Abstract
Glycemic excursions, independent of average glucose, have been implicated in the development of diabetic complications. It is unknown whether low levels of 1,5-anhydroglucitol (1,5-AG) are associated with advanced stages of kidney disease independent of kidney function and glycemia. In the Atherosclerosis Risk in Communities Study (n = 13,277 from 4 US communities), we used structural equation modeling to estimate the association between serum 1,5-AG levels and end-stage renal disease (ESRD) from baseline (1990–1992) through 2013 with adjustment for demographics, risk factors, a latent variable for glycemia (diabetes status, fasting glucose, glycated hemoglobin (HbA1c), fructosamine, glycated albumin), and a latent variable for kidney function (creatinine, cystatin C, β2-microglobulin). After adjusting for demographics, risk factors, and the latent variable for kidney function, the linear spline terms representing 1,5-AG levels <6.0 μg/mL (incidence rate ratio (IRR) = 0.79, 95% confidence interval (CI): 0.70, 0.88) and 6.0–9.9 μg/mL (IRR = 0.80, 95% CI: 0.70, 0.92) were significantly associated with ESRD. After additionally adjusting for the latent variable for glycemia, low 1,5-AG levels (<6.0 μg/mL) were no longer significantly associated with ESRD (IRR = 0.92, 95% CI: 0.81, 1.05). In conclusion, low 1,5-AG levels are associated with higher risk of incident ESRD independent of baseline kidney function but not independent of glycemia.
Keywords: 1,5-anhydroglucitol; biomarkers; diabetes mellitus; end-stage renal disease; epidemiology; glomerular filtration rate; hyperglycemia
1,5-Anhydroglucitol (1,5-AG) is a glucose-like monosaccharide that is derived primarily from dietary sources (1). In normal physiology, 1,5-AG is freely filtered by the glomerulus, and >99% is reabsorbed by the renal tubules (2). When glucose levels exceed the renal threshold (approximately 160–180 mg/dL), reabsorption of 1,5-AG is inhibited, resulting in higher urinary excretion rates and lower levels of 1,5-AG in the circulation (3). As such, low 1,5-AG is a marker of hyperglycemia excursions over a period of approximately 1–2 weeks (4–9). To the extent that 1,5-AG is a useful biomarker of glycemic excursions and glucose variability, this novel marker may provide additional prognostic information beyond blood levels of glucose and glycated hemoglobin (HbA1c) (4).
Glucose variability, above average glucose levels, has been linked to kidney disease risk (10–12). Formally testing an a priori hypothesis, in one study there was a significant association between lower levels of 1,5-AG and subsequent onset of chronic kidney disease (13). In addition, a global, untargeted metabolomics study discovered that level of 1,5-AG, out of 204 metabolites examined, was an independent risk factor for chronic kidney disease in the Atherosclerosis Risk in Communities (ARIC) Study (14). However, the relationship between 1,5-AG and more advanced stages of kidney disease has not been characterized, and it is not known whether 1,5-AG is a biomarker of kidney disease, a biomarker of diabetes, or an influence on kidney disease risk through a separate, nonglycemic pathway.
Kidney function can be estimated in a number of ways. Most frequently, it is assessed by estimated glomerular filtration rate (eGFR) based on endogenous blood levels of creatinine. Serum creatinine and eGFR based on serum creatinine are imperfect measures of kidney function due to the influence of non-GFR determinants, including dietary protein intake and body composition as well as other sources of measurement error (15, 16). Recent studies have suggested that measurement of multiple filtration markers may represent kidney function better than creatinine alone (17).
The overarching objective of the present study was to evaluate whether low levels of 1,5-AG, a biomarker of glycemic excursions, is a risk factor for end-stage renal disease (ESRD) in addition to average glycemia and underlying kidney function. In our main analysis, we used a structural equation modeling approach, which allowed for more complete adjustment for mediation by glycemia and kidney function by incorporating multiple markers of an underlying construct (i.e., the latent variable) and, by combining multiple markers, reduces the influence of measurement error in individual markers (18–20). We compared these results with those obtained using standard regression methods.
METHODS
Study design and population
The ARIC Study is a prospective cohort of 15,792 participants recruited from 4 US communities: Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and suburbs of Minneapolis, Minnesota (21). Participants were initially recruited at 45–64 years of age in 1987–1989 (visit 1), and they returned for follow-up study visits in 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), and 2011–2013 (visit 5). In the present analysis, we included participants with measurements of 1,5-AG at study visit 2 (1990–1992, baseline for the present study), who reported information on diabetes diagnosis, and who were free of ESRD at baseline (n = 13,277). Written documentation of informed consent was obtained at each study visit, and procedures were approved by the institutional review board at each study center.
