Abstract
The conjoint association of gestational diabetes mellitus (GDM) and gestational hypertension (GH) with cardiometabolic disease has not been well studied. We evaluated a combined GDM/GH risk indicator in both mothers and fathers because of shared spousal behaviors and environments. In the present population-based retrospective cohort study, GH was identified in matched pairs of mothers with GDM or without GDM (matched on age group, health region, and year of delivery) who had singleton live births in Quebec, Canada (1990–2007). A total of 64,232 couples were categorized based on GDM/GH status (neither, either, or both). Associations with diabetes, hypertension, and a composite of cardiovascular disease (CVD) and mortality were evaluated using Cox proportional hazard models (from 12 weeks postpartum to March 2012). Compared with having neither GDM nor GH, having either was associated with incident diabetes (hazard ratio (HR) = 14.7, 95% confidence interval (CI): 12.9, 16.6), hypertension (HR = 1.9, 95% CI: 1.8, 2.0), and CVD/mortality (HR = 1.4, 95% CI: 1.2, 1.7). We found associations of greater magnitude among participants who had both (for diabetes, HR = 36.9, 95% CI: 26.0, 52.3; for hypertension, HR = 5.7, 95% CI: 4.9, 6.7; and for CVD/mortality, HR = 2.4, 95% CI: 1.6, 3.5). Associations with diabetes were also observed in fathers (for either, HR = 1.2, 95% CI: 1.1, 1.3; for both, HR = 1.8, 95% CI: 1.4, 2.3). In conclusion, we found associations of a combined GDM/GH indicator with cardiometabolic disease in mothers and with diabetes in fathers, with stronger associations when both GDM and GH were present.
Keywords: cardiometabolic disease, cardiovascular disease, diabetes, gestational diabetes, gestational hypertension, hypertension, spousal concordance
Editor’s note: An invited commentary on this article appears on page 1125, and the authors’ response appears on page 1129.
Globally, gestational diabetes mellitus (GDM) and gestational hypertension (GH) complicate up to 16% and 8% of pregnancies, respectively, impacting a large number of families (1, 2). Although both conditions confer important peripartum risks, they have implications beyond pregnancy. The American Heart Association recommends long-term surveillance and management of cardiovascular disease (CVD) risk factors in women with these pregnancy-related complications (3, 4). Women with GDM and GH have a high risk of type 2 diabetes mellitus, hypertension and, ultimately, CVD (5). However, the combined associations of GDM and GH with diabetes, hypertension, and CVD has been little-studied. In previous studies of GDM, investigators have adjusted for GH and vice versa; this has the potential to dilute observed associations with outcomes, given that both conditions frequently co-occur and often emerge from a common substrate of excess weight, physical inactivity, and insulin resistance (6).
In a single previous study, GDM alone was associated with a 12-fold higher risk of diabetes; when combined with GH, the risk was 19-fold higher (7). The combined associations of GDM and GH with hypertension and CVD have not previously been evaluated in mothers. This issue also merits attention in fathers. Data from the Framingham Heart Study and the English Longitudinal Study of Aging demonstrate that changes in physical activity level and eating habits in one spouse parallel changes in the other (8, 9). We recently determined that GDM predicts incident diabetes in partners (10). The conjoint association of GDM and GH with diabetes in fathers has not been studied. Given the emphasis that many women place on partner collaboration, shared couple risk is highly relevant because it has the potential to highlight the importance of postpartum monitoring and efforts to change health behaviors after these pregnancy-related complications for both mothers and fathers (11–13).
In the present analyses, we considered a combined indicator of GDM and GH (neither, either, or both). We examined the associations of this indicator with incident diabetes, hypertension, and a composite of CVD and mortality in mothers and in fathers.
METHODS
Study design and construction of cohorts
We performed a retrospective cohort study in Quebec, Canada, which has universal health care. Procedures were approved by the Quebec Access to Information Commission and the Institutional Review Board of the McGill University Health Centre. The health insurance body (Régie de l’Assurance Maladie du Québec; RAMQ) delineated a random sample of 40,000 women 20–44 years of age who, between April 1, 1990, and December 31, 2007, had a singleton live birth with 2 or more outpatient physician billing diagnoses of GDM within 6 months of delivery and/or a GDM diagnosis at hospitalization discharge. Each mother with GDM was matched to a mother without GDM by age group, delivery year, and health administrative territory. Corresponding fathers and offspring were identified. The Quebec Statistical Institute used a probabilistic exact matching strategy to identify the same mother–father–offspring triads in the birth registry and, when applicable, the death registry (14). We received anonymized data from the RAMQ (15) and Quebec Statistical Institute (16) that included demographic information, pregnancy characteristics, and diagnostic codes from outpatient and inpatient visits for the 3 years before the index delivery until March 31, 2012. Cohorts were constructed using SAS/STAT, version 9.4 (SAS Institute, Inc., Cary, North Carolina 2013).
When a participant fulfilled an exclusion criterion, both the partner and the matched couple were excluded. Exclusion criteria were absence of recorded gestational age of infant, 1 or more diagnoses of diabetes in either the father (3 years before delivery) or mother (3 years before estimated conception based on gestational age at delivery) and/or 1 or more diagnoses of hypertension in either the father (2 years before 12 weeks postdelivery) or mother (2 years before 22 weeks’ gestation). Death before postdelivery discharge was also an exclusion criterion.
