Abstract
We assessed the association between recent bacterial vaginosis (BV) and incident Mycoplasma genitalium, a sexually transmitted bacterium associated with adverse female reproductive health outcomes. Female sex workers in Mombasa, Kenya, completed a monthly sexual behavior interview and clinical examination. During February 2005–February 2006, vaginal fluid specimens collected from women every other month were tested for M. genitalium by nucleic acid amplification testing. Vaginal microbiota were assessed monthly and categorized by Nugent score (0–3 = normal microbiota, 4–6 = intermediate microbiota disruption, and 7–10 = BV). A discrete failure time analysis for multiple events using logistic regression was employed to estimate the odds of incident M. genitalium infection at follow-up visits among women with BV (vs. normal microbiota) at the preceding visit. Among the 280 women, 54.3% were positive for human immunodeficiency virus. At baseline, 16.1% had prevalent M. genitalium infection and 40.4% had prevalent BV. There were 59 incident M. genitalium infections among 50 women, for an incidence rate of 34.6 cases per 100 person-years. Following adjustment for age, human immunodeficiency virus status, and time, prior BV was associated with a 3.5-fold increase in odds of incident M. genitalium (adjusted odds ratio = 3.49, 95% confidence interval: 1.86, 6.56). This strong association suggests that BV may enhance susceptibility to M. genitalium infection.
Keywords: bacterial vaginosis, Mycoplasma genitalium, Nugent score, vaginal microbiota
Mycoplasma genitalium, a sexually transmitted bacterium, has been associated with increased risk of adverse female reproductive health outcomes, including mucopurulent cervicitis, pelvic inflammatory disease, infertility, and preterm birth (1), as well as increased risk of human immunodeficiency virus (HIV) infection (2–4). However, little is known about factors that increase susceptibility to M. genitalium infection. Bacterial vaginosis (BV), which is present in approximately 11%–37% of women (5–9), has been associated with increased risk of acquiring other sexually transmitted infections (STIs) (10–16) and may also be associated with increased risk for M. genitalium. However, data are limited and inconsistent, with some studies demonstrating increased risk of M. genitalium infection among women with BV (2, 17, 18), one demonstrating decreased risk (19), and others demonstrating no relationship (20–22).
One potential explanation for conflicting evidence about the association between BV and M. genitalium is inadequate assessment of the temporal relationship. Most prior analyses employed cross-sectional study designs (2, 17, 19–21). Furthermore, in the 2 published longitudinal studies (18, 22), time between BV assessment and M. genitalium testing ranged from several months to over a year. This may not be the correct time frame in which to assess an etiological link, as levels of vaginal microbiota fluctuate over time (23, 24).
A second explanation for these conflicting results may be differing methods of assessing BV, which is typically diagnosed using either clinical (Amsel's) or microbiological (Nugent) criteria. Under the criteria of Amsel et al. (25), BV is defined as 3 of the following 4 signs: 1) thin, homogenous, white vaginal discharge; 2) clue cells on wet mount; 3) amine odor when exposed to a drop of 10% potassium hydroxide; and 4) pH > 4.5. Amsel's criteria, however, fail to capture women who have disrupted vaginal microbiota but do not have clinically detectable signs. In contrast, a Nugent score is derived by scoring Gram-stained vaginal smears based on the quantity of Lactobacillus morphotypes, Gram-variable rods, and curved rods, allowing for assessment of vaginal microbiota in the absence of clinical signs. The Nugent score (26) represents a continuum of increasing disruption of vaginal microbiota, with scores of 0–3 being classified as normal microbiota, scores of 4–6 as intermediate, and scores of 7–10 as BV. Most studies have compared women with BV (score ≥7) to women without BV (score 0–6), which combines the normal and intermediate categories. However, increasing evidence suggests that an intermediate level of microbiota disruption is associated with adverse outcomes (27, 28). Therefore, this categorization may miss the contribution of milder disruptions of the microbiota to adverse outcomes, such as STI acquisition, attenuating estimates of an underlying association.
Given the high prevalence of BV and adverse outcomes associated with M. genitalium, it is critical to determine whether BV enhances a woman's susceptibility to M. genitalium. To address this question, we investigated the association between recent BV and incident M. genitalium infection in a longitudinal study with frequent assessment of the vaginal microbiota using the Nugent score.
METHODS
Study population
Participants were women in the Mombasa Cohort, a prospective open cohort study of female sex workers (FSWs) in Mombasa, Kenya, with ongoing enrollment. The cohort was created in 1993 to identify risk factors for HIV and STI acquisition (29). There is no set follow-up period. Women were enrolled in the cohort if they were at least 16 years of age and reported exchanging sex for cash or in-kind payments.
The Kenyatta National Hospital/University of Nairobi Ethics and Research Committee and the University of Washington Institutional Review Board approved the study protocol. All participants provided written informed consent for participation.