Data collection
At the study visits, trained staff administered questionnaires to collect information on demographic characteristics (date of birth, sex, race) and health history (medication use, medical conditions, current smoking). Anthropometric measurements were taken while participants wore light clothing, without shoes. Body mass index was calculated as measured weight (kg)/height (m)2. Three seated blood pressure measurements were taken by a certified technician using a random-zero sphygmomanometer, and the mean of the second and third measurements was calculated.
Fasting blood samples were collected at each study visit, centrifuged within 30 minutes, and stored at −70°C for future laboratory analysis. Blood glucose was measured by the hexokinase method. HbA1c was measured using high-performance liquid chromatography with instruments standardized to the Diabetes Control and Complications Trial assay (Tosoh 2.2 Plus and Tosoh G7 Glycohemoglobin Analyzer, Tosoh Bioscience, Inc., San Francisco, California). In 2012–2013, serum levels of fructosamine (using reagents from Roche Diagnostics Corporation, Indianapolis, Indiana) and glycated albumin (Lucica GA-L, Asahi Kasei Corporation, Tokyo, Japan) were measured using the Roche Modular P800 Chemistry Analyzer (Roche Diagnostics Corporation). Diabetes was defined as a history of diagnosed diabetes or current diabetes medication use. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or current antihypertensive medication use.
Serum creatinine was measured by the modified Jaffé method. In 2012–2013, cystatin C was measured using the Gentian immunoassay (Gentian AS, Moss, Norway), and β2-microglobulin was measured using Roche reagents on the Roche Modular P800 Chemistry Analyzer (Roche Diagnostics Corporation) in stored serum samples collected at study visit 2 (1990–1992). Calculations were made separately, using the Chronic Kidney Disease Epidemiology Collaboration equations, for creatinine and cystatin C (eGFRCr-Cys) and for β2-microglobulin (eGFRβ2M) (17, 22).
Measurement of 1,5-AG
Levels of 1,5-AG were measured in serum samples collected from participants in 1990–1992 (study visit 2) and stored at −70°C since collection. The assays were performed at the University of Minnesota Advanced Research and Diagnostic Laboratory in 2012–2013. 1,5-AG was measured with the GlycoMark assay (GlycoMark, Inc., New York, New York) on the Roche Modular P800 Chemistry Analyzer (Roche Diagnostics Corporation) using an enzymatic method (coefficient of variation = 5%). After pretreatment with glucokinase, pyranose oxidase was used to oxidize the second-position hydroxyl group of 1,5-AG. The amount of hydrogen peroxide was assessed by colorimetry to represent the concentration of 1,5-AG.
Ascertainment of ESRD cases
Incident ESRD was defined as entry into the US Renal Data System registry, from baseline for the present study (study visit 2, 1990–1992) through December 31, 2013. The US Renal Data System registry represents initiation of kidney dialysis or receipt of a kidney transplant as reported on the Centers for Medicare and Medicaid Services Medical Evidence Form 2728.
Statistical analysis
Given the variability in the distribution of 1,5-AG by diabetes status and the variability in the association between 1,5-AG and ESRD by diabetes status, it was important to account for diabetes in the analysis. Therefore, we created diabetes-specific exposure categories (which have been used in prior publications) and, in other instances, restricted the sample to individuals with diagnosed diabetes (13, 23). Descriptive statistics (mean, standard deviation, proportions) were used to describe participant characteristics at baseline for the overall study population and according to diabetes-specific categories of 1,5-AG (for participants with no diagnosed diabetes: <10.0, ≥10.0 μg/mL; for participants with diagnosed diabetes: <6.0, 6.0–9.9, ≥10.0 μg/mL). Differences in baseline characteristics by diabetes-specific categories of 1,5-AG were tested using linear regression, t tests, and χ2 tests. Spearman's rank correlation coefficients were calculated to assess the cross-sectional relationship between 1,5-AG and other glycemic markers (glucose, HbA1c, fructosamine, glycated albumin) as well as kidney filtration markers (eGFRCr-Cys, eGFRβ2M) for the overall study population and stratified by baseline category of eGFRCr-Cys (mL/min/1.73 m2: <60, 60–89, or ≥90) and diabetes. Restricted cubic splines with 4 knots were used to visually depict the shape of the relationship between 1,5-AG as a continuous variable and risk of ESRD.