Exposures
We classified GDM and GH status as neither, either, or both. For confidentiality, we were provided with the month and year of delivery but not the day; therefore, we considered the midmonth date as the delivery date. GH is diagnosed after 20 weeks’ gestation and resolves by 12 weeks postpartum. Hypertension before this is considered to be pre-existing (17). Given the birth date approximation, we defined GH as any hypertension diagnostic code recorded between 22 weeks’ gestation and 12 weeks postpartum.
Participant characteristics at baseline
We characterized mothers and fathers in terms of age group, comorbid conditions, cohabitation at offspring birth, ethnocultural background, socioeconomic status, and number of prior pregnancies with the partner. We defined the reference ethnocultural group (“Caucasian”) as those born in North America, Europe, or Australia whose first language was of European origin. We used the Institut National de Santé Publique du Québec material deprivation index, a quintile ranking derived from census dissemination area–level scores based on proportion without high school diploma, employment/population ratio, and average income (18).
We considered delivery before 37 weeks’ gestation to be preterm. Neonates were categorized as small (weight <10th percentile), appropriate (weight 10th–90th percentile), or large (weight >90th percentile) for gestational age (19). Preterm or small-for-gestational-age infant status has been associated with higher postpartum blood pressures and rates of CVD in mothers, likely because of vascular risk factors that lead to placental insufficiency, such as smoking (20–22). Higher waist circumference and glucose levels have been shown to occur in mothers with infants who were large for gestational age (LGA) (20).
Outcome measures
Using validated definitions, incident diabetes and hypertension were each defined as 2 or more outpatient diagnoses at least 2 months apart or 1 hospitalization discharge diagnosis within a 2-year period (23, 24). The composite CVD and mortality outcome was defined as 1 hospital discharge diagnosis or procedure code for coronary artery disease, coronary artery bypass graft, angioplasty, stroke, carotid endarterectomy, or all-cause mortality (25, 26). Codes from the International Classification of Diseases (Ninth and Tenth Revisions) that were used are reported in Appendix Table 1.
Statistical analysis
Analyses were conducted using R, version 3.2.5 (R Foundation for Statistical Computing, Vienna, Austria). We examined baseline characteristics stratified by GDM and GH status. Disease incidence was examined from 12 weeks postpartum to departure from Quebec, death, or March 31, 2012, whichever came first. For all groups, we computed incidence rates for diabetes, hypertension, and CVD combined with all-cause mortality and plotted Kaplan Meier curves.
We computed hazard ratios for diabetes, hypertension, and CVD/mortality in mothers and fathers using stratified Cox proportional hazards models (for GDM and GH: either vs. neither and both vs. neither). Variables included in the models were age group (in father models only; this variable was a matching criterion for mothers), preterm delivery, infant’s size at delivery, previous pregnancy with same partner, cohabitation at time of delivery, hospitalization in the 3 years before delivery, history of psychiatric disease, history of airway disease, deprivation index score, and ethnicity. History of psychiatric disease and airway disease were included in the models because these diagnoses were identified in more than 5% of our cohort before delivery.
In sensitivity analyses, we explored the effects of varying definitions of GH (beginning at 18 or 20 weeks’ gestation instead of 22 and ending 6 weeks postpartum instead of 12). We modeled the risks of GDM and GH separately, with and without an interaction term. We also analyzed GH with and without preeclampsia separately. We considered incident diabetes and hypertension to lie along the pathway to CVD and thus did not adjust for diabetes and hypertension when examining CVD and mortality outcomes. In addition to the matched analyses, we performed unmatched analyses in which we retained couples even if the matched pair had been excluded.
RESULTS
Participant characteristics
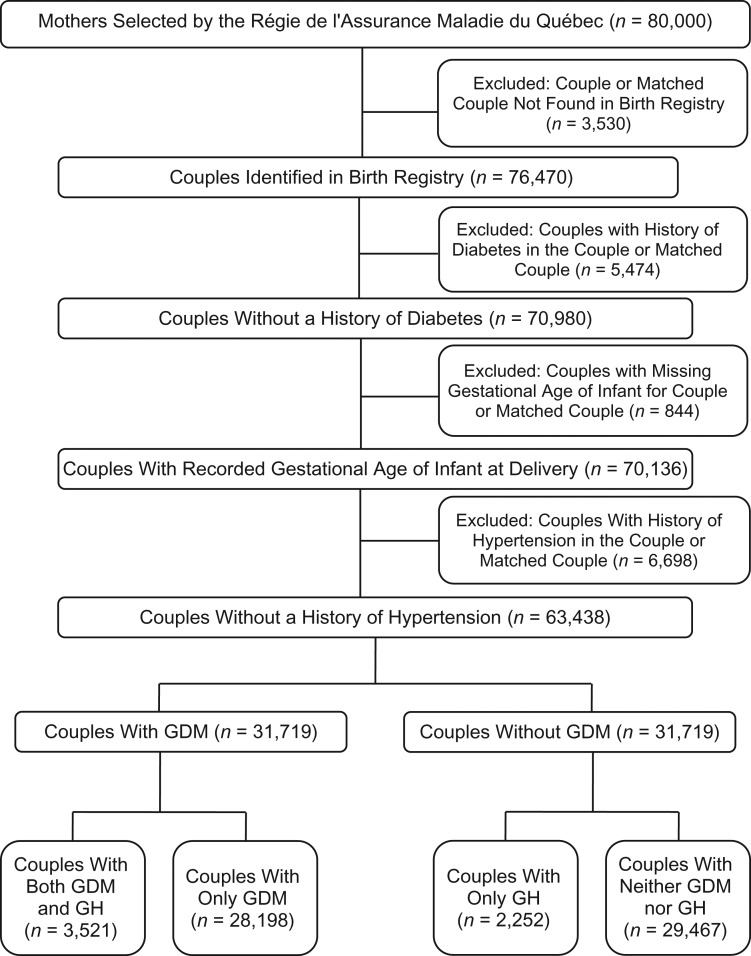
Among the 80,000 couples selected at the RAMQ, 76,470 couples were identified in the birth registry. After exclusions (Figure 1), 63,438 couples were retained. Overall, 44.4% (28,198) of mothers had GDM alone (i.e., a high proportion by design), 3.5% (2,252) had GH alone, and 5.6% (3,521) had both GDM and GH (Table 1). Approximately half of the mothers were younger than 30 years of age, and the remainder were 30–39 years old. Roughly one-third of fathers were younger than 30 years of age and over half were 30–39 years old. There was a stepwise increase in prematurity and LGA across the neither, either, and both categories. More than 90% of couples were living together and were from the same ethnocultural background. Higher deprivation levels were slightly more common in those with GDM and/or GH. Approximately one-fifth of the cohort was non-Caucasian. The either category had the highest proportion of non-Caucasians, mothers hospitalized in the 3 years before delivery, and previous pregnancies with the same partner.