Clinic procedures, specimen collection, and laboratory methods
At enrollment in the cohort and at monthly follow-up visits, women underwent a standardized face-to-face interview eliciting information on demographic and clinical characteristics and sexual behaviors. A clinical examination was performed at each visit, including collection of vaginal and cervical specimens for STI diagnosis. Women with STI symptoms were treated syndromically according to Kenyan national guidelines, which follow the World Health Organization's guidelines for syndromic management (30). Additional treatment for STIs was based on laboratory results. Asymptomatic BV was not treated, as there is currently no indication for treatment in asymptomatic nonpregnant women. HIV-positive women were offered antiretroviral therapy according to Kenyan national guidelines, which recommended treatment for persons with a CD4-positive T-lymphocyte (CD4 cell) count less than 200 cells/mm3 during the study period.
Vaginal swabs were collected for Gram staining and Nugent scoring (26). Cervical swab specimens were inoculated onto modified Thayer-Martin media for Neisseria gonorrhoeae culture. Cervicitis was defined as an average of ≥30 polymorphonuclear leukocytes in 3 high-power microscopic fields on a Gram-stained slide of cervical secretions. Trichomoniasis was diagnosed by detection of motile trichomonads on wet mount microscopy or positive culture of vaginal swab specimens inoculated into Diamond's media. In HIV-negative women, herpes simplex virus type 2 (HSV-2) was detected in serum using the HerpeSelect 2 ELISA immunoglobulin G test (Focus Diagnostics, Cypress, California) (31), with an optical density index value greater than 1.1 indicating positivity. Given the high prevalence of HIV/HSV-2 coinfection in this cohort (32), HIV-positive women did not undergo routine HSV-2 testing. Infection with HIV type 1 (HIV-1) was detected in serum using an enzyme-linked immunosorbent assay (Detect HIV-1/2; BioChem Immunosystems, Montreal, Quebec, Canada) and confirmed using a second enzyme-linked immunosorbent assay (Recombigen; Cambridge Biotech, Worchester, Massachusetts). In HIV-positive women, quantitation of CD4 cell count was performed every 3 months (FACScount; Becton Dickinson, San Jose, California).
Between February 2005 and February 2006, additional vaginal swabs were collected and stored at −80°C. Financial constraints prohibited testing of all specimens; therefore, we randomly sampled women for M. genitalium testing from among those with 2 or more additional vaginal specimens (approximately 1:1 HIV-positive to HIV-negative). Specimens from visits made every other month were shipped to Seattle, Washington, for testing with the Aptima TMA assay (Hologic, Inc., San Diego, California) using analyte-specific reagents for M. genitalium. Because testing was conducted several years after sample collection, women were not specifically treated for M. genitalium.
Measures
Prevalent M. genitalium was defined as a positive M. genitalium test at the baseline study visit. Incident M. genitalium was defined as a positive test at any follow-up visit that had been preceded by a negative M. genitalium test at the most recent prior visit. Persistent M. genitalium was defined as a positive test at more than 1 sequential visit with M. genitalium testing. Time to clearance of incident infections was defined as the number of days from the beginning of infection (midpoint between negative and positive tests) to the end of infection (midpoint between the last positive and first negative tests). Disruption of the vaginal microbiota was characterized by Nugent score (0–3 = normal microbiota (reference group), 4–6 = intermediate microbiota disruption, and 7–10 = BV). BV was considered persistent if it was detected at more than 1 sequential visit and recurrent if there was at least 1 visit with normal or intermediate microbiota between visits with BV.
Statistical methods
Baseline demographic, clinical, and sexual behavior characteristics of women with and without prevalent BV (Nugent scores of ≥7 and <7, respectively) and prevalent M. genitalium were compared using Fisher's exact test for categorical variables and Wilcoxon's rank-sum test for continuous variables to assess statistical significance. M. genitalium incidence and 95% confidence intervals were estimated using Poisson regression with robust standard errors (clustering by woman), allowing for multiple incident infections. Time at risk was defined using the beginning and end of M. genitalium infections as defined above. In sensitivity analyses, we also calculated M. genitalium incidence after excluding women with prevalent infections and censoring women after their first incident infection.
To evaluate the relationship between BV (either new or recurrent) at the visit prior to M. genitalium testing and incident M. genitalium, we employed a discrete failure time analysis for multiple events using logistic regression, clustering by woman (33). Women without M. genitalium at baseline entered the risk set at the first visit with exposure data (BV assessment at prior visit); women with prevalent M. genitalium at baseline entered the risk set at their first negative M. genitalium test. Women with incident infections reentered the risk set after their first negative M. genitalium test. All women were censored after their last M. genitalium test.
The models adjusted a priori for age (years; continuous), HIV-1 status, and time (visit number). Hormonal contraceptive use, presence of other STIs (Trichomonas vaginalis, N. gonorrhoeae), number of sex partners in the last week, frequency of protected and unprotected sex in the last week (no sex, all sex acts protected, some sex acts protected, no acts protected), and receipt of a doxycycline or metronidazole prescription at the prior visit were assessed as potential confounding factors. Variables associated with M. genitalium (P < 0.2) in bivariate models were added to the multivariable model one at a time and subsequently removed if the odds ratio for the association between BV and incident M. genitalium changed by less than 10% (after adjustment for age, HIV status, and time). No additional characteristics changed the estimates appreciably, and none were included in the final model. A term for interaction between BV and HIV-1 status was tested but was not statistically significant (Wald test: P = 0.87) and therefore not retained. We also performed a linear trend test to evaluate the relationship between increasing Nugent score (continuous) and the odds of incident M. genitalium infection. Data were analyzed using Stata 13.0 (StataCorp LP, College Station, Texas). Statistical significance was defined as P < 0.05 (2-sided) for all analyses.