We used a common modeling approach, Poisson regression, to characterize the association between categories of 1,5-AG and incident ESRD with progressive adjustment of individual covariates and groups of covariates of relevance through several regression models. Model 1 included no adjustments (no covariates) to show the crude association. Model 2 adjusted for demographic characteristics (age, sex, race) and provided the minimally adjusted results. Model 3 adjusted for variables in model 2 plus eGFRCr-Cys modeled using linear spline terms with knots at 60 mL/min/1.73 m2 and 90 mL/min/1.73 m2 in order to examine the influence of adjusting for this important measure of kidney function on the association between 1,5-AG and ESRD. Model 4 adjusted for all variables in model 3 plus traditional kidney disease risk factors (hypertension, body mass index, current smoking). Model 5a adjusted for all variables in model 4 plus fasting blood glucose levels, and Model 5b adjusted for all variables in model 4 plus HbA1c in an attempt to assess whether 1,5-AG levels were associated with ESRD independent of established glycemic markers. The trend across categories of 1,5-AG was tested using an ordinal variable. Harrell's C statistic was used to assess model discrimination, and the relative integrated discrimination statistic was calculated to evaluate improvement in risk classification (24, 25).
In addition to the Poisson regression models, generalized structural equation models were used to quantify the association of 1,5-AG level and incident ESRD before and after adjustment for latent variables for kidney function and glycemia as a means of incorporating multiple measures into a latent variable for kidney function (eGFRCr-Cys, eGFRβ2M) and a latent variable for glycemia (diabetes, fasting glucose, HbA1c, fructosamine, and glycated albumin) while avoiding the issue of collinearity. The variance was constrained to 1 for both latent variables, and we assumed no correlation between them. The 2 regression techniques were used in order to compare the results of the structural equation model with a more common analytic approach (Poisson regression). Analyses were conducted with Stata, version 14.1 (StataCorp LP, College Station, Texas).
RESULTS
Baseline participant characteristics
In the overall study sample, 44% of participants were men, 24% were African-American, and the mean age at baseline was 57 years (Web Table 1, available at https://academic.oup.com/aje). Those with lower serum levels of 1,5-AG were more likely to be women, African-American, or nonsmokers, and they had a higher mean body mass index. Only 6.5% (783 of 12,033) of those without diagnosed diabetes had serum levels of 1,5-AG below 10.0 μg/mL. The majority of those with diagnosed diabetes had low levels of 1,5-AG (1,5-AG <6.0 μg/mL: 53.1% (661/1,244); 1,5-AG 6.0–9.9 μg/mL: 13.0% (162/1,244)).
Cross-sectional analysis of 1,5-AG, filtration markers, and glycemic markers
Among those with diagnosed diabetes, there was a strong inverse correlation between 1,5-AG level and the other glycemic markers (fasting glucose: r = −0.77, P < 0.001; HbA1c: r = −0.84, P < 0.001; fructosamine: r = −0.83, P < 0.001; glycated albumin: r = −0.83, P < 0.001; Table 1). In the overall study population, 1,5-AG was moderately positively correlated with eGFRCr-Cys (r = 0.25, P < 0.001) and eGFRβ2M (r = 0.16, P = 0.002) among those with reduced kidney function at baseline (eGFRCr-Cys < 60 mL/min/1.73 m2).
Table 1.
Spearman's Correlationa Between 1,5-Anhydroglucitol, Other Glycemic Markers, and Kidney Filtration Markers in the Overall Study Population, According to Diabetes Status and Categories of Estimated Glomerular Filtration Rateb, Atherosclerosis Risk in Communities Study, United States, 1990–1992
| Marker | Overall | eGFRCr-Cys <60 | eGFRCr-Cys 60–89 | eGFRCr-Cys 90–134 | eGFRCr-Cys ≥135 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | r | P Value | r | P Value | |
| Total Study Populationc | ||||||||||