Figure 1.
Flow chart showing participant inclusion, Quebec, Canada, 1990–2007. We included matched pairs with and without gestational diabetes mellitus (GDM) who had singleton live births. GH, gestational hypertension with or without preeclampsia.
Table 1.
Couples’ Characteristics at Baseline, by Gestational Diabetes and Gestational Hypertension Status of the Mother, Quebec, Canada, 1990–2007
| Characteristic | Neither GDM or GH (n = 29,467) |
Either GDM or GH (n = 30,450) |
Both GDM and GH (n = 3,521) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Ages of fathers, years | ||||||
| <30 | 9,887 | 33.6 | 9,401 | 30.9 | 1,243 | 35.3 |
| 30–39 | 16,213 | 55.0 | 17,324 | 56.9 | 1,884 | 53.5 |
| ≥40 | 3,367 | 11.4 | 3,725 | 12.2 | 394 | 11.2 |
| Ages of mothers, years | ||||||
| <30 | 13,534 | 45.9 | 13,865 | 45.5 | 1,743 | 49.5 |
| 30–39 | 15,024 | 51.0 | 15,616 | 51.3 | 1,660 | 47.1 |
| ≥40 | 909 | 3.1 | 969 | 3.2 | 118 | 3.4 |
| Preterm delivery | 1,624 | 5.5 | 2,394 | 7.9 | 531 | 15.1 |
| Infants’ sizes at deliverya | ||||||
| Small for gestational age (<10th percentile) | 3,183 | 10.8 | 2,647 | 8.7 | 458 | 13.0 |
| Appropriate for gestational age (10th–90th percentile) | 24,144 | 81.9 | 24,084 | 79.1 | 2,559 | 72.7 |
| Large for gestational age (>90th percentile) | 2,140 | 7.3 | 3,719 | 12.2 | 504 | 14.3 |
| GDM during index pregnancy | 0 | 0 | 28,198 | 92.6 | 3,521 | 100 |
| GH during index pregnancy | 0 | 0 | 2,252 | 7.4 | 3,521 | 100 |
| Previous pregnancy with partner | 8,458 | 28.7 | 15,057 | 49.4 | 1,271 | 36.1 |
| Living with partner at delivery | 26,965 | 91.5 | 28,310 | 93.0 | 3,294 | 93.6 |
| Fathers’ comorbidity indicators | ||||||
| Hospitalization in prior 3 years | 2,800 | 9.5 | 2,909 | 9.6 | 350 | 9.9 |
| Diagnoses in 5% or more | ||||||
| Psychiatric disorders | 2,804 | 9.5 | 2,886 | 9.5 | 332 | 9.4 |
| Airway disease | 2,308 | 7.8 | 2,677 | 8.8 | 292 | 8.3 |
| Mothers’ co-morbidity indicators | ||||||
| Hospitalization in prior 3 years | 5,892 | 20.0 | 10,239 | 33.6 | 963 | 27.4 |
| Diagnoses in 5% or more | ||||||
| Psychiatric disorders | 5,008 | 17.0 | 5,373 | 17.6 | 676 | 19.2 |
| Airway disease | 3,219 | 10.9 | 4,006 | 13.2 | 569 | 16.2 |
| Deprivation indexb | ||||||
| Fathers in the 2 most-deprived quintiles | 11,037 | 37.5 | 13,155 | 43.2 | 1,582 | 44.9 |
| Mothers in the 2 most-deprived quintiles | 11,191 | 38.0 | 13,320 | 43.7 | 1,613 | 45.8 |
| Ethnocultural backgroundc | ||||||
| Non-Caucasian fathers | 5,380 | 18.3 | 7,026 | 23.1 | 675 | 19.2 |
| Non-Caucasian mothers | 5,206 | 17.7 | 6,876 | 22.6 | 642 | 18.2 |
| Ethnocultural group same as partner | 26,799 | 90.9 | 27,868 | 91.5 | 3,222 | 91.5 |
Abbreviations: GDM, gestational diabetes mellitus; GH, gestational hypertension with or without preeclampsia.
a Based on Canadian birth data (17).
b Based on neighborhood-level indicator of material deprivation.
c Based on primary language and country of birth. The ethnocultural groups considered non-Caucasian included South Asian, Southeast Asian, East Asian, West Asian, Afro-Caribbean, Central/South American, and Aboriginal.