To further investigate the timing of BV episodes, we conducted a secondary analysis using the same discrete failure time analysis approach and categorized women on the basis of their BV status at both the visit with M. genitalium testing and the visit prior to M. genitalium testing. Women without BV at either visit served as the referent group and were compared with 3 groups of women: 1) no BV at the prior visit but BV at the visit with M. genitalium testing; 2) BV at the prior visit but no BV at the visit with M. genitalium testing; and 3) BV at both the visit prior to and the visit with M. genitalium testing. For this analysis, women with intermediate microbiota were excluded.
RESULTS
Baseline
These analyses included 280 women with 2 or more M. genitalium tests during follow-up. The median age of participants was 35.3 years (interquartile range (IQR), 31.3–39.2), and 54.3% (152/280) were HIV-positive (Table 1). At baseline for this study, 40.4% (113/280) of women had BV, 18.2% (51/280) had intermediate microbiota disruption, and 41.4% (116/280) had normal vaginal flora. In addition, 16.1% had M. genitalium infection. Women with BV (Nugent score ≥7) were more likely to be HIV-positive than women without BV (Nugent score 0–6) (63.7% vs. 47.9%; P = 0.01). Similarly, women with M. genitalium were more likely to be HIV-positive (68.9% vs. 51.5%; P = 0.04) and younger (median age, 33.5 years vs. 35.5 years; P = 0.003) than those without M. genitalium. The distributions of all other baseline factors were similar between women with and without M. genitalium and with and without BV.
Table 1.
Baseline Demographic, Clinical, and Sexual Behavior Characteristics of 280 Female Sex Workers Enrolled in a Study of Bacterial Vaginosis and Incident Mycoplasma genitalium Infection Between February 2005 and February 2006, Mombasa, Kenya
| Baseline Characteristic | No. of Women | % | Median (IQR) |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years | 35.3 (31.3–39.2) | ||
| Smoking, cigarettes/weeka | |||
| 0 | 247 | 88.2 | |
| 1–9 | 27 | 9.6 | |
| ≥10 | 6 | 2.1 | |
| Alcohol consumption, drinks/week | |||
| 0 | 61 | 21.8 | |
| 1–7 | 147 | 52.5 | |
| ≥8 | 72 | 25.7 | |
| Clinical characteristicsb | |||
| M. genitalium | 45 | 16.1 | |
| Vaginal microbiota | |||
| Bacterial vaginosis (Nugent score ≥7) | 113 | 40.4 | |
| Intermediate disruption (Nugent score 4–6) | 51 | 18.2 | |
| Normal flora (Nugent score 0–3) | 116 | 41.4 | |
| HIV-1 seropositivity | 152 | 54.3 | |
| CD4 cell count, cells/mm3c | 308 (195–482) | ||
| Initiation of antiretroviral therapy (ever)d | 20 | 13.2 | |
| HSV-2 seropositivity | 148 | 92.5 | |
| Trichomonas vaginalis | 10 | 3.6 | |
| Neisseria gonorrhoeae | 2 | 0.7 | |
| Cervicitise | 10 | 3.6 | |
| Vaginal washing in past week | |||
| None | 8 | 2.9 | |
| Water | 60 | 21.6 | |
| Soap/otherf | 210 | 75.5 | |
| Method of contraception | |||
| None | 185 | 66.3 | |
| Oral contraceptive pills | 19 | 6.8 | |
| Medroxyprogesterone acetate injectable | 53 | 19.0 | |
| Intrauterine device | 2 | 0.7 | |
| Implant | 6 | 2.2 | |
| Other | 14 | 5.0 | |
| Hormonal contraception | 80 | 28.7 | |
| Vaginal itching/burning | 24 | 8.6 | |
| Vaginal discharge | 8 | 2.9 | |
| Sexual behavior | |||
| No. of sex partners in past weekg | 1 (0–1) | ||
| Frequency of sex in past week | 1 (0–2) | ||
| Protected and unprotected sex in past weekh | |||
| No sex | 128 | 45.7 | |
| All sex acts protected | 104 | 37.1 | |
| Some sex acts protected | 9 | 3.2 | |
| No sex acts protected | 39 | 13.9 | |
Abbreviations: HIV, human immunodeficiency virus; HIV-1, human immunodeficiency virus type 1; HSV-2, herpes simplex virus type 2; IQR, interquartile range.
a Participants were asked about smoking and alcohol use at enrollment in the Mombasa female sex worker cohort. Median time from enrollment to baseline for this study was 2.9 years (IQR, 0.82–7.76).
b Baseline data were available for 142 participants for CD4 cell count, for 160 participants for HSV-2, for 279 participants for N. gonorrhoeae infection, method of contraception, and hormonal contraception, and for 278 for cervicitis and vaginal washing in the past week.
c Quantitation of CD4 cell count was performed every 3 months. For HIV-positive participants who did not have a measured CD4 cell count at the baseline of this study (dates of which varied by woman), we carried forward their most recent Mombasa cohort CD4 cell count to baseline.
d HIV-positive participants who had ever initiated antiretroviral therapy (receipt of antiretroviral therapy at the Mombasa clinic or self-report of receipt of antiretroviral therapy at another location) by baseline of this study.
e ≥30 cervical peripheral mononuclear cells.
f “Other” includes detergent and antiseptic solution.
g “Number of partners” includes both regular partners and paying partners.
h “Protected sex” was defined as condom use during the sex act.