| Fasting glucose | −0.17 | <0.001 | −0.35 | <0.001 | −0.17 | <0.001 | −0.16 | <0.001 | −0.68 | <0.001 |
| HbA1c | −0.25 | <0.001 | −0.53 | <0.001 | −0.25 | <0.001 | −0.24 | <0.001 | −0.66 | <0.001 |
| Fructosamine | −0.28 | <0.001 | −0.47 | <0.001 | −0.27 | <0.001 | −0.27 | <0.001 | −0.70 | <0.001 |
| Glycated albumin | −0.29 | <0.001 | −0.41 | <0.001 | −0.27 | <0.001 | −0.28 | <0.001 | −0.70 | <0.001 |
| eGFRCr-Cys | −0.11 | <0.001 | 0.25 | <0.001 | −0.001 | 0.5 | −0.12 | <0.001 | −0.25 | 0.05 |
| eGFRβ2M | −0.11 | <0.001 | 0.16 | 0.002 | −0.06 | <0.001 | −0.12 | <0.001 | −0.15 | 0.2 |
| Participants Without Diagnosed Diabetesd | ||||||||||
| Fasting glucose | −0.02 | 0.03 | −0.08 | 0.2 | −0.03 | 0.06 | −0.02 | 0.1 | −0.28 | 0.06 |
| HbA1c | −0.09 | <0.001 | −0.14 | 0.02 | −0.09 | <0.001 | −0.09 | <0.001 | −0.35 | 0.02 |
| Fructosamine | −0.13 | <0.001 | −0.11 | 0.07 | −0.13 | <0.001 | −0.14 | <0.001 | −0.30 | 0.04 |
| Glycated albumin | −0.14 | <0.001 | −0.02 | 0.7 | −0.12 | <0.001 | −0.15 | <0.001 | −0.29 | 0.05 |
| eGFRCr-Cys | −0.12 | <0.001 | 0.13 | 0.03 | −0.03 | 0.05 | −0.11 | <0.001 | −0.21 | 0.2 |
| eGFRβ2M | −0.14 | <0.001 | −0.002 | 0.9 | −0.10 | <0.001 | −0.13 | <0.001 | −0.24 | 0.1 |
| Participants With Diagnosed Diabetese | ||||||||||
| Fasting glucose | −0.77 | <0.001 | −0.60 | <0.001 | −0.76 | <0.001 | −0.79 | <0.001 | −0.28 | 0.3 |
| HbA1c | −0.84 | <0.001 | −0.76 | <0.001 | −0.83 | <0.001 | −0.86 | <0.001 | −0.58 | 0.01 |
| Fructosamine | −0.83 | <0.001 | −0.65 | <0.001 | −0.81 | <0.001 | −0.85 | <0.001 | −0.32 | 0.2 |
| Glycated albumin | −0.83 | <0.001 | −0.62 | <0.001 | −0.81 | <0.001 | −0.86 | <0.001 | −0.28 | 0.2 |
| eGFRCr-Cys | −0.19 | <0.001 | 0.07 | 0.4 | −0.02 | 0.8 | −0.17 | <0.001 | −0.24 | 0.3 |
| eGFRβ2M | −0.17 | <0.001 | 0.17 | 0.07 | −0.002 | 0.9 | −0.14 | <0.001 | −0.008 | 0.9 |
Abbreviations: eGFRβ2M, estimated glomerular filtration rate based on β2-microglobulin; eGFRCr-Cys, estimated glomerular filtration rate based on creatinine and cystatin C; HbA1c, glycated hemoglobin.
a Spearman's rank correlation coefficients and P values.
b eGFRCr-Cys in units of mL/min/1.73 m2; 87 participants were missing data for cystatin C.
c The overall number of subjects was 13,277; for the categories of eGFRCr-Cys <60, 60–89, 90–134, and ≥135, the numbers of participants were 370, 4,207, 8,547, and 66, respectively.
d The overall number of subjects without diagnosed diabetes was 12,033; for the categories of eGFRCr-Cys <60, 60–89, 90–134, and ≥135, the numbers of participants were 260, 3,828, 7,825, and 47, respectively.
e The overall number of subjects with diagnosed diabetes was 1,244; for the categories of eGFRCr-Cys <60, 60–89, 90–134, and ≥135, the numbers of participants were 110, 379, 722, and 19, respectively.
Prospective analysis of 1,5-AG and incident ESRD
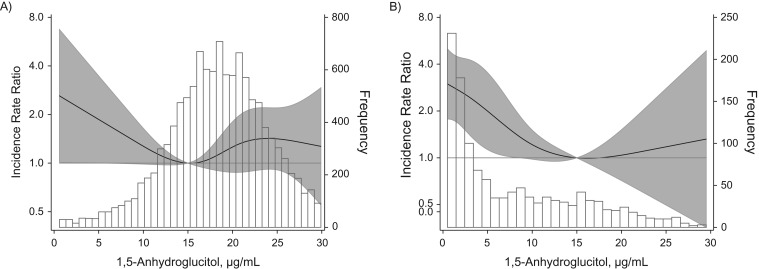
During a median follow-up of 22 years, there were 299 incident ESRD cases. When 1,5-AG was modeled continuously using restricted cubic spline terms, risk of ESRD increased approximately linearly at 1,5-AG levels below 15.0 μg/mL among participants without diagnosed diabetes (Figure 1A) as well as for participants with diagnosed diabetes (Figure 1B).
Figure 1.
Distribution of 1,5-anhydroglucitol (1,5-AG) and adjusted incidence rate ratios (IRRs) for end-stage renal disease according to serum levels of 1,5-AG among study participants without diagnosed diabetes (A) and with diagnosed diabetes (B), Atherosclerosis Risk in Communities Study, United States, 1990–2013. The solid black line represents IRR for end-stage renal disease according to serum level of 1,5-AG, modeled as restricted cubic spline terms with knots at the 5th, 35th, 65th, and 95th percentiles. The white bars with black outline depict the histogram for the distribution of 1,5-AG. IRRs were adjusted for age, sex, race, and estimated glomerular filtration rate (creatinine-cystatin C), modeled as linear spline terms with knots at 60 mL/min/1.73 m2 and 90 mL/min/1.73 m2. The shaded area around the solid black line represents 95% confidence intervals.
