Abstract
The objective of this study was to evaluate the association of vitamin D intake and serum levels with fracture risk in children under 6 years of age. A case-control study was conducted in Toronto, Ontario, Canada. Cases were recruited from the fracture clinic at the Hospital for Sick Children, and matched controls were obtained from the TARGet Kids! primary-care research network. Controls were matched to cases on age, sex, height, and season. Fracture risk was estimated from conditional logistic regression, with adjustment for skin type, fracture history, waist circumference, outdoor free play, neighborhood income, soda consumption, and child's birth weight. A total of 206 cases were recruited during May 2009–April 2013 and matched to 343 controls. Serum 25-hydroxyvitamin D concentration (per 10-nmol/L increment: adjusted odds ratio (aOR) = 0.95, 95% confidence interval (CI): 0.88, 1.03) and intake of cow's milk (<2 cups/day vs. 2 cups/day: aOR = 0.95 (95% CI: 0.60, 1.52); >2 cups/day vs. 2 cups/day: aOR = 1.39 (95% CI: 0.85, 2.23)) were not significantly associated with reduced odds of fracture. A statistically significant association was observed between child use of vitamin D supplements and decreased odds of fracture (yes vs. no: aOR = 0.42, 95% CI: 0.25, 0.69). Vitamin D supplementation, but not serum 25-hydroxyvitamin D level or milk intake, was associated with reduced fracture risk among these healthy young children.
Keywords: bone fractures, child injury, dietary supplements, 25-hydroxyvitamin D, milk, vitamin D
Approximately one-third of children have a bone fracture before the age of 17 years (1). Among girls, the risk of fracture ranges from 27% to 40%, and among boys it ranges from 42% to 64% (2). Known risk factors for childhood fracture include being overweight, lack of exposure to sunlight, milk avoidance, lack of physical activity, use of medications that lead to bone thinning, lower bone mass, and previous history of fracture (3–9). Fractures in healthy children often occur due to injury or trauma, from falls or collisions, or during play or sport (2, 10).
Vitamin D is involved in the regulation of calcium absorption and is important for bone health (11). It has long been known that severe vitamin D deficiency leads to rickets, bone deformities, and poor bone mineralization (9, 11, 12). Low vitamin D levels may also be associated with reduced bone mineralization and low bone density in children and adolescents (13). Yet it is unclear whether lower levels of vitamin D in early childhood are associated with increased risk of fracture (2). Twenty-five-hydroxyvitamin D (25(OH)D) is the preferred biomarker of serum vitamin D level, reflecting both cutaneous production following sunlight exposure and dietary sources of vitamin D (11). The US Recommended Dietary Allowance for vitamin D is 600 IU/day for all children over 1 year of age (14). Vitamin D supplementation and intake of cow's milk are the 2 main modifiable determinants of 25(OH)D concentration in Canadian children (15). Few previous studies have evaluated the association between vitamin D, or its main determinants, and fracture risk in children (2).
Our primary objective in the current study was to evaluate the association between serum 25(OH)D concentration and fracture risk among children younger than 6 years of age. Our secondary objectives were to evaluate the associations between children's intake of both vitamin D-fortified cow's milk and supplements containing vitamin D and fracture risk.
METHODS
Study design and participants
A case-control study was conducted among children younger than 6 years of age in Toronto, Ontario, Canada. Ethical approval for the study was obtained from the Research Ethics Board at the Hospital for Sick Children and St. Michael's Hospital, and consent was obtained from the parents of all participating children.
Cases
Cases were recruited from the pediatric fracture clinic at the Hospital for Sick Children in Toronto from May 2009 to April 2013. Children were eligible if they were less than 6 years of age with a bone fracture in a lower extremity (femur, tibia, fibula, talus, and metatarsal) or upper extremity (humerus, olecranon, condyle, radius, ulna, clavicle, elbow, forearm, and radius). Fractures were confirmed radiographically.
