 | n = 671 |
| Robust | Referent | Referent | Referent | Referent | Referent |
| Pre-frail | −5.3 (−8.1, −2.4)*** | −2.5 (−4.1, −0.9)** | −3.2 (−5.0, −1.4)*** | 0.00062 (−0.031, 0.032) | −0.0021 (−0.024, 0.020) |
| Frail | −17.4 (−25.4, −9.4)*** | −10.2 (−14.6, −5.7)*** | −11.4 (−16.4, −6.5)*** | 0.0044 (−0.096, 0.10) | −0.084 (−0.15, −0.014)* |
| Frailty index category | n = 2442 | n = 2442 | n = 2442 | n = 581 | n = 580 |
| Low | Referent | Referent | Referent | Referent | Referent |
| Medium | −8.7 (−12.6, −4.7)*** | −5.4 (−7.6, −3.2)*** | −5.7 (−8.2, −3.3)*** | 0.032 (−0.014, 0.078) | −0.0049 (−0.037, 0.027) |
| High | −11.6 (−18.9, −4.3)** | −7.0 (−11.1, −3.0)* | −7.8 (−12.3, −3.2)** | −0.13 (−0.24, −0.024)* | −0.019 (−0.0034, −0.0015) |
| Frialty indexb | −44.8 (−59.5, −30.0)*** | −25.5 (−33.7, −17.3)*** | −28.7 (−37.9, −19.6)*** | −0.027 (−0.21, 0.16) | −0.083 (−0.21, 0.044) |
Lumbar spine and femoral neck BMD is measured in g/cm2.*P < 0.05; **P < 0.01; ***P < 0.001.
aAdjusted for age, BMI and centre. Results are presented as adjusted β coefficients with robust/low frailty category as the referent group. These results represent the expected difference in bone parameters for each group, compared to the robust category.
bFI expressed as a continuous measure.
Figure 1.
Marginal mean values (95% CI) of QUS and DXA parameters by FP and FI category
FI and bone health
An increase in FI, assessed as a continuous measure, was significantly associated with lower BUA, SOS and QUI in the adjusted model, (β coefficient −25.5; 95% CI −33.7, −17.3, β coefficient −44.8; 95% CI −59.5, −30.0, and β coefficient −28.7; 95% CI −37.9, −19.6, respectively), see Figure 1 and Table 2. Compared to those with low FI, those who were categorised as high FI (FI > 0.35) had significantly lower SOS (β coefficient = −11.6; 95% CI −18.9, −4.3), BUA (β coefficient −7.0; 95% CI −11.1, −3.0), QUI (β coefficient −7.8; 95% CI −12.3, −3.2) and lumbar spine BMD (β coefficient −0.13; 95% CI −0.24, −0.024).
Discussion
In this population survey we found a significant association between frailty and bone health parameters including low BUA, SOS, QUI and also femoral neck BMD using the Fried categorisation and lumbar spine BMD using the FI categorisation, though the magnitude of these effects was relatively small. There was some evidence of a dose-response effect with those classified as pre-frail having BUA, SOS and QUI levels intermediate between those who were robust and those who were frail. All the component phenotype criteria were associated with reduced BUA and/or SOS/QUI though the effect appeared to be more marked for the slow walking speed and exhaustion criteria.
Epidemiological studies in men and women have shown that frailty is linked with an increased risk of falls and future fractures [19, 24–27]. There are, however, surprisingly few studies which have looked at the link between bone health and frailty, particularly in men. To our knowledge there are no data looking at the association between frailty and QUS parameters. Most cross-sectional studies have shown, after adjustment for age, no association between frailty and calcaneal, lumbar spine or femoral neck BMD [6, 8]. One study of older (>65 years) community-dwelling participants (82% female) found that frailty, defined using a modified Vulnerable Elders Survey (VES-13), was associated with lower BMD of the calcaneus, though the prevalence of frailty was higher than in other comparative studies at 44% [4]. A prospective study of community-dwelling men aged 70–97 years found no association between baseline frailty, defined by the Fried FP, and total hip BMD over a mean follow-up of 2.2 years after adjusting for age [5]. In contrast, a study of 235 community-dwelling women aged >70 years, found that frailty at baseline, defined by the VES-13, predicted lower total hip and lumbar spine BMD 1 year later, although no association was seen between baseline frailty and baseline total hip or lumbar spine BMD [9]. The inconsistency in the literature could, in part, be explained by differences in study design, sex, age and ethnicity of participants and choice of frailty instrument. Our data are consistent with an association between frailty and bone health parameters. The mechanism linking frailty and bone health is likely to be multifactorial and include a reduction in muscle mass and strength, reduced loading due to immobility, a decline in sex hormones, impaired nutrition including protein intake, the presence of chronic disease and also dysregulated inflammation [28].