ESRD risk was substantially higher for those with diagnosed diabetes and 1,5-AG levels <6.0 μg/mL relative to those without diagnosed diabetes and 1,5-AG levels ≥10.0 μg/mL (incidence rate ratio (IRR) = 16.78, 95% confidence interval (CI): 12.93, 21.78; Table 2). Adjusting for age, sex, race, eGFRCr-Cys, hypertension, body mass index, current smoking status, and, separately, fasting glucose and HbA1c substantially attenuated the risk estimates, but they remained statistically significant in the overall population. Among study participants with diagnosed diabetes, there was a significantly higher risk of ESRD associated with lower levels of 1,5-AG after adjusting for demographics, eGFRCr-Cys, traditional risk factors, and fasting glucose (all P for trend < 0.01) but not after adjusting for HbA1c (Model 5b: P for trend across diabetes-specific 1,5-AG categories = 0.3).
Table 2.
Incidence Rate Ratios for Incident End-Stage Renal Disease According to Categories of 1,5-Anhydroglucitol Level and Diabetes Status, Atherosclerosis Risk in Communities Study, United States, 1990–2013
| Model | 1,5-AG Without Diagnosed Diabetes | 1,5-AG With Diagnosed Diabetes | P for Trend Among All Categories | P for Trend in Diabetes Categories | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥10.0 μg/mL (n = 11,250) | <10.0 μg/mL (n = 783) | ≥10.0 μg/mL (n = 421) | 6.0–9.9 μg/mL (n = 162) | <6.0 μg/mL (n = 661) | ||||||||
| IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | |||
| 1a | 1 | Referent | 2.44 | 1.56, 3.84 | 6.28 | 4.15, 9.49 | 11.45b | 7.00, 18.73 | 16.78b | 12.93, 21.78 | <0.001 | <0.001 |
| 2c | 1 | Referent | 2.24 | 1.42, 3.52 | 5.12 | 3.38, 7.77 | 8.80 | 5.34, 14.49 | 12.78b | 9.75, 16.73 | <0.001 | <0.001 |
| 3d | 1 | Referent | 1.96 | 1.24, 3.09 | 3.88 | 2.54, 5.93 | 6.97 | 4.21, 11.54 | 9.63b | 7.24, 12.80 | <0.001 | <0.001 |
| 4e | 1 | Referent | 1.85 | 1.16, 2.96 | 3.30 | 2.14, 5.09 | 5.93 | 3.56, 9.89 | 8.62b | 6.45, 11.52 | <0.001 | <0.001 |
| 5af | 1 | Referent | 1.71 | 1.07, 2.74 | 3.09 | 2.00, 4.78 | 4.63 | 2.69, 7.96 | 5.74b | 3.83, 8.60 | <0.001 | 0.007 |
| 5bg | 1 | Referent | 1.38 | 0.83, 2.27 | 2.85 | 1.84, 4.42 | 3.99 | 2.33, 6.82 | 3.54 | 2.22, 5.64 | <0.001 | 0.3 |
Abbreviations: 1,5-AG, 1,5-anhydroglucitol; CI, confidence interval; IRR, incidence rate ratio.
a Model 1: no covariates (unadjusted).
b Among those with diagnosed diabetes, P < 0.05 for the estimate for the respective category of 1,5-anhydroglucitol (6.0–9.9 μg/mL or <6.0 μg/mL) relative to ≥10.0 μg/mL.
c Model 2 adjusted for demographics (age, sex, race).
d Model 3: model 2 with the addition of estimated glomerular filtration rate based on creatinine and cystatin C (modeled as linear spline terms with knots at 60 mL/min/1.73 m2 and 90 mL/min/1.73 m2).
e Model 4: model 3 with the addition of hypertension, body mass index, current smoking.
f Model 5a: model 4 with the addition of fasting glucose.
g Model 5b: model 4 with the addition of glycated hemoglobin.
In the overall study population, lower levels of 1,5-AG (<6.0 μg/mL and 6.0–9.9 μg/mL relative to ≥10.0 μg/mL) were significantly associated with higher risk of incident ESRD even after adjusting for demographics, eGFRCr-Cys, hypertension, body mass index, current smoking, and traditional markers of glycemia (Table 3). 1,5-AG did not improve prediction of incident ESRD when added to models including established glycemic markers (fasting glucose and HbA1c) and risk factors (age, sex, race, eGFRCr-Cys, hypertension, body mass index, and current smoking) (Table 4).
Table 3.