Controls
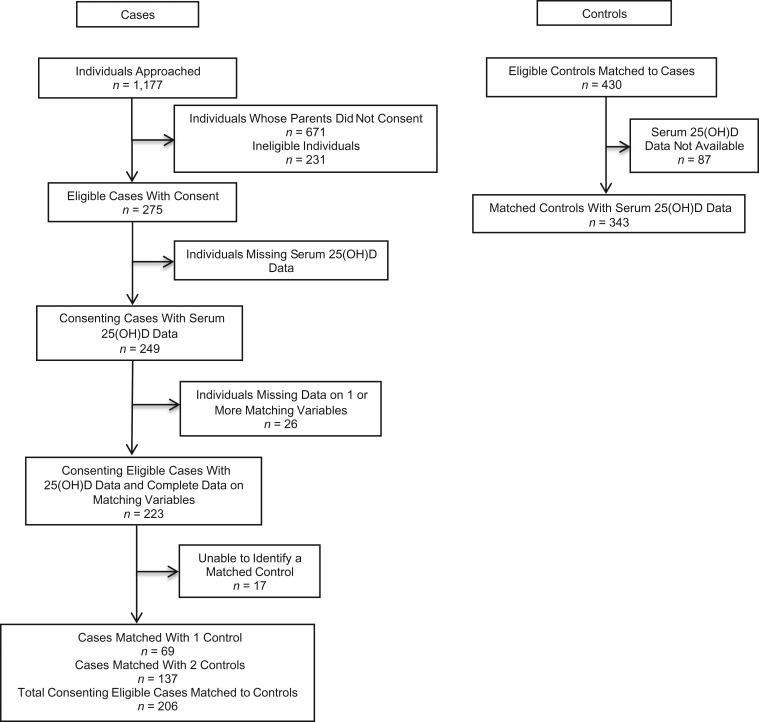
Controls were obtained from the TARGet Kids! primary health-care practice-based research network (16). Children under 6 years of age were recruited at their scheduled well-child visit at one of 8 pediatric or family practice primary-care clinics in Toronto (16). Controls were recruited between the years 2008–2012, and a subset were selected for this study by matching them 2:1 to cases on season of blood draw, age, sex, and height. For cases for whom 2 controls could not be matched, 1 matched control was used (see Figure 1).
Figure 1.
Identification of cases and controls for a study of vitamin D intake and serum levels and early-childhood fracture risk, Toronto, Ontario, Canada, 2009–2013.
Exclusion criteria
Children (both cases and controls) were excluded if they had severe chronic health conditions (with the exception of asthma and high-functioning autism), severe developmental delays, or conditions affecting growth, such as cystic fibrosis. Cases and controls were also excluded if they had conditions known to affect bone health, such as osteogenesis imperfecta or Marfan's syndrome, or if they were using medications such as barbiturates and corticosteroids, which may affect 25(OH)D concentrations or bone health.
Exposure variables
The primary exposure, total serum 25(OH)D concentration, was measured from blood samples collected at the fracture clinic for the cases (within a week of injury) and during well-child primary health-care visits for controls. Serum samples for both cases and controls were batch-analyzed for total 25(OH)D using liquid chromatography–tandem mass spectrometry at the Hospital for Sick Children. Samples were analyzed using the 4000 Q TRAP LC/MS/MS system (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, Massachusetts), which was regularly calibrated to the Vitamin D External Assessment Scheme (17). Interassay imprecision was less than 7.5% at 25(OH)D concentrations of 28 nmol/L, 79 nmol/L, and 171 nmol/L, and intraassay imprecision was 3.2%. We evaluated serum 25(OH)D as both a continuous variable and a dichotomous variable using 50 nmol/L as the cutpoint, as recommended by the US Institute of Medicine (14).
The secondary exposures, cow's milk intake and vitamin D supplementation, were measured from the same parent-completed questionnaire for both cases and controls based on the Canadian Community Health Survey (18). Information on intake of cow's milk was obtained by asking parents, “How many cups of each drink does your child have currently in a typical day? (1 cup = 8 ounces = 250 mL).” Response options included 7 checkboxes ranging from zero to 5+. Cow's milk categories were further collapsed based on the recommended daily intake of milk (2 cups/day) into 3 categories: <2 cups/day, 2 cups/day (reference category), and >2 cups/day (19). Children's use of vitamins containing vitamin D was derived from parental responses to the question, “Does your child take any vitamins or supplements regularly?,” with a list of vitamins and supplements which included both “vitamin D-containing multivitamin” and “vitamin D supplement.”
Other variables
Potential confounders and other covariates were identified a priori through a literature review. Cases and controls were matched on: season of blood draw (winter was defined as October–April and summer was defined as May–September), age (±3 months), sex, and height (±5 cm). Height was measured by trained research assistants using a calibrated stadiometer. We were unable to obtain data on child weight in most fracture cases, as the children were wearing a cast; thus, waist circumference was used as a proxy for adiposity. Waist circumference was age- and sex-standardized to obtain z scores using data on 5,000 children in the full TARGet Kids! cohort, and the mean values and standard deviations were similar to those from the US National Health and Nutrition Examination Survey for children ≥2 years of age (20).