Associations with diabetes, hypertension, and CVD/mortality in mothers and fathers
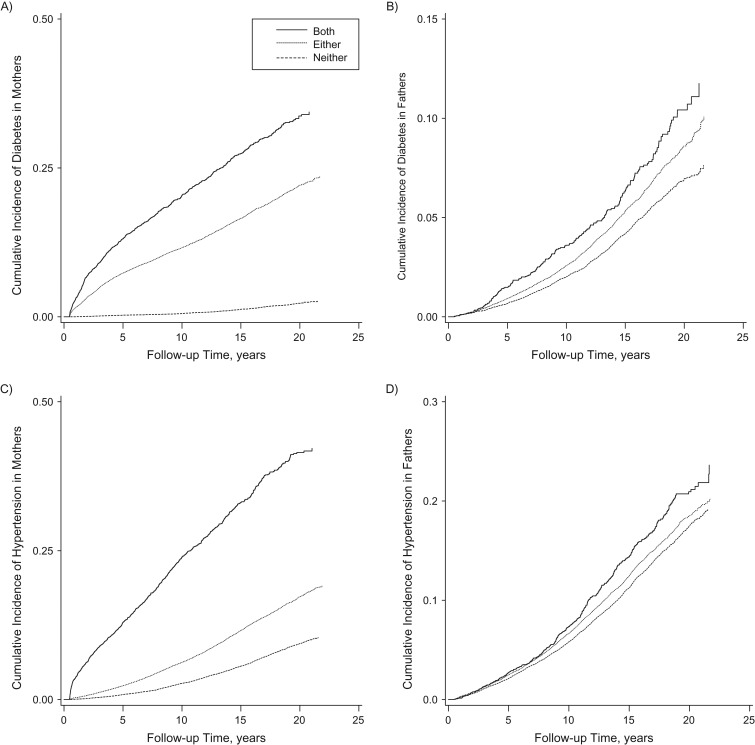
Over a mean of 13.0 (standard deviation, 5.4) years, mothers with GDM, GH, or both conditions had higher incidences of diabetes, hypertension, and CVD/mortality compared with those with neither (Figure 2; Table 2). Incidence rates were higher in those with both GDM and GH compared with those with either. There was a stepwise increase in incidence rates of diabetes and hypertension in fathers across groups, but CVD/mortality rates were comparable across exposure categories.
Figure 2.
Kaplan Meier curves for time to disease diagnosis of diabetes and hypertension in mothers and fathers, stratified by gestational diabetes mellitus and gestational hypertension status (both, either, or neither) of the mother, Quebec, Canada, 1990–2012. The Figure shows cumulative incidence of diabetes in A) mothers and B) fathers and cumulative incidence of hypertension in C) mothers and D) fathers.
Table 2.
Incidence Rates of Diabetes, Hypertension, and a Composite Cardiovascular Disease and Mortality Outcome per 1,000 Person-Years Among Mothers (n = 63,438) and Fathers (n = 63,438) With Neither, Either, or Both Gestational Diabetes and Gestational Hypertension During Index Delivery, Quebec, Canada, 1990–2012
| Parent and Disease | Neither GDM or GH n = 29,467 |
Either GDM or GH n = 30,450 |
Both GDM and GH n = 3,251 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Who Developed Disease | % Who Developed Disease | No. of Cases per 1,000 Person-Years | 95% CI | No. Who Developed Disease | % Who Developed Disease | No. of Cases per 1,000 Person-Years | 95% CI | No. Who Developed Disease | % Who Developed Disease | No. of Cases per 1,000 Person-Years | 95% CI | |
| Mothers | ||||||||||||
| Diabetes mellitus | 349 | 1.2 | 0.9 | 0.8, 1.0 | 4,622 | 15.2 | 12.5 | 12.2, 12.9 | 877 | 24.9 | 22.1 | 20.7, 23.6 |
| Hypertension | 1,527 | 5.2 | 3.9 | 3.7, 4.1 | 3,131 | 10.3 | 8.00 | 7.7, 8.3 | 1,041 | 29.6 | 26.8 | 25.2, 28.5 |
| Cardiovascular disease/mortality | 330 | 1.1 | 0.8 | 0.8, 0.9 | 506 | 1.7 | 1.3 | 1.1, 1.4 | 96 | 3.0 | 2.1 | 1.7, 2.5 |
| Fathers | ||||||||||||
| Diabetes mellitus | 1,106 | 3.8 | 2.9 | 2.7, 3.0 | 1,431 | 4.7 | 3.6 | 3.4, 3.8 | 207 | 5.9 | 4.6 | 4.0, 5.2 |
| Hypertension | 2,999 | 10.2 | 7.9 | 7.6, 8.2 | 3,354 | 11.0 | 8.6 | 8.4, 8.9 | 441 | 12.5 | 9.9 | 9.0, 10.9 |
| Cardiovascular disease/mortality | 1,011 | 3.4 | 2.6 | 2.4, 2.8 | 1,210 | 4.0 | 3.0 | 2.9, 3.2 | 126 | 3.9 | 2.7 | 2.3, 3.2 |
Abbreviations: CI, confidence interval; GDM, gestational diabetes mellitus; GH, gestational hypertension with or without preeclampsia.
