Abstract
The concept of multimorbidity has attracted growing interest over recent years, and more latterly with the publication of specific guidelines on multimorbidity by the National Institute for Health and Care Excellence (NICE). Increasingly it is recognised that this is of particular relevance to practitioners caring for older adults, where multimorbidity may be more complex due to the overlap of physical and mental health disorders, frailty and polypharmacy. The overlap of frailty and multimorbidity in particular is likely to be due to the widespread health deficit accumulation, leading in some cases to functional impairment. The NICE guidelines identify ‘target groups’ who may benefit from a tailored approach to care that takes their multimorbidity into account, and make a number of research recommendations. Management includes a proactive individualised assessment and care plan, which improves quality of life by reducing treatment burden, adverse events, and unplanned or uncoordinated care.
Keywords: multimorbidity, long-term conditions, frailty, older people
Introduction
Multimorbidity is defined by the presence of two or more long-term conditions (LTCs), which are those that cannot currently be cured but can be controlled through medications or other treatments. Multimorbidity increases with both social deprivation and age, with almost a quarter of the UK population affected as a whole and two-thirds of people aged 65 years or over [1]. Compared to those with one or no LTCs, people with multimorbidity have increased risk of functional decline [2, 3], poorer quality of life [4, 5], greater healthcare use [6, 7] and increased mortality [8, 9].
Numerically, most people with multimorbidity are middle-aged, and community-dwelling. Reflecting this, the concept of multimorbidity arose in the context of primary care as a remedy to both a recognised narrow focus on single diseases and as means of underscoring the need for generalism in the management of people, rather than diseases. Much of the multimorbidity research to date has focused on combinations of chronic, frequently synergistic diseases such as diabetes and cardiac disease in primary care.
Geriatricians are accustomed to managing multiple chronic conditions on a regular basis, yet until recently, there has been little to support multimorbidity as an entity in its own right. The publication of guidelines on multimorbidity by the National Institute for Health and Care Excellence (NICE) [10] and supporting editorials in both Age and Ageing [11] and the Journal of the American Geriatrics Society [12] have highlighted its importance to both clinicians and researchers, and emphasised the role of geriatricians and specialist practitioners in its recognition and management. Additionally, the NICE guidelines have contributed to advancing understanding by codifying frailty as a clinical entity in the wider context of multimorbidity.
This review will focus on recent findings in multimorbidity and discuss the interrelationship between multimorbidity and frailty, particularly in older people. Neurodegenerative disease in older people will be highlighted as an exemplar of a target group from the NICE guidelines.
Multimorbidity: applicability to geriatricians
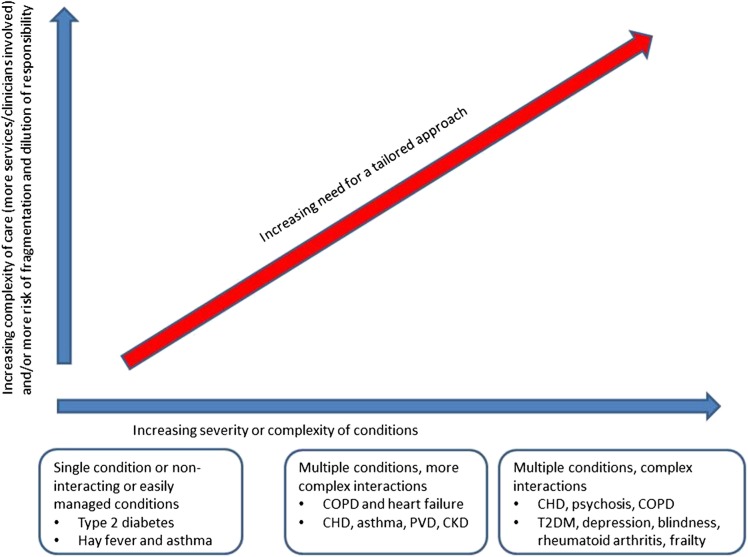
Multimorbidity is of particular relevance to geriatricians, as the number of morbidities and proportion of the population with multimorbidity increases substantially with age [1]. In addition, the complexity of multimorbidity in the context of coexisting conditions such as frailty and dementia, plus associated polypharmacy that is widespread in older adults, has a considerable burden at an individual level, and major implications from a health service, social care and policy perspective (Figure 1).