Incidence Rate Ratios for Incident End-Stage Renal Disease According to Categories of 1,5-Anhydroglucitol Level, Atherosclerosis Risk in Communities Study, United States, 1990–2013
| Model | 1,5-Anhydroglucitol Level, μg/mL | P for Trend | |||||
|---|---|---|---|---|---|---|---|
| ≥10.0 (n = 11,671) | 6.0–9.9 (n = 690) | <6.0 (n = 916) | |||||
| IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | ||
| 1a | 1 | Referent | 3.54 | 2.42, 5.18 | 10.53 | 8.24, 13.45 | <0.001 |
| 2b | 1 | Referent | 3.09 | 2.11, 4.53 | 8.18 | 6.37, 10.52 | <0.001 |
| 3c | 1 | Referent | 2.47 | 1.68, 3.64 | 6.52 | 5.00, 8.51 | <0.001 |
| 4d | 1 | Referent | 2.37 | 1.60, 3.51 | 5.93 | 4.53, 7.77 | <0.001 |
| 5ae | 1 | Referent | 2.07 | 1.38, 3.10 | 3.36 | 2.31, 4.90 | <0.001 |
| 5bf | 1 | Referent | 1.86 | 1.24, 2.80 | 2.04 | 1.32, 3.15 | <0.001 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
a Model 1: no covariates (unadjusted).
b Model 2 adjusted for demographics (age, sex, race).
c Model 3: model 2 with the addition of estimated glomerular filtration rate based on creatinine and cystatin C (modeled as linear spline terms with knots at 60 mL/min/1.73 m2 and 90 mL/min/1.73 m2).
d Model 4: model 3 with the addition of hypertension, body mass index, current smoking.
e Model 5a: model 4 with the addition of fasting glucose.
f Model 5b: model 4 with the addition of glycated hemoglobin.
Table 4.
Prediction of Incident End-Stage Renal Disease With Established and Novel Glycemic Markers, Atherosclerosis Risk in Communities Study, United States, 1990–2013
| Modela | C Statistic | 95% CI | Difference | 95% CI | P Value | IDI | 95% CI | P Value |
|---|---|---|---|---|---|---|---|---|
| Base model + fasting glucose | 0.879 | 0.858, 0.900 | ||||||
| Base model + fasting glucose + 1,5-AG | 0.875 | 0.852, 0.897 | −0.004 | −0.010, 0.003 | 0.2 | −0.025 | −0.081, 0.030 | 0.4 |
| Base model + HbA1c | 0.886 | 0.865, 0.906 | ||||||
| Base model + HbA1c + 1,5-AG | 0.883 | 0.861, 0.905 | −0.003 | −0.006, 0.001 | 0.1 | −0.017 | −0.048, 0.014 | 0.3 |
Abbreviations: 1,5-AG, 1,5-anhydroglucitol; CI, confidence interval; HbA1c, glycated hemoglobin; IDI, integrated discrimination improvement statistic.
a Base model adjusted for age, sex, race, estimated glomerular filtration rate based on creatinine and cystatin C (modeled as linear spline terms with knots at 60 mL/min/1.73 m2 and 90 mL/min/1.73 m2), hypertension, body mass index, current smoking.