Follow-up
Participants contributed 2,454 study visits during 196.9 person-years of observation between February 2005 and February 2006. The median number of visits per woman was 10 (IQR, 5–12), and the median time between visits was 29 days (IQR, 28–34). After excluding time in which women had M. genitalium infections, there were 170.5 person-years at risk for acquiring M. genitalium.
BV was detected at least once in 73.9% (207/280) of women at 38.4% (940/2,448) of visits with BV data. Only 8.4% (79/940) of visits with BV were accompanied by symptoms (vaginal discharge and/or itching), and metronidazole was prescribed at only 2.6% (24/940) of visits with BV. Among women with BV, 55.1% (114/207) had at least 1 recurrence of BV, with a maximum of 4 recurrences.
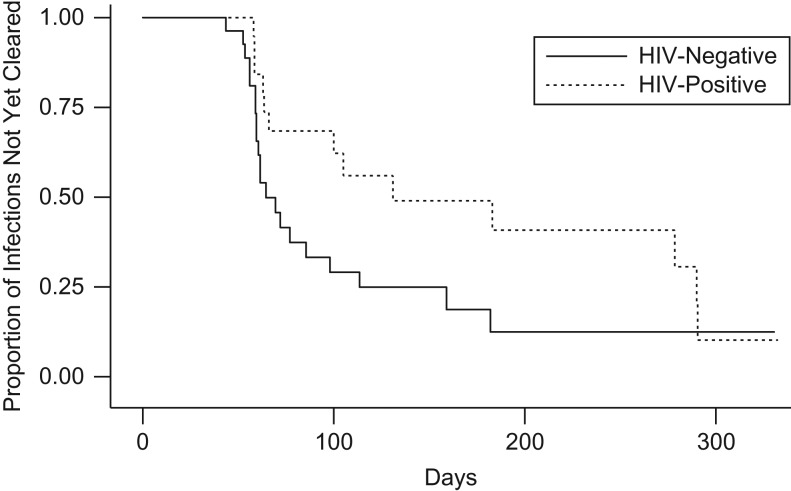
During a median follow-up time of 318.5 days, 50 women experienced an incident M. genitalium infection, and 9 women experienced 2 episodes, for a total of 59 incident infections and an incidence rate of 34.6 per 100 person-years (95% confidence interval (CI): 26.0, 42.0). In sensitivity analyses excluding women with prevalent M. genitalium infections and censoring women after their first incident infection, the incidence rate was 25.0 per 100 person-years (95% CI: 18.2, 34.4). Of the 104 M. genitalium infections (prevalent or incident), 45 (43.3%) were persistent, and the maximum duration of infection was 332.5 days. The median time to clearance of incident M. genitalium infections was 85.5 days (IQR, 59.5–183). Although time to clearance was longer for HIV-positive women, this difference was not statistically significant (Figure 1; log-rank P = 0.09). Doxycycline was prescribed at 2.5% (54/2,174) of follow-up visits for syndromic treatment of STI, but not specifically for M. genitalium.
Figure 1.
Number of days to clearance of incident Mycoplasma genitalium infection by human immunodeficiency virus (HIV) status among female sex workers in Mombasa, Kenya, 2005–2006. The Kaplan-Meier plot includes all 59 incident M. genitalium infections (30 HIV-negative and 29 HIV-positive, allowing for multiple incident infections). Infections were censored at the first negative test (clearance) or at the woman's exit from the study, whichever occurred first. At day 100, 7 HIV-negative and 11 HIV-positive women remained infected and in follow-up. At day 200, 2 HIV-negative and 5 HIV-positive women remained infected and in follow-up. At day 300, only 3 women under follow-up (2 HIV-negative, 1 HIV-positive) had not yet cleared their infection. Fifty percent (25/50) of women with any incident infection had a positive M. genitalium test at their last study visit. Only 5 of the HIV-positive women had baseline CD4 cell counts less than 200 cells/mm3.
Recent BV and incident M. genitalium
In bivariate analysis, compared with women with normal vaginal microbiota (Nugent score 0–3) at the visit prior to M. genitalium testing, BV (Nugent score ≥7) at the preceding visit was associated with a 3.5-fold increase (odds ratio = 3.48, 95% CI: 1.87, 6.48) in the odds of incident M. genitalium infection (Table 2). Intermediate microbiota disruption (Nugent score 4–6) was associated with a 1.7-fold increase in the odds of incident M. genitalium infection (odds ratio = 1.70, 95% CI: 0.69, 4.19), but this was not statistically significant. After adjustment for age, HIV status, and time (visit number), BV at the prior visit remained associated with an increase in the odds of subsequent incident M. genitalium infection (adjusted odds ratio (aOR) = 3.49, 95% CI: 1.86, 6.56). Considering the continuous Nugent score, the odds of incident M. genitalium increased by 16% for each incremental point increase, after adjustment (aOR = 1.16, 95% CI: 1.07, 1.26; P-trend < 0.001).