Figure 1.
Diagram to indicate the need for an approach to care that takes account of multimorbidity [10]. Reproduced with permission National Guideline Centre (2016) Multimorbidity: clinical assessment and management (NG56). Published by the National Guidelines Centre at The Royal College of Physicians, 11 St Andrews Place, Regent's Park, London NW1 4LE. Copyright © NGC. Reproduced by permission.
Prior to the NICE guidelines, a key difficulty in prioritising multimorbidity was that, by definition, it affects approximately 25% of the UK population, which equates to around 15 million people; this is an insurmountably large number to target for intervention [1, 11]. Furthermore, traditional multimorbidity measures such as disease counts [13] or comorbidity indices [14] may fail to characterise the common characteristics of multimorbidity in old age. A key strength of the NICE guidelines is the identification of target groups with multimorbidity who have especially high levels of complexity, so need an approach to care that takes account of multimorbidity (Box 1). The large majority of the target groups are closely aligned with the patient populations seen by geriatricians on a daily basis, e.g. people who are frail in virtue of accumulated deficits that often include combinations of functional impairment, falls, coexisting physical and mental health conditions, and polypharmacy [10, 11, 15].
Box 1. NICE ‘target groups’ of people who may benefit from an approach to care that takes account of their multimorbidity.
they find it difficult to manage their treatments or day-to-day activities;
they receive care and support from multiple services and need additional services;
they have both long-term physical and mental health conditions;
they have frailty or falls;
they frequently seek unplanned or emergency care; and
they are prescribed multiple regular medicines.
As advancing age is associated with increasing multimorbidity, some older people can be particularly challenged to maintain health and well-being with increasing number and severity of individual conditions, and associated frailty [16]. Despite significant levels of disease, however, baseline findings from the Newcastle 85 + Study demonstrated that many 85-year olds rated their health as good or very good [17], highlighting that different disease-related factors may be of particular importance. Multimorbidity may have a greater impact on overall health and well-being in some people if it is in disparate conditions; e.g. conditions affecting physical and mental health compared to closely related comorbidities (such as ischaemic heart disease, hypertension and diabetes); the former encompasses neurodegenerative diseases which disproportionately affect older adults [10].
The majority of older adults have two or more LTCs [1], so use more primary and secondary care services [18] and can expect more fragmented care compared to those with only one or two recognised illnesses [6]. Care home residents are almost universally affected by multimorbidity, with a mean number of chronic conditions found to be 17 in one German study [19]. When compared with those with single chronic conditions, older adults with multimorbidity are at much greater risk of becoming care dependent, with almost a third requiring care home placement over a 5-year period in contrast to a quarter of the non-multimorbid population [20]. Finally in terms of relevance to geriatricians, multimorbidity increases the risk of experiencing intrusive problems such as pain, incontinence, falls, pressure ulcers and delirium. Specific diseases such as Parkinson's disease (PD), cerebrovascular disease and peripheral artery disease appear to be especially problematic coexisting LTCs as they are associated with particularly high levels of these ageing syndromes [21].