Our study has a number of strengths; it was large, population based, and used standard methods in both conduct and assessment. There are though a number of limitations which need to be considered in interpreting the analysis. The response rate for participation 41%, with those who declined to take part being older, more likely to be current smokers and reporting experiencing less pain lasting at least one day in the past month, than those who participated [12]. It seems unlikely, however that any such selection factors would impact on the findings reported, which were based on internal comparison of those who took part. The Fried phenotype definition of frailty developed in EMAS was adapted from the original definition, utilising the data available and instruments used in EMAS. It has though been shown to be associated with falls, impaired quality of life [19], and mortality [29]. Among those with BMD measurements performed (N = 735) the prevalence of frailty was lower than those who did not have the measurements performed (1.5% versus 2.4%). However it seems unlikely that this would have influenced findings concerning the association between BMD and frailty which was based on an internal comparison of those in whom measurements were performed.
Men with missing data for one or more components of the Fried FP were not included in the analysis; again it seems unlikely that this would have had an influence on our findings relating to the association between frailty and bone parameters. Our study was cross-sectional and therefore it is not possible to determine the temporal nature of the observed associations. It seems unlikely though that reduced bone density per se would lead to an increase in the risk of frailty. Our cohort included younger men (age <65 years) which may explain the lower proportion of frailty than observed in other cohorts. Finally, our study focused on a European population and so the results should be extrapolated beyond this group with caution.
Our findings are consistent with the view that in addition to an increased susceptibility to falls among frail men, reduced bone strength contributes to susceptibility to fracture risk. Data from a recent trial among female nursing home residents suggest that treatment with long-acting bisphosphonate (zoledronic acid) is linked with an increase in bone density in this vulnerable group and provides therefore a real opportunity for prevention of fractures in this group based on targeting bone [30, 31].
In conclusion, a reduction in bone strength may in part explain the increased susceptibility to fracture among frail older people. Prevention of fractures in frail older people should include consideration of optimising bone health as well as preventing falls.
Key points.
Frailty is associated with reduced bone density and heel ultrasound parameters.
Pre-frail men have ultrasound parameters intermediate between robust and frail men.
Optimisation of bone health should be considered as a strategy to reduce fractures in frail older people.
Supplementary data
Supplementary data mentioned in the text are available to subscribers in Age and Ageing online.
Supplementary Material
Acknowledgements
The Principal Investigator of the European Male Ageing Study is Prof. Frederick Wu, MD; Dept of Endocrinology, Manchester Royal Infirmary, Manchester, UK. EMAS is supported by the Manchester Academic Health Sciences Centre (MAHSC). The authors thank the men who participated in the eight countries, the research/nursing staff in the eight centres: C. Pott, Manchester, E. Wouters, Leuven, M. Nilsson, Malmö, M. del Mar Fernandez, Santiago de Compostela, M. Jedrzejowska, Łódź, H.-M. Tabo, Tartu, A. Heredi, Szeged for their data collection, C. Moseley, Manchester for data entry and project coordination.
Conflicts of interest
None declared.
Funding
This work was supported by the Commission of the European Communities Fifth Framework Programme ‘Quality of Life and Management of Living Resources’ (grant number QLK6-CT-2001-00258) and by Arthritis Research UK (grant number 20380) and the UK National Osteoporosis Society. This report includes independent research supported by the National Institute for Health Research Biomedical Research Unit Funding Scheme. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. Dr K.A.W. is a senior research scientist working within the Nutrition and Bone Health Core Program at MRC Human Nutrition Research, funded by the UK Medical Research Council (grant number U105960371). Dr D.V. is a senior clinical investigator supported by the Clinical Research Fund of the University Hospitals Leuven, Belgium. The financial sponsors played no role in the design, execution, analysis and interpretation of data, or writing of this study.