Table 2.
Association Between Prior Bacterial Vaginosis and Incident Mycoplasma genitalium Infection Among Female Sex Workers in Mombasa, Kenya, 2005–2006
| Nugent Score Category | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Normal (0–3) | 1.00 | Referent | 1.00 | Referent | ||
| Intermediate (4–6) | 1.70 | 0.69, 4.19 | 0.25 | 1.70 | 0.69, 4.18 | 0.25 |
| Bacterial vaginosis (≥7) | 3.48 | 1.87, 6.48 | <0.001 | 3.49 | 1.86, 6.56 | <0.001 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Adjusted for age, human immunodeficiency virus status, and time (visit number). Further adjustment for hormonal contraceptive use, presence of other sexually transmitted infections, number of sex partners in the past week, frequency of protected and unprotected sex in the past week, and receipt of an antibiotic prescription at the prior visit did not appreciably change the estimates and were not included in the final adjusted model.
Secondary analysis
Relative to women with normal microbiota at 2 sequential visits, BV at the prior visit but not at the visit with M. genitalium testing was associated with substantially increased odds of M. genitalium infection (aOR = 4.51, 95% CI: 1.76, 11.6), as was BV at both the prior visit and the visit with M. genitalium testing (aOR = 3.93, 95% CI: 1.87, 8.27), after adjustment (Table 3). In contrast, there was no significant increase in the odds of M. genitalium when BV was identified only at the visit with M. genitalium testing.
Table 3.
Associations Between Prior and Concurrent Bacterial Vaginosis and Incident Mycoplasma genitalium Infection Among Female Sex Workers in Mombasa, Kenya, 2005–2006
| BV Statusa | No. of MG Infections (n = 47)b | % of MG Infections | Unadjusted | Adjustedc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BV at Prior Visit | Concurrent BV | No. of Visits (n = 1,465) | % of Visits | OR | 95% CI | P Value | OR | 95% CI | P Value | ||
| No | No | 684 | 46.7 | 10 | 21.3 | 1.00 | Referent | 1.00 | Referent | ||
| No | Yes | 152 | 10.4 | 5 | 10.6 | 2.23 | 0.74, 6.70 | 0.15 | 2.31 | 0.77, 6.91 | 0.14 |
| Yes | No | 152 | 10.4 | 9 | 19.1 | 4.44 | 1.73, 11.38 | <0.01 | 4.51 | 1.76, 11.6 | <0.01 |
| Yes | Yes | 477 | 32.6 | 23 | 48.9 | 3.76 | 1.81, 7.82 | <0.001 | 3.93 | 1.87, 8.27 | <0.001 |
Abbreviations: BV, bacterial vaginosis; CI, confidence interval; MG, Mycoplasma genitalium; OR, odds ratio.
a BV (Nugent score ≥7) was compared with normal vaginal microbiota (Nugent score 0–3). Visits at which women had intermediate microbiota disruption (Nugent score 4–6) were excluded from this analysis.
b This does not reflect the total number of incident M. genitalium infections (n = 59), since visits at which women had intermediate microbiota disruption were excluded.
c Adjusted for age, human immunodeficiency virus status, and time (visit number). Further adjustment for hormonal contraceptive use, presence of other sexually transmitted infections, number of sex partners in the past week, frequency of protected and unprotected sex in the past week, and receipt of an antibiotic prescription at the prior visit did not appreciably change the estimates and were not included in the final adjusted model.
DISCUSSION
In this cohort of high-risk Kenyan women, the prevalences of M. genitalium (16.1%) and BV (40.4%) at baseline were high. The incidence of M. genitalium infection was also high, at nearly 35 per 100 person-years. Women with BV had 3.5 times the odds of acquiring M. genitalium by their next study visit compared with women with normal vaginal microbiota. The odds of incident M. genitalium infection increased by 16% with each 1-point increase in the Nugent score, suggesting a dose-response effect of vaginal dysbiosis on the odds of acquiring M. genitalium.
The incidence of M. genitalium in this study was higher than in previous observations. In low-risk populations of women in the United Kingdom and Australia, M. genitalium incidence rates ranged from a cumulative annual incidence of 0.9% to a rate of 1.3 per 100 person-years (18, 34). In higher-risk populations in Kenya and the United States, incidence ranged from 22.7 per 100 person-years to 33.6 per 100 person-years (22, 35). Unlike the case in previous studies, we used a recurrent event analysis, allowing women to have multiple incident infections over the course of follow-up. This may partially explain our higher observed incidence rate.
The median time to clearance of M. genitalium infection in these women (85.5 days or 2.8 months) was also higher than that observed among FSWs in either Nairobi, Kenya (median duration, 1 month) (22) or Kampala, Uganda (median time to clearance, 2.1 months) (36). This difference may be partly due to our bimonthly sampling interval for M. genitalium testing. More frequent M. genitalium testing may have increased our ability to detect a shorter time to clearance. Antibiotic use may also have influenced time to clearance, and more women in the Nairobi and Kampala studies received antibiotics for other conditions during follow-up than women in our study (22, 36). The higher median time to M. genitalium clearance among HIV-positive women that we observed was consistent with results seen among Ugandan FSWs (36), but our finding was not statistically significant, probably reflecting low statistical power.