The overlap of multimorbidity and frailty
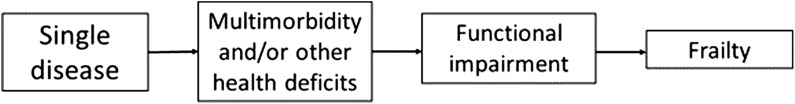
Most older people with frailty have multimorbidity, but the majority of people with multimorbidity are not phenotypically frail [22], despite being at greater risk for adverse health outcomes than their age peers. Although separate concepts, it is apparent that there is a large overlap between frailty and multimorbidity [23], and this is recognised in the recent NICE guidelines [10]. At younger ages, a single disease process characteristically dominates which, over time, can become part of a wider multimorbidity complex. The accumulation of multiple LTCs alongside other health deficits (clinical signs, symptoms, impairments) can lead to the development of frailty, which identifies people at especially high risk of adverse outcomes such as falls, disability, nursing home admission, hospitalisation and mortality [15, 24] (Fig. 2). Frailty can usefully be considered therefore as a method of identifying older people with multimorbidity who are especially vulnerable to a wide range adverse outcomes that are important from an individual and societal perspective.
Figure 2.
Flowchart of the evolution of a single disease process to multimorbidity, functional impairment and finally frailty.
Importantly, established models of frailty such as the phenotype model and cumulative deficit model incorporate aspects of function such as evidence of mobility impairment or activities of daily living, which are not ordinarily incorporated into multimorbidity models. This is especially relevant because functional impairments are often targeted as a core component of evidence-based interventions to improve outcomes for older people with frailty, such as Comprehensive Geriatric Assessment and exercise programmes. Furthermore, evidence indicates that the association between multimorbidity and mortality is lost when adjusted for functional impairment [25]. Taken together, this evidence highlights the critical importance of identifying frailty in older people with multimorbidity as a key method of targeting those who may benefit from provision of interventions to improve outcomes.
The underlying pathophysiology of frailty is characterised by a prominent decrease in physiological reserve and failure of homoeostatic mechanisms that are more pronounced than would be expected from normal ageing [15]. It is proposed that epigenetic mechanisms, in combination with both genetic and environmental factors, generate cumulative molecular and cellular damage, triggering a cascade of further negative insults [15]. At a cellular level, alterations in the neuroendocrine production of insulin-like growth factor-1 (IGF-1) [26], testosterone [27] and cortisol [28] have been implicated in frailty, together with abnormalities of the inflammatory response and associated changes in inflammatory cytokines [15, 29, 30]. Analogously, a recent discussion paper proposed that multimorbidity is the end result of failure of multiple physiological networks interacting with psychosocial and behavioural factors of the individual [31]. These networks include genomic, proteomic, metabolomic, neuroendocrine, immune and bioenergetics processes. Particular emphasis was placed on the autonomic nervous system, the hypothalamic–pituitary–adrenal axis and its subsequent influence on cytokine production, and on mitochondrial function; all of which may have important implications for interventions in multimorbidity [31]. This accumulating evidence has led to a proposed shift away from defining multimorbidity as a simple disease count to reflect a more widespread health deficit accumulation [10, 32].
In view of the overlap between frailty and multimorbidity, the NICE guidelines suggest that we should consider the identification of frailty as one way to identify those with multimorbidity who may benefit from a tailored approach to care [10]. In hospital outpatient settings, the use of self-reported health status, the ‘Timed Up and Go’ test, gait speed, PRISMA-7 questionnaire or self-reported physical activity were all recommended tools for identifying frailty. Although ostensibly pragmatic, the difficulty faced with the use of frailty as a targeting characteristic in the NICE guidelines is that these physical performance measures of frailty are not advocated in acute settings, thereby limiting these instruments for geriatricians working with in-patients.
The use of frailty to target interventions for people with multimorbidity was also prominent in the NICE guideline research recommendations. For example, frailty was identified as one method of identifying a target group for a clinical trial evaluation of a holistic assessment and management intervention for people in the community living with high levels of multimorbidity. Furthermore, frailty was identified as a method of targeting for future randomised controlled trial (RCT) evaluations of deprescribing interventions to reduce treatment burden for people with multimorbidity.