Skin type for the cases and controls was determined by trained research assistants on the basis of the Fitzpatrick scale (21). Neighborhood median after-tax household income was determined using the Statistics Canada Postal Code Conversion Files and information from the 2006 Canadian census (22). Outdoor free play time, child's history of fracture, carbonated soda consumption, and child's birth weight were measured by parental report.
Sample size
Based on preliminary data, the sample size was calculated a priori for the primary outcome, assuming a mean 25(OH)D concentration of 50 nmol/L with a standard deviation of 19.65 nmol/L, using a simple 2-tailed t test with a type I error probability of 0.05. A sample size of 250 cases and a minimum of 250 controls would have 80% power to detect a small difference of only 5 nmol/L between cases and controls. Only 125 cases and 125 controls would be required to detect a larger difference in 25(OH)D of 7 nmol/L.
Statistical analysis
Data were analyzed using R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria; www.R-project.org). Descriptive statistics were calculated separately for cases and controls, and differences were evaluated using χ2 tests for categorical variables and t tests for continuous variables. Statistical significance was defined as P < 0.05, and all tests were 2-sided. Odds ratios and 95% confidence intervals were obtained from conditional logistic regression, to account for the matching variables, using the “survival” package in R (23). Both unadjusted and fully adjusted multivariable models were created separately for each of the primary and secondary exposures: 1) continuous 25(OH)D; 2) categorical 25(OH)D (<50 nmol/L vs. ≥50 nmol/L); 3) cow's milk intake; 4) vitamin D supplement use; and 5) multivitamin supplement use. All multivariable models included all potential confounders identified a priori: skin type, standardized waist circumference, history of fracture, outdoor free play time, income based on postal code, birth weight, and soda intake. Missing data were imputed using the multivariate imputation by chained equations (MICE) procedure in R, specifying 15 maximum iterations and 20 multiple imputations (24). The amount of missing data was less than 15% for each variable.
RESULTS
Of the 249 eligible cases whose parents gave consent for them to participate, 206 had 25(OH)D measured and were successfully matched to controls. Of the 430 control children who were eligible for matching, 343 had 25(OH)D data available and were successfully matched to cases (Figure 1). Descriptive statistics for the cases and controls are shown in Table 1. The average age of cases and matched controls was 43 months, and 44% were female. Fracture cases were more likely to have darker skin pigmentation than controls (P = 0.01) and more likely to have a history of fracture (P = 0.01).
Table 1.
Baseline Characteristics of Fracture Cases and Controls in a Study of Vitamin D Intake and Serum Levels and Early-Childhood Fracture Risk, Toronto, Ontario, Canada, 2009–2013
| Cases (n = 206) | Controls (n = 343) | P Valuea | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | No. of Childrenb | % | Mean (SD) | No. of Children | % | ||
| Age, months | 43.03 (18.79) | 42.84 (18.86) | 0.91c | ||||
| Neighborhood median after-tax household income, Can$/year | 63,133 (29,890) | 57,013 (20,693) | 0.01 | ||||
| Height, cm | 99.90 (13.60) | 99.35 (13.19) | 0.64c | ||||
| Outdoor free play, minutes/day | 69.93 (57.83) | 60.73 (61.59) | 0.09 | ||||
| Waist circumference z scored | 0.01 (1.47) | 0.06 (0.95) | 0.64 | ||||
| Child's birth weight, kg | 3.39 (0.57) | 3.27 (0.67) | 0.04 | ||||
| Sex | |||||||
| Male | 116 | 56 | 191 | 56 | 0.89c | ||
| Female | 90 | 44 | 152 | 44 | |||
| Age, months | |||||||
| <18 | 20 | 10 | 30 | 9 | 0.75c | ||
| 18–35 | 63 | 31 | 93 | 27 | |||
| 36–60 | 69 | 33 | 128 | 37 | |||
| >60 | 54 | 26 | 92 | 27 | |||
| Seasone | |||||||
| Winter | 112 | 54 | 193 | 56 | 0.66c | ||
| Summer | 94 | 46 | 150 | 44 | |||
| History of fracture | |||||||
| No | 186 | 92 | 321 | 97 | 0.01 | ||
| Yes | 17 | 8 | 11 | 3 | |||
| Fitzpatrick skin typef | |||||||
| I or II (lightest) | 81 | 39 | 185 | 54 | 0.01 | ||
| III | 56 | 27 | 84 | 24 | |||
| IV | 35 | 17 | 38 | 11 | |||
| V or VI (darkest) | 30 | 15 | 32 | 9 | |||
| Typical soda intake, cups/day | |||||||
| 0 | 181 | 88 | 292 | 85 | 0.04 | ||
| ≥1 | 19 | 9 | 14 | 4 | |||
| Location of fractureg | |||||||
| Lower extremity | 77 | 37 | NA | ||||
| Upper extremity | 129 | 63 | NA | ||||
Abbreviations: NA, not applicable; SD, standard deviation.