Diabetes
The presence of either GDM or GH was associated with diabetes in both mothers and fathers. Specifically, in adjusted models, the hazard ratio was 14.7 in mothers (95% confidence intervals (CI): 12.9, 16.6) and 1.2 in fathers (95% CI: 1.1, 1.3) (Table 3). Hazard ratios rose further with both GDM and GH, to 36.9 in mothers (95% CI: 26.0, 52.3) and 1.8 in fathers (95% CI: 1.4, 2.3). Median time to diagnosis of diabetes was 4.6 years (interquartile range (IQR), 4.1–5.2 years) in mothers with both GDM and GH, 5.3 years (IQR, 2.3–11.0 years) in mothers with either GDM or GH, and 11.2 years (IQR, 5.5–15.0 years) in mothers with neither condition. In fathers, the median time to diagnosis of diabetes was 9.0 years (IQR, 4.9–14.5 years) among those whose partners had both GDM and GH, 10.4 years (IQR, 6.0–14.3 years) among those whose partners had either GDM or GH, and 10.5 years (IQR, 6.4–14.4 years) among those whose partners had neither.
Table 3.
Hazard Ratios for Diabetes, Hypertension, and a Composite Cardiovascular Disease and Mortality Outcome Among Mothers and Fathers, by Gestational Diabetes and Gestational Hypertension Status,a Quebec, Canada, 1990–2007
| GDM/GH Status in Mothera | Mothers | Fathers | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjustedc | Unadjusted | Adjustedc | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Diabetes | ||||||||
| Either | 15.5 | 13.7, 17.3 | 14.7 | 12.9, 16.6 | 1.3 | 1.2, 1.4 | 1.2 | 1.1, 1.3 |
| Both | 41.8 | 29.5, 59.1 | 36.9 | 26.0, 52.3 | 1.7 | 1.4, 2.2 | 1.8 | 1.4, 2.3 |
| Hypertension | ||||||||
| Either | 2.0 | 1.8, 2.1 | 1.9 | 1.8, 2.0 | 1.1 | 1.1, 1.7 | 1.1 | 1.0, 1.2 |
| Both | 6.0 | 5.1, 7.0 | 5.7 | 4.9, 6.7 | 1.2 | 1.0, 1.4 | 1.2 | 1.0, 1.4 |
| Cardiovascular disease/mortality | ||||||||
| Either | 1.5 | 1.3, 2.7 | 1.4 | 1.2, 1.7 | 1.2 | 1.1 1.3 | 1.2 | 1.1, 1.3 |
| Both | 2.3 | 1.6, 3.2 | 2.4 | 1.6, 3.5 | 1.1 | 0.8, 1.4 | 1.1 | 0.8, 1.4 |
Abbreviations: GDM, gestational diabetes mellitus; GH, gestational hypertension with or without preeclampsia.
a Computed using stratified Cox proportional hazard models.
b The reference group was “neither” (no GDM or GH).
c Adjusted for age, gestational age and size of infants at birth, deprivation level, ethnocultural background, co-morbid conditions, prior pregnancy in partner, and living with partner at time of delivery.
Hypertension
The hazard ratio for incident hypertension among mothers with either GDM or GH was nearly double that of mothers without GDM or GH (hazard ratio (HR) = 1.9, 95% CI: 1.8, 2.0) and was close to 6-fold higher in mothers with a history of both GDM and GH (HR = 5.7, 95% CI: 4.9, 6.7). The associations of GDM and/or GH with hypertension in fathers were not conclusive (for either, HR = 1.1, 95% CI: 1.0, 1.2; for both, HR = 1.2, 95% CI: 1.0, 1.4). Median time to diagnosis of hypertension was 6.0 years (IQR, 2.3–10.2 years) in mothers with both GDM and GH, 9.3 years (IQR, 5.4–13.6 years) in mothers with either GDM or GH, and 10.8 years (IQR, 6.9–14.6 years) in mothers with neither GDM nor GH. In fathers, the median time to diagnosis of hypertension was 9.8 years (IQR, 5.7–13.5 years) among those whose partners had both GDM and GH, 9.4 years (IQR, 5.4–13.4 years) among those whose partners had either GDM or GH, and 9.9 years (IQR, 5.6–14.0 years) among those whose partners had neither GDM or GH.
CVD/mortality
In mothers, a history of either GDM or GH resulted in a hazard ratio for the composite CVD and mortality outcome of 1.4 (95% CI: 1.2, 1.7), and a history of both increased the hazard ratio to 2.4 (95% CI: 1.6, 3.5). In fathers, the presence of either GDM or GH in mothers resulted in a hazard ratio of 1.2 (95% CI: 1.1, 1.3). The presence of both GDM and GH in mothers was not conclusively associated with a higher hazard ratio in fathers (HR = 1.1, 95% CI: 0.8, 1.4). Median time to death or diagnosis of CVD was 10.8 years (IQR, 7.6–14.7 years) in mothers with both GDM and GH, 11.4 years (IQR, 6.7–15.2 years) in mothers with either GDM or GH, and 11.5 years (IQR, 6.6–15.1 years) in mothers with neither GDM nor GH. In fathers, the median time to death or diagnosis of CVD was 11.0 years (IQR, 6.2–13.6 years) among those whose partners had both GDM and GH, 10.4 years (IQR, 5.9–14.7 years) among those whose partners had either GDM or GH, and 10.4 years (IQR, 6.3–14.8 years) among those whose partners had neither GDM nor GH.
Sensitivity analyses
In sensitivity analyses, varying GH definitions or conducting an unmatched analysis produced estimates similar to those of the main analyses. The interaction term in models with GDM and GH as independent risk factors was nonsignificant. When preeclampsia and other forms of GH were evaluated separately, the risk estimates were similar to those of the combined GH exposure used in the main analyses.