Multimorbidity in LTCs: the example of neurodegenerative disease and multimorbidity
The majority of people with one LTC have multiple LTCs, yet most clinical guidelines focus almost exclusively on single conditions [33]. If guidelines are followed for each comorbidity in older adults, invariably polypharmacy ensues, leading to probable drug–drug interactions and their associated consequences, which may be more pronounced in frailer and/or cognitively impaired people [33, 34]. The preventative effect of each individual drug is likely to be less in those with multiple drug use, and is reduced again in those with a limited life expectancy [33]. Additionally, the evidence for the individual guidelines often comes from younger, fitter patients, as older adults with multimorbidity are often excluded from clinical trials, limiting the generalisability to the typical population seen by practitioners working with older people [35, 36].
Neurodegenerative diseases provide a useful example of the NICE guideline target group of people with multimorbidity living with physical and mental health problems. Parkinson's disease (PD) is the second commonest neurodegenerative disease in economically developed countries and is an example of an age-related multisystem disease encompassing both physical and mental health problems such as dementia [37], mild cognitive impairment (MCI) [38], depression [39] and psychosis [40]. A recent study on the impact of a large number of differing comorbidities on quality of life in people with multimorbidity reported that PD had the most pronounced negative effect [41], whilst additional work in Germany on multimorbidity and long-term care dependency highlighted that the conditions with the highest risk for care home admission were PD and dementia [20]. General polypharmacy is common in PD, with UK primary healthcare data demonstrating that 19.2% of PD patients aged 55 and over are on 10 or more medications compared to 6.2% of controls [MacLean G et al., under review]. The same study showed that compared to 22.9% of controls, only 7.4% of PD subjects had no other comorbidities. Taken together, these studies illustrate that multimorbidity in PD also has a significant impact on healthcare expenditure in terms of institutionalisation and medication use, with evidence that excess expenditures associated with PD are at least partly driven by coexisting LTCs [42]. Moreover, there is evidence that multimorbidity may also drive disease progression or complications in PD. One study found evidence for a small negative effect of cardiovascular and diabetes comorbidities on cognition, which was independent of age, PD duration and severity, and medication use [43]. In the general (non-PD) population, multimorbidity has been associated with greater longitudinal risk of MCI or dementia [44], and can accelerate cognitive decline in those with underlying dementia [45].
Managing multimorbidity
Familiar to geriatricians, the NICE guidelines advocate an approach to care that takes account of multimorbidity, involving a personalised assessment and the development of an individualised management plan [10]. The aim is to improve quality of life by reducing treatment burden, adverse events and unplanned or uncoordinated care. The approach takes account of a person's individual needs, preferences for treatments, health priorities and lifestyle. It aims to improve coordination of care across services, particularly if this has become fragmented. This approach should be considered if the person requests it or if any of the issues apply as outlined in Box 1 [10].
The NICE guidelines expand on a previous approach detailed by the American Geriatrics Society (AGS) [46]. The guidelines encouraged the use of a treatment plan in primary care for older adults with multiple chronic conditions, where consideration had been given to prognosis, treatment/condition interactions, benefits and harms of treatments and subsequent reassessment [46]. Although the AGS and NICE guidelines do not specifically consider situations in which patients are unable to participate in care discussions due to acute severe illness, delirium, dementia or severe frailty, a proactive approach to treatment plan development prior to crises or loss of capacity should be considered good practice.
An additional challenge in the management of multimorbidity is the relative paucity of evidence to date on the effectiveness of interventions to improve outcomes in this construct, in contrast to the more robust evidence available for a Comprehensive Geriatric Assessment in the management of frailty [47]. A recent Cochrane review of interventions in multimorbidity in primary care and community settings highlighted the limited number of RCTs and their mixed results [48]. The interventions were largely focussed on care delivery, which translated into probable improvements in mental health outcomes and patient-reported outcomes, but not into clinical outcomes or health service use. Further intervention studies are required in this area.