a The t test was used for continuous variables, and the χ2 test was used for categorical variables.
b Values may not sum to totals because of missing data.
c Matched variable; no significant difference expected.
d Age- and sex-standardized.
e Winter was defined as October–April and summer as May–September.
f Skin type was determined by trained research assistants on the basis of the Fitzpatrick scale (21).
g Lower-extremity fractures included fractures of the femur, tibia, fibula, talus, and metatarsal; upper-extremity fractures included fractures of the humerus, olecranon, condyle, radius, ulna, clavicle, elbow, forearm, and radius.
The mean serum 25(OH)D concentration in the cases was 88.5 (standard deviation, 22.4) nmol/L, while the mean concentration in controls was 91.6 (standard deviation, 31.1) nmol/L (Table 2). Among the fracture cases, 16% of parents reported that their child regularly took a vitamin D supplement, as opposed to 31% among the controls.
Table 2.
Conditional Logistic Regression Analysis of the Association Between Vitamin D Intake and Serum Levels and Early-Childhood Fracture Risk, Toronto, Ontario, Canada, 2009–2013
| Cases (n = 206) | Controls (n = 343) | OR | 95% CI | Adjusted ORa | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| No.b | % | No. | % | |||||
| Serum 25(OH)D concentration, nmol/Lc | 88.5 (22.4) | 91.6 (31.1) | ||||||
| Serum 25(OH)D concentration, per 10-nmol/L increase | 0.96 | 0.90, 1.03 | 0.95 | 0.88, 1.03 | ||||
| Intake of cow's milk, cups/day | ||||||||
| <2 | 66 | 32 | 126 | 37 | 0.97 | 0.64, 1.49 | 0.95 | 0.60, 1.52 |
| 2 | 63 | 31 | 118 | 34 | 1 | Referent | 1 | Referent |
| >2 | 72 | 35 | 87 | 25 | 1.49 | 0.96, 2.33 | 1.39 | 0.85, 2.23 |
| Serum 25(OH)D status, nmol/L | ||||||||
| ≥50 | 195 | 95 | 329 | 96 | 1 | Referent | 1 | Referent |
| <50 | 11 | 5 | 14 | 4 | 1.30 | 0.58, 2.95 | 1.36 | 0.55, 3.38 |
| Child use of single vitamin D supplement | ||||||||
| No | 170 | 84 | 229 | 69 | 1 | Referent | 1 | Referent |
| Yes | 32 | 16 | 105 | 31 | 0.42 | 0.26, 0.66 | 0.42 | 0.25, 0.69 |
| Child use of multivitamins | ||||||||
| No | 127 | 63 | 185 | 55 | 1 | Referent | 1 | Referent |
| Yes | 75 | 37 | 149 | 45 | 0.71 | 0.47, 1.06 | 0.76 | 0.49, 1.18 |
Abbreviations: CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio.
a Adjusted for fracture history, skin type, age- and sex-standardized waist circumference, outdoor free play time, parental income (based on postal code), birth weight, and soda intake.
b Values may not sum to totals because of missing data.
c Values are presented as mean (standard deviation).
No statistically significant association was observed between serum 25(OH)D level and fracture risk, and the odds ratio was close to the null (per 10-nmol/L increment in 25(OH)D concentration, adjusted odds ratio (aOR) = 0.95, 95% confidence interval (CI): 0.88, 1.03) (Table 2). The association between low serum 25(OH)D level and fracture risk was also not statistically significant, although the odds ratio was in the expected direction and the 95% confidence interval was very wide (<50 nmol/L vs. ≥50 nmol/L: aOR = 1.36, 95% CI: 0.55, 3.38). Similarly, no statistically significant association was found between cow's milk intake and fracture risk (<2 cups/day vs. 2 cups/day: aOR = 0.95 (95% CI: 0.60, 1.52)); >2 cups/day vs. 2 cups/day: aOR = 1.39 (95% CI: 0.85, 2.23)) (Table 2). However, child use of vitamin D supplements was significantly associated with decreased odds of fracture risk (yes vs. no: aOR = 0.42, 95% CI: 0.25, 0.69), whereas child multivitamin use was not significantly associated with fracture risk (yes vs. no: aOR = 0.76, 95% CI: 0.49, 1.18). The odds ratios were similar in conditional logistic regression models with no adjustment and those with full adjustment (Table 2). We also evaluated a model with minimal adjustment, adjusting only for variables that were independently associated with both vitamin D exposure and fracture outcome: skin type and soda intake. The results of the minimally adjusted model were not different from those of the fully adjusted model (data not shown).