DISCUSSION
GDM and GH as risk indicators of incident diabetes, hypertension, and CVD/mortality in mothers
Our results demonstrate that having either GDM or GH during pregnancy is associated with postpartum diabetes, hypertension, and CVD/mortality in mothers. A history of both GDM and GH increases the magnitudes of the associations further across these important outcomes. Specifically, a history of either GDM or GH was associated with 15-fold higher risk of postpartum diabetes, and a history of both was associated with a more than 37-fold higher risk. Having either GDM or GH was associated with a doubling of postpartum hypertension risk, and having both was associated with a 6-fold greater risk of postpartum hypertension. With either GDM or GH, there was a 40% risk increase for CVD/mortality in the years after pregnancy and a doubling of risk with both GDM and GH.
It has been demonstrated in meta-analyses that GDM is associated with a 7-fold higher risk of type 2 diabetes in affected mothers, and GH is associated with doubling of postpartum diabetes risk in mothers (5, 27). In a previous study, Feig et al. (7) reported that GDM alone was associated with a 13-fold higher risk of postpartum diabetes, similar to the 15-fold higher risk that we demonstrated with GDM or GH; they also reported a 16-fold increase in diabetes risk among women with GDM and preeclampsia and a 19-fold increase when a pregnancy was complicated by GDM and hypertension (excluding preeclampsia). We report a hazard ratio of 37 for postpartum diabetes in women with a history of GDM and GH versus neither, regardless of the presence or absence of preeclampsia. Although the confidence interval is wide, its lower limit indicates at least a 26-fold higher risk of postpartum diabetes. Unlike in previous studies, our access to data on gestational age permitted us to more precisely apply GH definitions to accurately distinguish among preexisting hypertension, GH, and incident hypertension. Additionally, we used a validated algorithm to identify GDM that differed from the definition in the previous study (28). Nonetheless, both studies indicated a stronger association between pregnancy-related cardiometabolic disease and diabetes when both GDM and GH occurred.
We also determined that in mothers, GDM or GH alone nearly doubled hypertension risk, whereas the presence of both increased the risk by almost 6-fold. In a meta-analysis, investigators reported that preeclampsia was associated with higher risk of postpartum hypertension (HR = 3.7, 95% CI: 2.7, 5.1) (5). GDM has also been identified as a risk factor for hypertension in mothers in observational studies; in a Canadian population, nonoverweight women with GDM had a 2-fold higher risk of chronic hypertension than did women who had normoglycemic pregnancies, and in a Finnish study, there was a comparable signal for increased hypertension in women with GDM (29, 30). To our knowledge, our study is the first in which the joint association of GDM and GH with hypertension in women has been examined and in which it has been demonstrated that the presence of both is associated with a marked increase in risk for the future development of hypertension.
Results from other large population-based studies have demonstrated that a history of GDM or GH increases a woman’s risk of premature vascular disease (5, 31–33). Ours is the first to identify the combined association of these risk factors with CVD/mortality in mothers; we estimated a 1.4-fold increase in CVD/mortality risk among women with either GDM or GH and a 2.4-fold increase in risk among women with both conditions.
We did not have data on maternal weight; however, we were able to determine whether the offspring was LGA, a condition that is driven by prepartum obesity and excess gestational weight gain (20). The proportion of mothers with LGA offspring was lowest among those with neither GDM nor GH (7.3%), higher among those with either GDM or GH (12.2%), and highest among mothers with both conditions (14.3%) (Table 1). Accounting for LGA offspring in our analyses provided some indirect adjustment for maternal weight. Results from previous studies support the association of GDM with diabetes outcomes in both normal-weight and overweight mothers. In a large Finnish cohort study, GDM in normal-weight women was associated with a 10-fold risk increase for diabetes in normal weight women (95% CI: 4.2, 27) and a 47-fold increase in overweight women (95% CI: 25, 87) (30). For the hypertension outcome, there was a trend towards an association in normal-weight mothers with GDM (HR = 1.5, 95% CI: 0.7, 3.2) and a conclusive association in overweight mothers with GDM (HR = 9.2, 95% CI: 6.1, 13.9) (30). In women who did not have GDM, being overweight was associated with a 13-fold risk increase for diabetes (95% CI: 7.4, 21.6) and a 3-fold risk increase for hypertension (95% CI: 2.1, 3.9). Similarly, in a Canadian study, over a median follow-up of 5 years, diabetes incidence was 36% among overweight women with GDM, 18.8% among women with GDM only, 4.8% among overweight women, and 1.1% in the reference group (29). For hypertension and CVD/mortality outcomes, the incidence was similar in subgroups of participants with GDM or who were overweight (14.5%) and higher than in the control group (1.5%). Incidence was highest in those who both had GDM and were overweight (29). Thus, pregnancy-related metabolic complications (i.e., GDM, GH) and overweight conditions have both independent associations with postpartum conditions, as well as important combined effects.
GDM and GH as risk indicators of incident diabetes, hypertension, and CVD/moratlity in fathers
In our large retrospective cohort study, we demonstrated the individual and combined associations of GDM and GH with diabetes incidence in not only mothers but also fathers. Having a partner who had either GDM or GH was associated with a 20% higher risk of diabetes in fathers. If the partner had both conditions, the risk was 80% than that among men whose partners had neither condition. For hypertension, the corresponding risk increases in fathers were inconclusive and lower in magnitude. For CVD/mortality, there was a conclusive 20% higher risk among fathers whose partners had either GDM or GH, but findings were not conclusive for fathers whose partners had both conditions, perhaps because of the smaller sample size.