A more nuanced approach to multimorbidity management has been highlighted in a recent interesting discussion paper [31]. The authors emphasise the importance of bio-psycho-social factors in the context of physiological dysregulation of the neuroendrocrine, immune and mitochondrial systems. They postulate that future care of the multimorbid patient might include small molecule therapeutics to target the complex molecular and cellular dysfunction, together with environmental factors such as health behaviour, emotional and social support and stress management [31].
Conclusions and future directions
Multimorbidity is a highly prevalent, highly relevant concept for specialists working with older people. Although research has been traditionally centred in primary care, multimorbidity is synonymous with the challenges faced by geriatricians, where the complexity of disease processes and polypharmacy is particularly onerous for individuals, their families and for the health service. While previous research has investigated the cross-sectional overlap between comorbidity and frailty, there is a paucity of research on the temporal relationships between single LTCs, multimorbidity, frailty and disability. Furthermore, there is a paucity of research involving people with neurodegenerative disease who have multiple LTCs, with the exception of limited work in dementia.
The development of a cohesive research strategy on how to meet the needs of older people with multimorbidity is a key priority. In the UK, the National Institute for Health Research has prioritised this research area, indicating ongoing commitment to strengthening the evidence base for older people with complex health needs (http://www.nihr.ac.uk/funding-and-support/themed-calls/). NICE has recommended that key topics for research in this field are the organisation of care, holistic assessment in the community, stopping preventive medicines and predicting life expectancy [10]. These key topic areas require further refinement to align with the priorities of older people, the public and healthcare practitioners; views which are currently actively being sought and prioritised in a James Lind Alliance priority setting partnership (https://research.ncl.ac.uk/jlaprioritysetting/ageingmultimorbidity/).
Future research should focus on these areas, with definitive RCTs of suitably targeted interventions, together with the exploration of putative common underlying physiological process to identify novel therapeutic targets. As geriatricians, we are ideally placed to advance this field of research and ensure that the new horizon in multimorbidity is informed by a robust evidence base that is aligned with the complex health needs of older people.
Key points.
Multimorbidity is defined by two or more long-term conditions and increases with age.
A considerable overlap can be seen between frailty and multimorbidity.
The recent National Institute for Health and Care Excellence (NICE) guidelines on multimorbidity highlight target groups who require an individualised assessment and care plan.
The complexity of multimorbidity in the context of frailty, dementia and polypharmacy bears a substantial healthcare burden.
Acknowledgements
The research was supported by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding
None.
Conflict of interest
Kenneth Rockwood is President and Chief Science Officer of DGI Clinical, which has contracts with pharma on individualised outcome measurement. In July 2015 he gave a lecture at the Alzheimer Association International Conference in a symposium sponsored by Otsuka and Lundbeck. At that time he presented at an Advisory Board meeting for Nutricia. In 2017 he attended an advisory board meeting for Lundbeck. He is a member of the Research Executive Committee of the Canadian Consortium on Neurodegeneration in Aging, which is funded by the Canadian institutes of Health Research, with additional funding from the Alzheimer Society of Canada and several other charities, as well as from Pfizer Canada and Sanofi Canada. He receives career support from the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research, and research support from the Nova Scotia Health Research Foundation, the Capital Health Research Fund and the Fountain Family Innovation Fund of the Nova Scotia Health Authority Foundation. The other authors have no conflicts of interest to report.