We conducted a sensitivity analysis evaluating the removal of all children under 18 months of age (n = 50); this included only 4 children younger than 12 months of age, who ranged in age from 7 months to 11 months. The adjusted odds ratios for 25(OH)D, cow's milk intake, and child vitamin D supplement use were very similar to those from our primary analysis. Further, we explored a model that simultaneously adjusted for both cow's milk intake and vitamin D supplement use, and the adjusted odds ratios for these 2 exposures also did not change (data not shown).
DISCUSSION
Although vitamin D is known to be important for bone health, it has been unclear whether vitamin D is associated with fracture risk in early childhood. We found no statistically significant association between concurrent 25(OH)D concentration and fracture risk. Further, the main dietary source of vitamin D, milk intake, was also not statistically significantly associated with reduced risk of fractures. However, parent-reported children's vitamin D supplement use was associated with a significant reduction in the odds of fracture, and this association was not observed for multivitamin use. The American Academy of Pediatrics Committee on Nutrition recommends supplementation with 400 IU of vitamin D per day for children who drink less than 1 L of vitamin D-fortified milk per day (25).
Despite a large body of literature on the importance of vitamin D for bone health, very few studies have evaluated the association between 25(OH)D concentration and fracture risk (2). In 3 different case series, a high prevalence of 25(OH)D deficiency (<50 nmol/L), ranging from 8% to 59%, was reported among children with fractures; however, none of these studies included a comparison group of children without fractures (9, 26, 27). In one cross-sectional study, Ceroni et al. (28) compared 25(OH)D concentrations among Swiss adolescents (aged 10–16 years) with upper limb fracture, those with lower limb fracture, and healthy controls, and they did not find a statistically significant difference in 25(OH)D across the 3 outcome groups. The only previous case-control study of vitamin D and fracture risk was conducted in African-American children aged 5–9 years (76 cases and 74 controls); in that study, Ryan et al. (29) reported that low serum 25(OH)D concentration was associated with higher fracture risk (aOR = 3.46, 95% CI: 1.09, 10.94). Our null finding is not consistent with this previous study; however, the prevalence of vitamin D deficiency (<50 nmol/L) in that study was high (41% for controls and 47% for cases) (29) relative to our study, where only 5% of cases and 4% of controls had 25(OH)D concentrations less than 50 nmol/L.
Our study also found no statistically significant association between intake of vitamin D-fortified cow's milk and reduced fracture risk. This finding is consistent with several other studies carried out in older children (30, 31), although 2 studies that focused on milk-avoidant children (7) and children with milk allergies (32) found that a complete lack of milk intake was associated with increased fracture risk. Adult studies have had inconsistent findings, with some studies identifying a negative association between cow's milk intake and fracture risk (33) and others finding a positive association with adult (34) or adolescent (35) milk consumption, while a meta-analysis found no overall association (36).
Consistent with our hypothesis, we found that use of vitamin D supplements was statistically significantly associated with a 58% reduction in the odds of fracture in early childhood. Our results may be supported by those of a previous study showing that vitamin D supplementation in children leads to increased bone mineral density (37). It is somewhat counterintuitive that vitamin D supplementation but not 25(OH)D concentration was associated with reduced fracture risk; however, patterns of supplement use in children may be relatively stable, and supplementation may reflect 25(OH)D level in an earlier time window of bone mineralization, which may not be reflected by current 25(OH)D concentration. It is also possible that our finding of a significant protective association for vitamin D supplements was due to residual confounding. Although we adjusted for numerous potential confounders hypothesized a priori and the adjusted results did not differ substantially from the unadjusted results, use of vitamin D supplements may be associated with some unmeasured healthy lifestyle characteristics or protective parenting. If the association was due to residual confounding, a significant inverse association might also be expected for multivitamin use; however, this was not observed. The difference between vitamin D supplements and multivitamins may also be explained by differences in vitamin D dose or frequency of supplement intake, although this information was not available in our study. Further, it may be hypothesized that children who take vitamin D supplements have different patterns of milk consumption; in our study, the mean daily intake of cow's milk was 2.07 cups/day in non–supplement users and 1.83 cups/day in supplement users, and this difference was borderline statistically significant (P = 0.05).