References
- 1. Walston J, Hadley EC, Ferrucci L et al. . Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54: 991–1001. [DOI] [PubMed] [Google Scholar]
- 2. Fransen M, Woodward M, Norton R, Robinson E, Butler M, Campbell AJ. Excess mortality or institutionalization after hip fracture: men are at greater risk than women. J Am Geriatr Soc 2002; 50: 685–90. [DOI] [PubMed] [Google Scholar]
- 3. Ensrud KE, Ewing SK, Taylor BC et al. . Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 2007; 62: 744–51. [DOI] [PubMed] [Google Scholar]
- 4. Ma SL, Oyler J, Glavin S, Alavi A, Vokes T. Self-reported frailty is associated with low calcaneal bone mineral density in a multiracial population of community-dwelling elderly. Osteoporosis Int 2009; 20: 1837–46. [DOI] [PubMed] [Google Scholar]
- 5. Bleicher K, Cumming RG, Naganathan V et al. . Predictors of the rate of BMD loss in older men: findings from the CHAMP study. Osteoporosis Int 2013; 24: 1951–63. [DOI] [PubMed] [Google Scholar]
- 6. Kenny AM, Waynik IY, Smith J, Fortinsky R, Kleppinger A, McGee D. Association between level of frailty and bone mineral density in community-dwelling men. J Clin Densitom 2006; 9: 309–14. [DOI] [PubMed] [Google Scholar]
- 7. Orwoll E. Assessing bone density in men. J Bone Miner Res 2000; 15: 1867–70. [DOI] [PubMed] [Google Scholar]
- 8. Frisoli A, Chaves PH, Ingham SJM, Fried LP. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the Women's Health and Aging Study (WHAS) II. Bone 2011; 48: 952–7. [DOI] [PubMed] [Google Scholar]
- 9. Sternberg SA, Levin R, Dkaidek S, Edelman S, Resnick T, Menczel J. Frailty and osteoporosis in older women-a prospective study. Osteoporosis Int 2014; 25: 763–8. [DOI] [PubMed] [Google Scholar]
- 10. Verschueren S, Gielen E, O'Neill TW et al. . Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporosis Int 2013; 24: 87–98. [DOI] [PubMed] [Google Scholar]
- 11. Amin S, Felson DT. Osteoporosis in men. Rheum Dis Clin N Am 2001; 27: 19. [DOI] [PubMed] [Google Scholar]
- 12. Lee DM, O'Neill TW, Pye SR et al. . The European Male Ageing Study (EMAS): design, methods and recruitment. Int J Androl 2009; 32: 11–24. [DOI] [PubMed] [Google Scholar]
- 13. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical-Activity Scale for the Elderly (PASE)—development and evaluation. J Clin Epidemiol 1993; 46: 153–62. [DOI] [PubMed] [Google Scholar]
- 14. Reuben DB, Siu AL. An objective-measure of physical function of elderly outpatients—the physical performance-test. J Am Geriatr Soc 1990; 38: 1105–12. [DOI] [PubMed] [Google Scholar]
- 15. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–71. [DOI] [PubMed] [Google Scholar]
- 16. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med 1986; 80: 429–34. [DOI] [PubMed] [Google Scholar]
- 17. Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int 1995; 5: 262–70. [DOI] [PubMed] [Google Scholar]
- 18. Reid DM, Mackay I, Wilkinson S et al. . Cross-calibration of dual-energy X-ray densitometers for a large, multi-center genetic study of osteoporosis. Osteoporos Int 2006; 17: 125–32. [DOI] [PubMed] [Google Scholar]
- 19. O'Connell M, Tajar A, O'Neill T et al. . Frailty is associated with impaired quality of life and falls in middle-aged and older European men. J Frailty Aging 2013; 2: 77–83. [DOI] [PubMed] [Google Scholar]
- 20. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62: 722–7. [DOI] [PubMed] [Google Scholar]
- 22. Tajar A, O'Connell MD, Mitnitski AB et al. . Frailty in relation to variations in hormone levels of the hypothalamic-pituitary-testicular axis in older men: results from the European male aging study. J Am Geriatr Soc 2011; 59: 814–21. [DOI] [PubMed] [Google Scholar]
- 23. Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc 2008; 56: 898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kennedy CC, Ioannidis G, Rockwood K et al. . A frailty index predicts 10-year fracture risk in adults age 25 years and older: results from the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int 2014; 25: 2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Vries OJ, Peeters GMEE, Lips P, Deeg DJH. Does frailty predict increased risk of falls and fractures? A prospective population-based study. Osteoporosis Int 2013; 24: 2397–403. [DOI] [PubMed] [Google Scholar]
- 26. Ensrud KE, Ewing SK, Taylor BC et al. . Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med 2008; 168: 382–9. [DOI] [PubMed] [Google Scholar]
- 27. Ensrud KE, Ewing SK, Cawthon PM et al. . A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57: 492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dawson A, Dennison E. Measuring the musculoskeletal aging phenotype. Maturitas 2016; 93: 13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ravindrarajah R, Lee DM, Pye SR et al. . The ability of three different models of frailty to predict all-cause mortality: results from the European Male Aging Study (EMAS). Arch Gerontol Geriatr 2013; 57: 360–8. [DOI] [PubMed] [Google Scholar]
- 30. Greenspan SL, Perera S, Ferchak MA, Nace DA, Resnick NM. Efficacy and safety of single-dose zoledronic acid for osteoporosis in frail elderly women a randomized clinical trial. JAMA Intern Med 2015; 175: 913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rolland Y, Van Kan GA, Gillette-Guyonnet S, Roux C, Boonen S, Vellas B. Strontium ranelate and risk of vertebral fractures in frail osteoporotic women. Bone 2011; 48: 332–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.