The totality of our analyses in fathers indicates a need to evaluate, follow, and advise the partners of women with GDM and/or GH. The higher rates of diabetes in fathers may be explained in part by shared eating patterns and physical activity levels between partners forged during the course of the relationship or similarities that existed at the time of union (9). Women with a prior GDM history have a strong desire for partner collaboration to achieve health behavior changes (11–13). Trials of changes in health behaviors have reported indirect “ripple effects” on spouses; in the Look AHEAD (Action for Health in Diabetes) trial, approximately 25% of the spouses of participants in the intensive intervention arm lost 5% or more of baseline weight compared with less than 10% of spouses of participants in the control arm (34). Explicitly aiming for partner collaboration after a GDM and/or GH pregnancy has strong potential for achieving behavior change in both partners.
Strengths and limitations
Our study has limitations. The health administrative definition used could not distinguish between type 1 and type 2 diabetes; however, more than 95% of all cases of diabetes in adults are type 2 diabetes. Women diagnosed with GDM and/or GH may have more interactions with the health-care system than those without such a history. This could lead to more testing and diagnoses of diabetes, hypertension, and CVD. Such a surveillance bias may in part have contributed to both more frequent and earlier diagnoses of the outcomes of interest. However, the risk increase observed in our study was substantial and thus unlikely to be explained by surveillance bias alone. Individuals with non-European ancestry born in North America, Europe, or Australia who reported a European first language were classified in the reference ethnocultural category and thus possibly misclassified, particularly for second-generation immigrants and beyond. However, individuals who reported a European language as their first language likely shared some behaviors with other members of the reference group through acculturation. We did not have data on health behaviors to confirm the mechanisms of higher risks in partners of women with GDM and/or GH. However, prior studies have indicated concordance of health behaviors within couples (8, 9). We did not have access to data on parent weight status but did adjust for the offspring being LGA, which is driven by prepartum obesity and excess gestational weight gain (18). Our analyses extend these findings, demonstrating concordance of diabetes that often results from less than optimal health behaviors. Strengths of our study included a cohort design, a large number of subjects, and indicators of ethnocultural background, deprivation level, cohabitation status, and size and gestational age of infant, which are not typically available in health administrative database studies.
Clinical implications and conclusions
We have demonstrated the utility of jointly considering GDM and GH history when evaluating future risks of cardiometabolic disease. Although these pregnancy-related complications are associated with cardiometabolic disease when evaluated alone, they have important combined associations in mothers. With respect to diabetes and CVD/mortality outcomes, GDM and GH are of relevance in fathers as well. Pregnancy offers an early window of opportunity to engage young mothers and fathers in collaborative efforts to change their future health and prevent chronic disease by focusing on diabetes, hypertension, and CVD risk surveillance and prevention. Our findings clearly signal to patients and clinicians that although GDM and GH alone merit longer-term follow-up and prevention efforts, the urgency is compounded when both occur and the risks and benefits are not limited to the mother.
ACKNOWLEDGMENTS
Author affiliations: Division of Clinical Epidemiology, Department of Medicine, McGill University, Montreal, Quebec, Canada (Romina Pace, Anne-Sophie Brazeau, Elham Rahme, Kaberi Dasgupta); Division of Internal Medicine, Department of Medicine, McGill University, Montreal, Quebec, Canada (Romina Pace, Kaberi Dasgupta); Centre for Outcomes Research and Evaluation, Research Institute of the McGill University Health Centre, Montreal, Quebec, Canada (Anne-Sophie Brazeau, Elham Rahme, Kaberi Dasgupta); and Division of Endocrinology, Department of Medicine, McGill University, Montreal, Quebec, Canada (Sara Meltzer, Kaberi Dasgupta).
This study was conducted with the support of Diabetes Canada and the Lawson Foundation. R.P. was supported by a clinician scientist in training award from the Fonds de Recherche du Québec–Santé. K.D. was supported by a senior clinician scientist award from the Fonds de Recherche du Québec–Santé.
We thank the Quebec Statistical Institute (particularly Isabelle Leroux), the Régie de l’Assurance Maladie du Québec (RAMQ), and their teams for working with us to ensure that data were appropriately selected, collated, and transmitted. This was critical to ensuring a high-quality study. We thank Youssef Habel for merging the data sets we received from the Quebec Statistical Institute and RAMQ. We also thank Hacene Nedjar for his assistance in preparing the graphics.
Conflict of interest: none declared.
Appendix
Appendix Table 1.
Diagnostic Codes From the International Classification of Diseases, Ninth and Tenth Revisions, Used to Identify Outcomes
| Outcome | ICD-9 Codes | ICD-10 Codes |
|---|---|---|
| Diabetes | 250.x | E10–E14 |
| Hypertension | 401.x–405.x | I10–I15 |
| Cardiovascular disease | ||
| Coronary artery diseasea | 410.x, 411.x, 412.x, 413.x, 414.x, 429.2, V4581, V4582 | I20–I25 |
| Strokeb | 431.x,433.x–438.x | G46, I61–I69 |
Abbreviations: ICD-9, International Classification of Diseases, Ninth Revision; ICD-10, International Classification of Diseases, Tenth Revision.
a ICD-9 procedure codes: 480.2, 480.3, 481.x; ICD-10 procedure codes: 1.IJ.50, 1.IJ.54.GQ-AZ, 1.IJ.56,1.IJ.57.GQ.
b ICD-9 procedure codes: 38.12; ICD-10 procedure codes: 03.BK.x, 03.BL.x.