Structural equation model
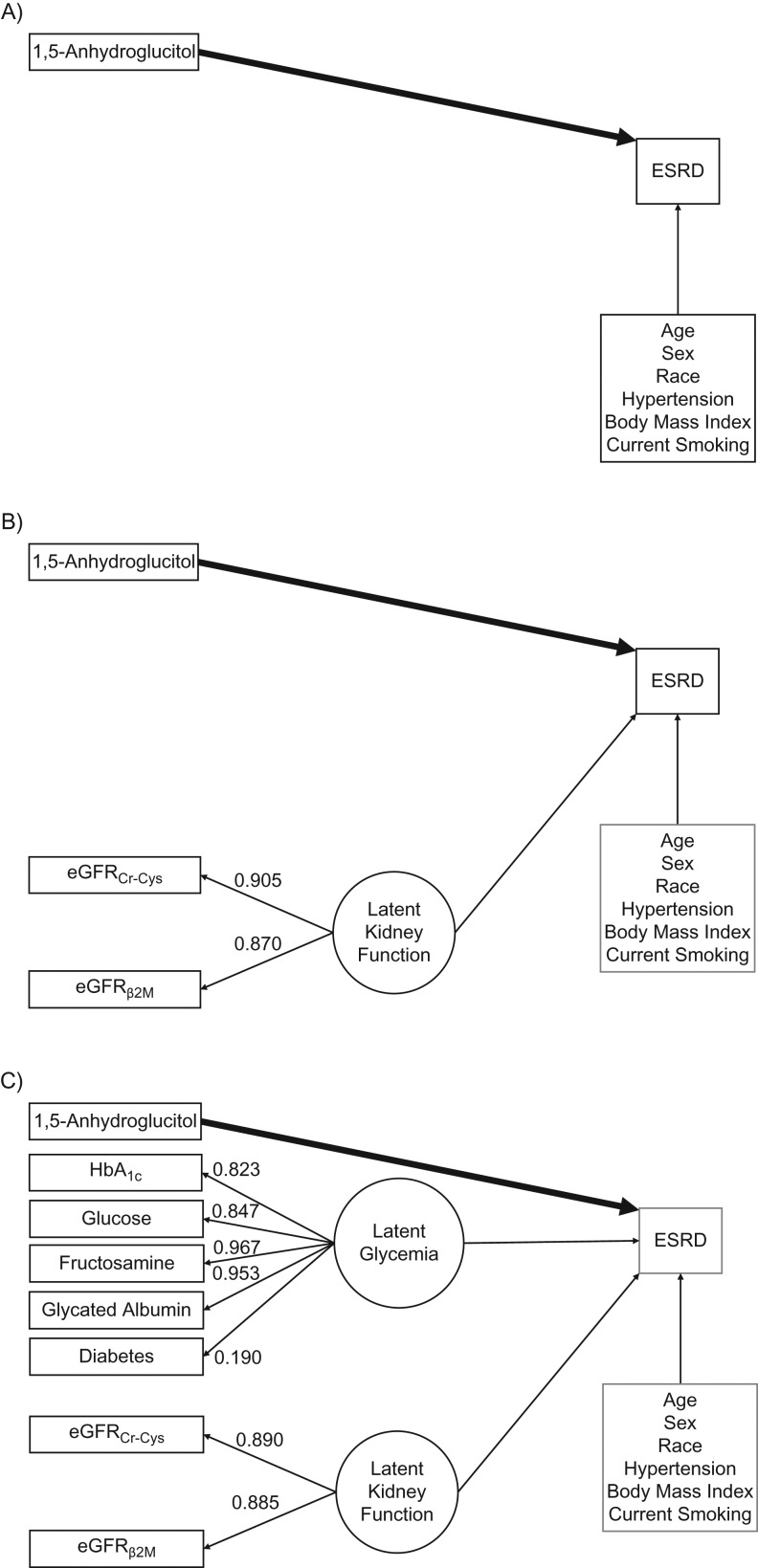
The latent variable for kidney function was estimated using the 3 filtration markers with high standardized coefficients (for eGFRCr-Cys, 0.890; for eGFRβ2M, 0.885), suggesting good fit. After adjustment for the latent variable for kidney function and accounting for diabetes status through multivariable adjustment or restriction of the study population, none of the filtration markers were independently associated with risk of incident ESRD. For the glycemia latent variable, all standardized path coefficients for other biomarkers were high (for HbA1c, 0.823; for fasting glucose, 0.847; for fructosamine, 0.967; for glycated albumin, 0.953), and the standardized path coefficient for diabetes was moderate (0.190).
In the structural equation model adjusting for demographics and traditional risk factors, but not for the kidney function and glycemia latent variables, the linear spline terms representing 1,5-AG levels <6.0 μg/mL and 6.0–9.9 μg/mL were significantly associated with ESRD (per 1.0-μg/mL increase in 1,5-AG <6.0 μg/mL, IRR = 0.88, 95% CI: 0.79, 0.98 (P = 0.02); in the category 6.0–9.9 μg/mL, IRR = 0.69, 95% CI: 0.61, 0.79 (P < 0.001)), and higher 1,5-AG levels were not associated with ESRD (per 1.0-μg/mL increase in 1,5-AG ≥10 μg/mL, IRR = 1.00, 95% CI: 0.97, 1.02; P = 0.8; Figure 2A). After additionally adjusting for the kidney-function latent variable, lower levels of 1,5-AG remained significantly associated with risk of ESRD (per 1.0-μg/mL increase in 1,5-AG <6.0 μg/mL, IRR = 0.79, 95% CI: 0.70, 0.88 (P < 0.001); in the category 6.0–9.9 μg/mL, IRR = 0.80, 95% CI: 0.70, 0.92 (P = 0.002)), and higher 1,5-AG levels remained unassociated with ESRD (per 1.0-μg/mL increase in 1,5-AG ≥10 μg/mL, IRR = 0.98, 95% CI: 0.96, 1.01; P = 0.3; Figure 2B). In the model additionally adjusting for the glycemia latent variable, 1,5-AG levels between 6.0 and 9.9 μg/mL were associated with ESRD risk (per 1.0-μg/mL increase in 1,5-AG 6.0–9.9 μg/mL, IRR = 0.85, 95% CI: 0.74, 0.98; P = 0.02), but the other categories of 1,5-AG were not independently associated with risk of incident ESRD (per 1.0-μg/mL increase in 1,5-AG <6.0 μg/mL, IRR = 0.92, 95% CI: 0.81, 1.05 (P = 0.2); in the category ≥10.0 μg/mL IRR = 0.99, 95% CI: 0.96, 1.02 (P = 0.4); Figure 2C).
Figure 2.