References
- 1. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380: 37–43. [DOI] [PubMed] [Google Scholar]
- 2. Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011; 10: 430–9. [DOI] [PubMed] [Google Scholar]
- 3. Marengoni A, von Strauss E, Rizzuto D, Winblad B, Fratiglioni L. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J Intern Med 2009; 265: 288–95. [DOI] [PubMed] [Google Scholar]
- 4. Fortin M, Bravo G, Hudon C, Lapointe L, Almirall J, Dubois MF et al. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual Life Res 2006. Feb; 15: 83–91. [DOI] [PubMed] [Google Scholar]
- 5. Tyack Z, Frakes KA, Barnett A, Cornwell P, Kuys S, McPhail S. Predictors of health-related quality of life in people with a complex chronic disease including multimorbidity: a longitudinal cohort study. Qual Life Res 2016; 25: 2579–92. [DOI] [PubMed] [Google Scholar]
- 6. Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2011; 61: e12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med 2002; 162: 2269–76. [DOI] [PubMed] [Google Scholar]
- 8. Gijsen R, Hoeymans N, Schellevis FG, Ruwaard D, Satariano WA, van den Bos GAM. Causes and consequences of comorbidity: a review. J Clin Epidemiol 2001; 54: 661–74. [DOI] [PubMed] [Google Scholar]
- 9. Nunes BP, Flores TR, Mielke GI, Thume E, Facchini LA. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriat 2016; 67: 130–8. [DOI] [PubMed] [Google Scholar]
- 10. NICE Multimorbidity: Clinical Assessment and Management. CG56, 2016. https://wwwniceorguk/guidance/ng56.
- 11. Stott DJ, Young J. ‘Across the pond’-a response to the NICE guidelines for management of multi-morbidity in older people. Age Ageing 2017. 10.1093/ageing/afx031. [DOI] [PubMed] [Google Scholar]
- 12. Applegate WB. Across the pond. J Am Geriatr Soc 2017. 10.1111/jgs.14803). [DOI] [PubMed] [Google Scholar]
- 13. O’Halloran J, Miller GC, Britt H. Defining chronic conditions for primary care with ICPC-2. Fam Pract 2004; 21: 381–6. [DOI] [PubMed] [Google Scholar]
- 14. Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic co-morbidity in longitudinal-studies—development and validation. J Chron Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 15. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nutzel A, Dahlhaus A, Fuchs A, Gensichen J, Konig HH, Riedel-Heller S et al. Self-rated health in multimorbid older general practice patients: a cross-sectional study in Germany. BMC Fam Pract 2014; 15: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collerton J, Davies K, Jagger C, Kingston A, Bond J, Eccles MP et al. Health and disease in 85 year olds: baseline findings from the Newcastle 85+cohort study. Br Med J 2009; 339: b4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palladino R, Lee JT, Ashworth M, Triassi M, Millett C. Associations between multimorbidity, healthcare utilisation and health status: evidence from 16 European countries. Age Ageing 2016; 45: 431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akner G. Analysis of multimorbidity in individual elderly nursing home residents. Arch Gerontol Geriatr 2009; 49: 413–9. [DOI] [PubMed] [Google Scholar]
- 20. Koller D, Schon G, Schafer I, Glaeske G, van den Bussche H, Hansen H. Multimorbidity and long-term care dependency—a five-year follow-up. BMC Geriatr 2014; 14: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vetrano DL, Foebel AD, Marengoni A, Brandi V, Collamati A, Heckman GA et al. Chronic diseases and geriatric syndromes: the different weight of comorbidity. Eur J Intern Med 2016; 27: 62–7. [DOI] [PubMed] [Google Scholar]
- 22. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M56. [DOI] [PubMed] [Google Scholar]
- 23. Villacampa-Fernandez P, Navarro-Pardo E, Tarin JJ, Cano A. Frailty and multimorbidity: two related yet different concepts. Maturitas 2017; 95: 31–5. [DOI] [PubMed] [Google Scholar]
- 24. Rockwood K, Song XW, MacKnight C, Bergman H, Hogan DB, McDowell I et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005; 173: 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. St John PD, Tyas SL, Menec V, Tate R. Multimorbidity, disability, and mortality in community-dwelling older adults. Can Fam Physician 2014; 60: E272–E80. [PMC free article] [PubMed] [Google Scholar]