To our knowledge, our study was the first to evaluate the associations of vitamin D intake and serum levels with fracture risk in a multiethnic population of young, healthy North American children. Strengths of our study included a relatively large sample of young children with fracture and the inclusion of a healthy control group matched to cases on important suspected confounders. Furthermore, fracture was confirmed by radiography. The matched case-control study design we used allowed us to overcome the feasibility issues involved in obtaining a sufficient number of cases in a prospective cohort study, as fractures in early childhood are relatively rare.
Our study had a number of limitations. Although the sample size was calculated a priori with the goal of 80% power, it is possible that a larger sample size may have revealed more statistically significant associations; however, for the primary exposure, mean 25(OH)D concentrations were similar in cases and controls and the odds ratio was close to 1.0, suggesting no association. Recall bias is possible if the parents of cases differentially reported cow's milk intake or vitamin D supplementation relative to the parents of controls. Further, we did not have detailed information on the duration and frequency of supplement use or the dose of vitamin D. Data on bone mineral density and cause of fracture were also not available. The possibility of selection bias is also a limitation. Controls were recruited through routine primary health-care clinics in the same jurisdiction as the fracture clinic, and they may have been healthier than cases, who may or may not have had the same degree of primary health care. However, controls appeared to have a lower family income than cases, which argues against this possibility. Lastly, 25(OH)D levels in this study population may have been sufficiently high to minimize fracture risk from vitamin D, and our results may not be generalizable to other populations with lower 25(OH)D levels.
Our findings suggest that serum 25(OH)D level at the time of fracture may not be associated with fracture risk among young children in a population with relatively high 25(OH)D concentrations. However, use of vitamin D supplements was significantly associated with reduced fracture risk; this may have reflected differential 25(OH)D levels at a past time point in bone development or protection against seasonal fluctuation. Future longitudinal studies are needed to elucidate the role of vitamin D in early-childhood fracture risk.
ACKNOWLEDGMENTS
Author affiliations: Department of Health Research Methods, Evidence, and Impact, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada (Laura N. Anderson); Child Health Evaluative Sciences Program, The Hospital for Sick Children, Toronto, Ontario, Canada (Laura N. Anderson, Catherine S. Birken, Patricia C. Parkin); Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (Sze Wing Heong, Kevin E. Thorpe); Applied Health Research Centre of the Li Ka Shing Knowledge Institute of St. Michael's Hospital, University of Toronto, Toronto, Ontario, Canada (Yang Chen, Kevin E. Thorpe, Jonathon L. Maguire); Clinical Biochemistry Division, Department of Paediatric Laboratory Medicine, The Hospital for Sick Children, Toronto, Ontario, Canada (Khosrow Adeli); Division of Orthopaedics, The Hospital for Sick Children, Toronto, Ontario, Canada (Andrew Howard); Department of Pediatrics, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada (Andrew Howard, Etienne Sochett, Catherine S. Birken, Patricia C. Parkin, Jonathon L. Maguire); Pediatric Outcomes Research Team, Division of Pediatric Medicine, Department of Pediatrics, The Hospital for Sick Children, Toronto, Ontario, Canada (Patricia C. Parkin, Catherine S. Birken, Jonathon L. Maguire); and Institute for Health Policy, Management and Evaluation, University of Toronto, Toronto, Ontario, Canada (Catherine S. Birken, Patricia C. Parkin, Jonathon L. Maguire).
This work was supported by the Canadian Institutes of Health Research and the Dairy Farmers of Canada.
We are grateful to all practitioners who are currently involved in the TARGet Kids! research network, including the Steering Committee (Drs. Tony Barozzino, Brian Chisamore, Mark Feldman, and Moshe Ipp), the Research Team (Kathleen Abreo, Dharma Dalwadi, Tarandeep Malhi, Antonietta Pugliese, Megan Smith, and Laurie Thompson), the Applied Health Research Centre (Dr. Gerald Lebovic, Magda Melo, and Patricia Nguyen), and Mount Sinai Services Laboratory (Dr. Azar Azad).