The strong association between BV and incident M. genitalium that we observed when women with BV were compared with women with normal microbiota (Nugent score ≥7 vs. 0–3), as well as the modest association between intermediate microbiota (Nugent score 4–6) and incident M. genitalium, suggests that some of the inconsistencies between prior studies may have been due to differences in BV measurement. Prior studies categorized vaginal microbiota disruption in a binary manner (BV vs. no BV), whether diagnosing BV clinically (2, 19, 21) or by scoring Gram stains of vaginal secretions (17, 18, 20, 22). In studies utilizing the Nugent score (17, 18, 22), investigators did not explicitly state how intermediate microbiota disruption was incorporated into their definition of BV, nor did they report the prevalence of intermediate microbiota. However, binary categorizations of vaginal microbiota typically classify women with intermediate microbiota as normal, potentially obscuring a relationship between BV and M. genitalium. In addition, studies in which BV was defined by clinical diagnosis (2, 19, 21) may have misclassified women with disrupted vaginal microbiota unaccompanied by clinical signs, again obscuring a relationship with M. genitalium.
Two other prospective studies have assessed the relationship between BV and incident M. genitalium (18, 22), and conflicting results were reported. Among FSWs in Nairobi, Kenya, there was no association between BV diagnosed during follow-up and incident M. genitalium (unadjusted hazard ratio = 1.14, 95% CI: 0.70, 1.94) (22). Visits occurred every 2 months, but neither the duration of follow-up nor the timing of BV detection in relation to M. genitalium testing was summarized, leaving doubt about the temporal relationship. In contrast, BV detected at baseline was associated with increased risk of subsequent detection of M. genitalium (unadjusted relative risk = 6.09, 95% CI: 1.98, 18.50) in a study of female students in the United Kingdom (18). Follow-up samples were collected a median of 16 months after baseline (18), yet despite this long interval, there was a strong association between BV and M. genitalium acquisition. Similarly, in our study, we observed a strong association between BV and incident M. genitalium. This finding, paired with the results from our secondary analysis assessing BV status at the visit prior to and the visit with M. genitalium testing, provide additional evidence that recent BV is a more significant driver of M. genitalium acquisition than concurrent BV.
The changes in the vaginal microbiota associated with BV offer biological plausibility for an increased risk of acquiring M. genitalium. Normal vaginal microbiota are dominated by Lactobacillus species that may decrease a woman's susceptibility to STIs (37). Lactic acid produced by lactobacilli contributes to an acidic vaginal pH, prohibiting the growth of acid-intolerant bacteria (38). In addition, some Lactobacillus species may inhibit vaginal colonization by select pathogens (37). Hydrogen peroxide can be produced by some Lactobacillus species and is hypothesized to play a role in inhibiting infection, but it is unclear whether hydrogen peroxide is produced in vivo (37). BV has been associated with acquisition of HIV (16) and other STIs, including HSV-2, T. vaginalis, N. gonorrhoeae, and Chlamydia trachomatis (10–15), suggesting that increased susceptibility to M. genitalium is plausible. Alternatively, the association between BV and STIs may reflect common sexual risk behaviors (39). However, our secondary analysis demonstrating the strongest synergy between acquisition of M. genitalium and recent BV rather than concurrent BV argues in favor of enhanced biological susceptibility.
These analyses were characterized by several strengths. The prospective study design allowed us to determine that BV preceded M. genitalium infection, and monthly assessment of BV permitted evaluation of a more specific temporal association. In addition, our analysis using the continuous Nugent score (0–10) allowed us to more precisely evaluate the incremental effect of disrupted microbiota on M. genitalium incidence. Finally, the discrete failure time analysis that allowed multiple M. genitalium infections per woman accounted for spontaneous clearance of M. genitalium and the multiple opportunities for acquiring pathogens experienced by these FSWs.
These analyses also had limitations. Women were not screened for C. trachomatis, the majority were not tested for HSV-2, and T. vaginalis was diagnosed using wet mount and/or culture, methods which are less sensitive than molecular assays (40). While C. trachomatis and T. vaginalis are both associated with BV (12–14), their relationship with M. genitalium is less consistent (19, 41). Nevertheless, there may have been residual confounding due to infection with these organisms. Social desirability bias may have influenced the FSWs’ reporting on condom use and number of sex partners, reducing our ability to completely control for the effects of these factors. Time to clearance may have been overestimated if regular sexual partners reinfected women with M. genitalium. In contrast, the bimonthly M. genitalium testing schedule may have missed short-duration infections; however, M. genitalium infections were not treated and antibiotic use was low, and thus our incidence rate was probably not markedly underestimated. Among women diagnosed with multiple incident M. genitalium infections, interim negative results may have reflected false-negative tests or been the result of fluctuation around the threshold for detection, which could have overestimated incidence. However, the frequency with which this occurs is unknown. While discrete failure time analysis is essentially equivalent to an interval-censored analysis, the odds ratios may overestimate relative risk and should not be interpreted as such. Lastly, despite a relatively low rate of partner change, risk of HIV/STI was high and HIV/HSV coinfection was common (31, 42); our findings may not be generalizable to lower-risk populations.