- 26. Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res 2004; 16: 153–7. [DOI] [PubMed] [Google Scholar]
- 27. Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TTL, Barrett-Connor E et al. Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. J Clin Endocr Metab 2009; 94: 3806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci 2008; 63: 190–5. [DOI] [PubMed] [Google Scholar]
- 29. Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med 2009; 13: 3103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev 2012; 133: 456–66. [DOI] [PubMed] [Google Scholar]
- 31. Sturmberg JP, Bennett JM, Martin CM, Picard M. ‘Multimorbidity’ as the manifestation of network disturbances. J Eval Clin Pract 2017; 23: 199–208. [DOI] [PubMed] [Google Scholar]
- 32. Melis RJF, Gijzel SMW, Rikkert MGMO. Moving beyond multimorbidity as a simple count of diseases. J Eval Clin Pract 2017; 23: 216–8. [DOI] [PubMed] [Google Scholar]
- 33. Guthrie B, Payne K, Alderson P, McMurdo MET, Mercer SW. Adapting clinical guidelines to take account of multimorbidity. Br Med J 2012; 345: e6341. [DOI] [PubMed] [Google Scholar]
- 34. Hughes LD, McMurdo MET, Guthrie B. Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Ageing 2013; 42: 62–9. [DOI] [PubMed] [Google Scholar]
- 35. McMurdo MET, Witham MD, Gillespie ND. Including older people in clinical research—benefits shown in trials in younger people may not apply to older people. Br Med J 2005; 331: 1036–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Spall HGC, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals—a systematic sampling review. J Am Med Assoc 2007; 297: 1233–40. [DOI] [PubMed] [Google Scholar]
- 37. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008; 23: 837–44. [DOI] [PubMed] [Google Scholar]
- 38. Yarnall AJ, Breen DP, Duncan GW, Khoo TK, Coleman S, Firbank MJ et al. Characterizing mild cognitive impairment in incident Parkinson's disease: The ICICLE-PD Study. Neurology 2014; 82: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aarsland D, Pahlhagen S, Ballard CG, Ehrt U, Svenningsson P. Depression in Parkinson disease—epidemiology, mechanisms and management. Nat Rev Neurol 2012. Jan; 8: 35–47. [DOI] [PubMed] [Google Scholar]
- 40. Fenelon G, Alves G. Epidemiology of psychosis in Parkinson's disease. J Neurol Sci 2010; 289: 12–7. [DOI] [PubMed] [Google Scholar]
- 41. Brettschneider C, Leight L, Bickel H, Dahlhaus A, Fuchs A, Gensichen J et al. Relative impact of multimorbid chronic conditions on health-related quality of life—results from the MultiCare Cohort Study. PLoS One 2013; 8: e66742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bhattacharjee S, Sambamoorthi U. Co-occurring chronic conditions and healthcare expenditures associated with Parkinson's disease: a propensity score matched analysis. Parkinsonism Relat Disord 2013; 19: 746–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jones JD, Malaty I, Price CC, Okun MS, Bowers D. Health comorbidities and cognition in 1948 patients with idiopathic Parkinson's disease. Parkinsonism Relat Disord 2012; 18: 1073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vassilaki M, Aakre JA, Cha RH, Kremers WK, St Sauver JL, Mielke MM et al. Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc 2015; 63: 1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Melis RJF, Marengoni A, Rizzuto D, Teerenstra S, Kivipelto M, Angleman SB et al. The Influence of multimorbidity on clinical progression of dementia in a population-based cohort. PLoS One 2013; 8: e84014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ickowicz E, Panel AGSE. Patient-centered care for older adults with multiple chronic conditions: a stepwise approach from the American Geriatrics Society. J Am Geriatr Soc 2012; 60: 1957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ellis G, Whitehead MA, Robinson D, O’Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. Br Med J 2011; 343: d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith SM, Wallace E, O’Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2016; 3: CD006560. [DOI] [PMC free article] [PubMed] [Google Scholar]