Members of the TARGet Kids! collaboration: Scientific Committee—Kawsari Abdullah, Laura N. Anderson, Catherine S. Birken, Cornelia M. Borkhoff, Sarah Carsley, Yang Chen, Mikael Katz-Lavigne, Kanthi Kavikondala, Christine Kowal, Jonathon L. Maguire, Dalah Mason, Jessica Omand, Patricia C. Parkin, Navindra Persaud, and Meta van den Heuvel; Site Investigators—Jillian Baker, Tony Barozzino, Joey Bonifacio, Douglas Campbell, Sohail Cheema, Brian Chisamore, Karoon Danayan, Paul Das, Mary Beth Derocher, Anh Do, Michael Dorey, Sloane Freeman, Keewai Fung, Charlie Guiang, Curtis Handford, Hailey Hatch, Sheila Jacobson, Tara Kiran, Holly Knowles, Bruce Kwok, Sheila Lakhoo, Margarita Lam-Antoniades, Eddy Lau, Fok-Han Leung, Jennifer Loo, Sarah Mahmoud, Rosemary Moodie, Julia Morinis, Sharon Naymark, Patricia Neelands, James Owen, Michael Peer, Marty Perlmutar, Navindra Persaud, Andrew Pinto, Michelle Porepa, Nasreen Ramji, Noor Ramji, Alana Rosenthal, Janet Saunderson, Rahul Saxena, Michael Sgro, Susan Shepherd, Barbara Smiltnieks, Carolyn Taylor, Thea Weisdors, Sheila Wijayasinghe, Peter Wong, Ethel Ying, and Elizabeth Young.
Conflict of interest: none declared.
Contributor Information
Collaborators: Kawsari Abdullah, Laura N. Anderson, Catherine S. Birken, Cornelia M. Borkhoff, Sarah Carsley, Yang Chen, Mikael Katz-Lavigne, Kanthi Kavikondala, Christine Kowal, Jonathon L. Maguire, Dalah Mason, Jessica Omand, Patricia C. Parkin, Navindra Persaud, Meta van den Heuvel, Jillian Baker, Tony Barozzino, Joey Bonifacio, Douglas Campbell, Sohail Cheema, Brian Chisamore, Karoon Danayan, Paul Das, Mary Beth Derocher, Anh Do, Michael Dorey, Sloane Freeman, Keewai Fung, Charlie Guiang, Curtis Handford, Hailey Hatch, Sheila Jacobson, Tara Kiran, Holly Knowles, Bruce Kwok, Sheila Lakhoo, Margarita Lam-Antoniades, Eddy Lau, Fok-Han Leung, Jennifer Loo, Sarah Mahmoud, Rosemary Moodie, Julia Morinis, Sharon Naymark, Patricia Neelands, James Owen, Michael Peer, Marty Perlmutar, Navindra Persaud, Andrew Pinto, Michelle Porepa, Nasreen Ramji, Noor Ramji, Alana Rosenthal, Janet Saunderson, Rahul Saxena, Michael Sgro, Susan Shepherd, Barbara Smiltnieks, Carolyn Taylor, Thea Weisdors, Sheila Wijayasinghe, Peter Wong, Ethel Ying, and Elizabeth Young
REFERENCES
- 1. Cooper C, Dennison EM, Leufkens HG, et al. Epidemiology of childhood fractures in Britain: a study using the General Practice Research Database. J Bone Miner Res. 2004;19(12):1976–1981. [DOI] [PubMed] [Google Scholar]
- 2. Clark EM. The epidemiology of fractures in otherwise healthy children. Curr Osteoporos Rep. 2014;12(3):272–278. [DOI] [PubMed] [Google Scholar]
- 3. Goulding A, Jones IE, Taylor RW, et al. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res. 2000;15(10):2011–2018. [DOI] [PubMed] [Google Scholar]
- 4. Wren TA, Shepherd JA, Kalkwarf HJ, et al. Racial disparity in fracture risk between white and nonwhite children in the United States. J Pediatr. 2012;161(6):1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ward LM. Osteoporosis due to glucocorticoid use in children with chronic illness. Horm Res. 2005;64(5):209–221. [DOI] [PubMed] [Google Scholar]
- 6. Wyshak G. Teenaged girls, carbonated beverage consumption, and bone fractures. Arch Pediatr Adolesc Med. 2000;154(6):610–613. [DOI] [PubMed] [Google Scholar]
- 7. Goulding A, Rockell JE, Black RE, et al. Children who avoid drinking cow's milk are at increased risk for prepubertal bone fractures. J Am Diet Assoc. 2004;104(2):250–253. [DOI] [PubMed] [Google Scholar]
- 8. Yeh FJ, Grant AM, Williams SM, et al. Children who experience their first fracture at a young age have high rates of fracture. Osteoporos Int. 2006;17(2):267–272. [DOI] [PubMed] [Google Scholar]
- 9. James JR, Massey PA, Hollister AM, et al. Prevalence of hypovitaminosis D among children with upper extremity fractures. J Pediatr Orthop. 2013;33(2):159–162. [DOI] [PubMed] [Google Scholar]
- 10. Goulding A. Risk factors for fractures in normally active children and adolescents. Med Sport Sci. 2007;51:102–120. [DOI] [PubMed] [Google Scholar]
- 11. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winzenberg T, Jones G. Vitamin D and bone health in childhood and adolescence. Calcif Tissue Int. 2013;92(2):140–150. [DOI] [PubMed] [Google Scholar]
- 14. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine, National Academy of Sciences Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 15. Maguire JL, Birken CS, Khovratovich M, et al. Modifiable determinants of serum 25-hydroxyvitamin D status in early childhood: opportunities for prevention. JAMA Pediatr. 2013;167(3):230–235. [DOI] [PubMed] [Google Scholar]
- 16. Carsley S, Borkhoff CM, Maguire JL, et al. Cohort profile: the applied research group for kids (TARGet Kids!). Int J Epidemiol. 2015;44(3):776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carter GD, Carter R, Jones J, et al. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem. 2004;50(11):2195–2197. [DOI] [PubMed] [Google Scholar]
- 18. Statistics Canada Canadian Community Health Survey. http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurveyandSDDS=3226. Published November 24, 2014. Modified April 20, 2015. Accessed April 22, 2016.