These analyses suggest a strong association between recent BV and M. genitalium acquisition in a cohort of FSWs in Mombasa, Kenya, and highlight the potential value of categorizing the vaginal microbiota using the full spectrum of the Nugent score. Additional studies will be needed to determine whether BV results in enhanced biological susceptibility to other STIs or whether susceptibility is elevated through common risk factors. If the former is true, effective screening and treatment of BV might reduce women's susceptibility to M. genitalium.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Erica M. Lokken, Jennifer E. Balkus, R. Scott McClelland, Lisa E. Manhart); Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, Washington (Jennifer E. Balkus); Department of Obstetrics and Gynaecology, College of Health Sciences, University of Nairobi, Nairobi, Kenya (James Kiarie); Kenyatta National Hospital, Nairobi, Kenya (James Kiarie); Department of Biostatistics, School of Public Health, University of Washington, Seattle, Washington (James P. Hughes); Department of Medical Microbiology, College of Health Sciences, University of Nairobi, Nairobi, Kenya (Walter Jaoko); Department of Global Health, Schools of Public Health and Medicine, University of Washington, Seattle, Washington (Jennifer E. Balkus, R. Scott McClelland, Lisa E. Manhart); Department of Medicine, School of Medicine, University of Washington, Seattle, Washington (Patricia A. Totten, R. Scott McClelland); and University of Nairobi Institute of Tropical and Infectious Diseases, Nairobi, Kenya (R. Scott McClelland).
This work was supported by the US National Institutes of Health (the National Institute of Child Health and Human Development (grant P01-HD64915) and the National Institute of Allergy and Infectious Diseases (grant R01-AI99106)) and by a developmental award from the Center for AIDS Research, a collaboration between the University of Washington and the Fred Hutchinson Cancer Research Center (grant P30-AI27757). Infrastructure and logistical support for the Mombasa field site were also received from the Center for AIDS Research (grant P30-AI27757).
We are grateful to our research, clinical, laboratory, outreach, and administrative staff in Mombasa, Kenya, and Seattle, Washington, for making this study possible.
Preliminary data from this study were presented at the 18th Meeting of the International Society for Sexually Transmitted Diseases Research, London, United Kingdom, June 28–July 1, 2009 (abstract P3.37).
The funders played no role in the study design, data collection, and analysis, the decision to publish, or the preparation of the manuscript. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
R.S.M. is currently conducting research sponsored by Hologic, Inc. (Marlborough, Massachusetts). L.E.M. has served on a scientific advisory board for Hologic and also received test kits and reagents from Hologic. J.E.B. received donated assay reagents from Hologic for Mycoplasma genitalium testing and received honoraria for consulting from Symbiomix Therapeutics, LLC (Newark, New Jersey).
REFERENCES
- 1. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis. 2015;61(3):418–426. [DOI] [PubMed] [Google Scholar]
- 2. Mavedzenge SN, Müller EE, Lewis DA, et al. Mycoplasma genitalium is associated with increased genital HIV type 1 RNA in Zimbabwean women. J Infect Dis. 2015;211(9):1388–1398. [DOI] [PubMed] [Google Scholar]
- 3. Mavedzenge SN, Weiss HA. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS. 2009;23(5):611–620. [DOI] [PubMed] [Google Scholar]
- 4. Vandepitte J, Weiss HA, Bukenya J, et al. Association between Mycoplasma genitalium infection and HIV acquisition among female sex workers in Uganda: evidence from a nested case-control study. Sex Transm Infect. 2014;90(7):545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel V, Weiss H, Mabey D, et al. The burden and determinants of reproductive tract infections in India: a population based study of women in Goa, India. Sex Transm Infect. 2006;82(3):243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walraven G, Scherf C, West B, et al. The burden of reproductive-organ disease in rural women in The Gambia, West Africa. Lancet. 2001;357(9263):1161–1167. [DOI] [PubMed] [Google Scholar]
- 7. Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004: associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34(11):864–869. [DOI] [PubMed] [Google Scholar]
- 8. Lan PT, Lundborg CS, Phuc HD, et al. Reproductive tract infections including sexually transmitted infections: a population-based study of women of reproductive age in a rural district of Vietnam. Sex Transm Infect. 2008;84(2):126–132. [DOI] [PubMed] [Google Scholar]
- 9. Tchoudomirova K, Bassiri M, Savova J, et al. Gynaecological and microbiological findings in women attending for a general health check-up. J Obstet Gynaecol. 1998;18(6):556–560. [DOI] [PubMed] [Google Scholar]
- 10. Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37(3):319–325. [DOI] [PubMed] [Google Scholar]
- 11. Gallo MF, Warner L, Macaluso M, et al. Risk factors for incident herpes simplex type 2 virus infection among women attending a sexually transmitted disease clinic. Sex Transm Dis. 2008;35(7):679–685. [DOI] [PubMed] [Google Scholar]
- 12. Ness RB, Kip KE, Soper DE, et al. Bacterial vaginosis (BV) and the risk of incident gonococcal or chlamydial genital infection in a predominantly black population. Sex Transm Dis. 2005;32(7):413–417. [DOI] [PubMed] [Google Scholar]