REFERENCES
- 1. Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. [DOI] [PubMed] [Google Scholar]
- 2. Guariguata L, Linnenkamp U, Beagley J, et al. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103(2):176–185. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 4. Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. Circulation. 2011;123(11):1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellamy L, Casas JP, Hingorani AD, et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wen SW, Xie RH, Tan H, et al. Preeclampsia and gestational diabetes mellitus: pre-conception origins? Med Hypotheses. 2012;79(1):120–125. [DOI] [PubMed] [Google Scholar]
- 7. Feig DS, Shah BR, Lipscombe LL, et al. Preeclampsia as a risk factor for diabetes: a population-based cohort study. PLoS Med. 2013;10(4):e1001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357(4):370–379. [DOI] [PubMed] [Google Scholar]
- 9. Jackson SE, Steptoe A, Wardle J. The influence of partner’s behavior on health behavior change: the English Longitudinal Study of Ageing. JAMA Intern Med. 2015;175(3):385–392. [DOI] [PubMed] [Google Scholar]
- 10. Dasgupta K, Ross N, Meltzer S, et al. Gestational diabetes mellitus in mothers as a diabetes predictor in fathers: a retrospective cohort analysis. Diabetes Care. 2015;38(9):e130–e131. [DOI] [PubMed] [Google Scholar]
- 11. Lie ML, Hayes L, Lewis-Barned NJ, et al. Preventing type 2 diabetes after gestational diabetes: women’s experiences and implications for diabetes prevention interventions. Diabet Med. 2013;30(8):986–993. [DOI] [PubMed] [Google Scholar]
- 12. Dasgupta K, Da Costa D, Pillay S, et al. Strategies to optimize participation in diabetes prevention programs following gestational diabetes: a focus group study. PLoS One. 2013;8(7):e67878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nicklas JM, Zera CA, Seely EW, et al. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a history of gestational diabetes. BMC Pregnancy Childbirth. 2011;11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fellegi IP, Sunter AB. A theory for record linkage. J Am Stat Assoc. 1969;64(328):1183–1210. [Google Scholar]
- 15. Régie de l’assurance maladie du Québec Data and statistics [in French]. http://www.ramq.gouv.qc.ca/fr/donnees-et-statistiques/chercheurs-affilies/Pages/chercheurs-affilies.aspx. Accessed March 21, 2017.
- 16. L’Institut de la statistique du Québec Banques de microdonnées. http://www.stat.gouv.qc.ca/produits-services/acces-donnees-recherche/banque-microdonnees.html. Accessed March 21, 2017.
- 17. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. [DOI] [PubMed] [Google Scholar]
- 18. Pampalon R, Hamel D, Gamache P, et al. A deprivation index for health planning in Canada. Chronic Dis Can. 2009;29(4):178–191. [PubMed] [Google Scholar]
- 19. Kramer MS, Platt RW, Wen SW, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108(2):E35. [DOI] [PubMed] [Google Scholar]
- 20. Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125(11):1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Friedlander Y, Manor O, Paltiel O, et al. Birth weight of offspring, maternal pre-pregnancy characteristics, and mortality of mothers: the Jerusalem perinatal study cohort. Ann Epidemiol. 2009;19(2):112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davey Smith G, Hyppönen E, Power C, et al. Offspring birth weight and parental mortality: prospective observational study and meta-analysis. Am J Epidemiol. 2007;166(2):160–169. [DOI] [PubMed] [Google Scholar]
- 23. Leong A, Dasgupta K, Bernatsky S, et al. Systematic review and meta-analysis of validation studies on a diabetes case definition from health administrative records. PLoS One. 2013;8(10):e75256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pace R, Peters T, Rahme E, et al. Validity of health administrative database definitions for hypertension: a systematic review. Can J Cardiol. 2017;33(8):1052–1059. [DOI] [PubMed] [Google Scholar]
- 25. McCormick N, Lacaille D, Bhole V, et al. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One. 2014;9(3):e92286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCormick N, Bhole V, Lacaille D, et al. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One. 2015;10(8):e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. [DOI] [PubMed] [Google Scholar]
- 28. Bowker SL, Savu A, Lam NK, et al. Validation of administrative data case definitions for gestational diabetes mellitus. Diabet Med. 2017;34(1):51–55. [DOI] [PubMed] [Google Scholar]
- 29. Kaul P, Savu A, Nerenberg KA, et al. Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: a population-level analysis. Diabet Med. 2015;32(2):164–173. [DOI] [PubMed] [Google Scholar]
- 30. Pirkola J, Pouta A, Bloigu A, et al. Prepregnancy overweight and gestational diabetes as determinants of subsequent diabetes and hypertension after 20-year follow-up. J Clin Endocrinol Metab. 2010;95(2):772–778. [DOI] [PubMed] [Google Scholar]
- 31. Goueslard K, Cottenet J, Mariet AS, et al. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc Diabetol. 2016;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Archambault C, Arel R, Filion KB. Gestational diabetes and risk of cardiovascular disease: a scoping review. Open Med. 2014;8(1):e1–e9. [PMC free article] [PubMed] [Google Scholar]
- 33. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gorin AA, Wing RR, Fava JL, et al. Weight loss treatment influences untreated spouses and the home environment: evidence of a ripple effect. Int J Obes (Lond). 2008;32(11):1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]