- 19. Health Canada Eating well with Canada's food guide. http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/order-commander/eating_well_bien_manger-eng.php. Published 2011. Modified November 18, 2011. Accessed April 22, 2016.
- 20. Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007–2010. Vital Health Stat. 2012;11(252):1–48. [PubMed] [Google Scholar]
- 21. Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–871. [DOI] [PubMed] [Google Scholar]
- 22. Wilkins R. PCCF+ Version 5E User's Guide. Automated Geographic Coding Based on the Statistics Canada Postal Code Conversion Files; Including Postal Codes through March 2009 Ottawa, Ontario, Canada: Statistics Canada; 2010. [Google Scholar]
- 23. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer Publishing Company; 2000. [Google Scholar]
- 24. van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2000;45(3):1–67. [Google Scholar]
- 25. Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding, et al. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. [DOI] [PubMed] [Google Scholar]
- 26. Ryan LM, Brandoli C, Freishtat RJ, et al. Prevalence of vitamin D insufficiency in African American children with forearm fractures: a preliminary study. J Pediatr Orthop. 2010;30(2):106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schilling S, Wood JN, Levine MA, et al. Vitamin D status in abused and nonabused children younger than 2 years old with fractures. Pediatrics. 2011;127(5):835–841. [DOI] [PubMed] [Google Scholar]
- 28. Ceroni D, Anderson de la Llana R, Martin X, et al. Prevalence of vitamin D insufficiency in Swiss teenagers with appendicular fractures: a prospective study of 100 cases. J Child Orthop. 2012;6(6):497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ryan LM, Teach SJ, Singer SA, et al. Bone mineral density and vitamin D status among African American children with forearm fractures. Pediatrics. 2012;130(3):e553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma D, Jones G. Soft drink and milk consumption, physical activity, bone mass, and upper limb fractures in children: a population-based case-control study. Calcif Tissue Int. 2004;75(4):286–291. [DOI] [PubMed] [Google Scholar]
- 31. Wyshak G, Frisch RE. Carbonated beverages, dietary calcium, the dietary calcium/phosphorus ratio, and bone fractures in girls and boys. J Adolesc Health. 1994;15(3):210–215. [DOI] [PubMed] [Google Scholar]
- 32. Konstantynowicz J, Nguyen TV, Kaczmarski M, et al. Fractures during growth: potential role of a milk-free diet. Osteoporos Int. 2007;18(12):1601–1607. [DOI] [PubMed] [Google Scholar]
- 33. Sahni S, Mangano KM, Tucker KL, et al. Protective association of milk intake on the risk of hip fracture: results from the Framingham Original Cohort. J Bone Miner Res. 2014;29(8):1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cumming RG, Klineberg RJ. Case-control study of risk factors for hip fractures in the elderly. Am J Epidemiol. 1994;139(5):493–503. [DOI] [PubMed] [Google Scholar]
- 35. Feskanich D, Bischoff-Ferrari HA, Frazier AL, et al. Milk consumption during teenage years and risk of hip fractures in older adults. JAMA Pediatr. 2014;168(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, et al. Milk intake and risk of hip fracture in men and women: a meta-analysis of prospective cohort studies. J Bone Miner Res. 2011;26(4):833–839. [DOI] [PubMed] [Google Scholar]
- 37. Zamora SA, Rizzoli R, Belli DC, et al. Vitamin D supplementation during infancy is associated with higher bone mineral mass in prepubertal girls. J Clin Endocrinol Metab. 1999;84(12):4541–4544. [DOI] [PubMed] [Google Scholar]