- 13. Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180(6):1863–1868. [DOI] [PubMed] [Google Scholar]
- 14. Brotman RM, Klebanoff MA, Nansel TR, et al. Bacterial vaginosis assessed by Gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis. 2010;202(12):1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36(5):663–668. [DOI] [PubMed] [Google Scholar]
- 16. Atashili J, Poole C, Ndumbe PM, et al. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22(12):1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vandepitte J, Bukenya J, Hughes P, et al. Clinical characteristics associated with Mycoplasma genitalium infection among women at high risk of HIV and other STI in Uganda. Sex Transm Dis. 2012;39(6):487–491. [DOI] [PubMed] [Google Scholar]
- 18. Oakeshott P, Aghaizu A, Hay P, et al. Is Mycoplasma genitalium in women the “new chlamydia?” A community-based prospective cohort study. Clin Infect Dis. 2010;51(10):1160–1166. [DOI] [PubMed] [Google Scholar]
- 19. Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis. 2003;187(4):650–657. [DOI] [PubMed] [Google Scholar]
- 20. Keane FE, Thomas BJ, Gilroy CB, et al. The association of Mycoplasma hominis, Ureaplasma urealyticum and Mycoplasma genitalium with bacterial vaginosis: observations on heterosexual women and their male partners. Int J STD AIDS. 2000;11(6):356–360. [DOI] [PubMed] [Google Scholar]
- 21. Lawton B, Rose SB, Bromhead C, et al. High prevalence of Mycoplasma genitalium in women presenting for termination of pregnancy. Contraception. 2008;77(4):294–298. [DOI] [PubMed] [Google Scholar]
- 22. Cohen CR, Nosek M, Meier A, et al. Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex Transm Dis. 2007;34(5):274–279. [DOI] [PubMed] [Google Scholar]
- 23. Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5(4):e10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4(132):132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amsel R, Totten P, Spiegel C, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14–22. [DOI] [PubMed] [Google Scholar]
- 26. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichonomas vaginalis acquisition among HIV-1 negative women. Sex Transm Dis. 2014;41(2):123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van de Wijgert JH, Morrison CS, Brown J, et al. Disentangling contributions of reproductive tract infections to HIV acquisition in African women. Sex Transm Dis. 2009;36(6):357–364. [DOI] [PubMed] [Google Scholar]
- 29. Martin HL, Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178(4):1053–1059. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization Guidelines for the Management of Sexually Transmitted Infections. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 31. Chohan V, Baeten JM, Benki S, et al. A prospective study of risk factors for herpes simplex virus type 2 acquisition among high-risk HIV-1 seronegative women in Kenya. Sex Transm Infect. 2009;85(7):489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mostad SB, Kreiss JK, Ryncarz AJ, et al. Cervical shedding of herpes simplex virus in human immunodeficiency virus-infected women: effects of hormonal contraception, pregnancy, and vitamin A deficiency. J Infect Dis. 2000;181(1):58–63. [DOI] [PubMed] [Google Scholar]
- 33. Fahrmeir L. Discrete survival-time models In: Armitage P, Colton T, eds. Encyclopedia of Biostatistics. 2nd ed New York, NY: John Wiley & Sons, Inc.; 2005. [Google Scholar]
- 34. Walker J, Fairley CK, Bradshaw CS, et al. Mycoplasma genitalium incidence, organism load, and treatment failure in a cohort of young Australian women. Clin Infect Dis. 2013;56(8):1094–1100. [DOI] [PubMed] [Google Scholar]
- 35. Balkus JE, Manhart LE, Lee J, et al. Periodic presumptive treatment for vaginal infections may reduce the incidence of sexually transmitted bacterial infections. J Infect Dis. 2016;213(12):1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vandepitte J, Weiss HA, Kyakuwa N, et al. Natural history of Mycoplasma genitalium infection in a cohort of female sex workers in Kampala, Uganda. Sex Transm Dis. 2013;40(5):422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spurbeck RR, Arvidson CG. Lactobacilli at the front line of defense against vaginally acquired infections. Future Microbiol. 2011;6(5):567–582. [DOI] [PubMed] [Google Scholar]
- 38. Boskey ER, Telsch KM, Whaley KJ, et al. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect Immun. 1999;67(10):5170–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hillier S, Marrazzo J, Holmes KK. Bacterial vaginosis In: Holmes KK, Sparling P, Stamm W, et al., eds. Sexually Transmitted Diseases. 4th ed New York, NY: McGraw-Hill Companies; 2007:737–768. [Google Scholar]
- 40. Nathan B, Appiah J, Saunders P, et al. Microscopy outperformed in a comparison of five methods for detecting Trichomonas vaginalis in symptomatic women. Int J STD AIDS. 2014;26(4):251–256. [DOI] [PubMed] [Google Scholar]
- 41. Huppert JS, Mortensen JE, Reed JL, et al. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis. 2008;35(3):250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Masese L, Baeten JM, Richardson BA, et al. Changes in the contribution of genital tract infections to HIV acquisition among Kenyan high-risk women from 1993 to 2012. AIDS. 2015;29(9):1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]