Structural equation model for serum levels of 1,5-anhydroglucitol (1,5-AG) and incident end-stage renal disease (ESRD), Atherosclerosis Risk in Communities Study, United States, 1990–2013. This figure displays the structural equation models for the association between 1,5-AG and ESRD, adjusted for demographics and established risk factors (A); adjusted for demographics, established risk factors, and latent kidney function (B); and adjusted for demographics, established risk factors, latent kidney function, and latent glycemia (C). These 3 models were used to assess the independence of the association between 1,5-AG and ESRD after accounting for known risk factors, markers of kidney function (using a latent variable), and markers of glycemia (using a separate latent variable). 1,5-AG, glycated hemoglobin (HbA1c), fasting glucose, estimated glomerular filtration rate based on serum creatinine and serum cystatin C (eGFRCr-Cys), and estimated glomerular filtration rate based on serum β2-microglobulin (eGFRβ2M) were standardized and log-transformed.
DISCUSSION
In this large, community-based study of middle-aged African-American and white men and women, baseline serum levels of 1,5-AG were strongly associated with subsequent development of incident ESRD over 20 years of follow-up. The association between 1,5-AG level and ESRD risk was independent of baseline kidney function. However, risk estimates were substantially attenuated, and not statistically significant in some cases, after adjusting for baseline markers of glycemia. 1,5-AG did not improve the prediction of ESRD beyond traditional markers of glycemia and established risk factors. Taken together, our findings suggest that 1,5-AG is a marker of hyperglycemia and glucose variability that increases the likelihood of future development of kidney disease.
It was previously shown, among ARIC Study participants diagnosed with diabetes, that serum levels of 1,5-AG <6.0 vs. ≥10.0 μg/mL were associated with a 2- to 3-fold increased risk of earlier stages of kidney disease (stage 3 or higher) (13). In a separate analysis of a subset of 1,921 African-American participants in the ARIC Study, a serum metabolomic profile was characterized using an untargeted, unbiased approach (14). 1,5-AG was one of 2 metabolites (out of a total of 204 examined) found to be significantly associated with risk of incident chronic kidney disease even after correcting for multiple testing and adjusting for demographic characteristics, diabetes status, baseline eGFR, and other established risk factors (quartile 4 vs. 1: odds ratio = 0.47, 95% CI: 0.31, 0.72; P for trend < 0.001). To the best of our knowledge, the present study is the first to examine the association of 1,5-AG with incident ESRD. Given the long-term follow-up, we were able to accrue a sufficient number of cases of this clinically significant outcome of advanced kidney disease.
It has been previously shown that glycemic variability or excursions, beyond average glucose levels, may injure the vasculature and could thereby contribute to the development of kidney disease (26). Postprandial glycemic spikes lead to endothelial dysfunction and oxidative stress due in part to increased production of peroxynitrite (27–29). Peroxynitrite can damage endothelial cells directly and has also been implicated in lipid peroxidation (30, 31). Other potential mechanisms through which glucose variability could be related to risk of kidney disease include activation of inflammatory markers, protein kinase C, and nicotinamide adenine dinucleotide phosphate oxidase (32–35).
We used 2 regression techniques in the present study to allow for a comparison of the results from the structural equation model with the results from the more frequently used Poisson regression. In general, results from the 2 regression techniques yielded similar interpretations. That is, lower serum levels of 1,5-AG were significantly associated with increased risk of ESRD even after accounting for demographic characteristics, established risk factors for kidney disease, and baseline eGFR, but this relationship was not independent of other measures of glycemic control. These findings suggest that 1,5-AG is a marker of hyperglycemia and glucose variability, which is an important pathway leading to higher risk of ESRD.
The key difference between the 2 regression techniques and the appeal of structural equation modeling is the ability to incorporate latent variables. We were able to assess the independence of the association between 1,5-AG and ESRD from baseline kidney function and glucose metabolism. We used structural equation models with latent variables for kidney function and glycemia, both based on multiple markers, to rigorously adjust for these constructs. The 2 analytic approaches produced different results, although the overall interpretation was consistent. In multivariable regression using diabetes-specific 1,5-AG categories, the association between 1,5-AG and ESRD remained significant after adjusting for fasting glucose but not after adjusting for HbA1c. Similarly, using Poisson regression in the overall study population, 1,5-AG remained significantly associated with ESRD after accounting for fasting glucose as well as HbA1c. In contrast, after adding the glycemia latent variable (estimated using glucose levels, HbA1c, fructosamine levels, glycated albumin levels, and diabetes status) using the structural equation model framework, low 1,5-AG level (<6.0 μg/mL) was no longer independently associated with ESRD. Incorporating the latent variables for glycemia and kidney function minimized the influence of collinearity and measurement error for the individual biomarkers and allowed for a more complete adjustment for these confounding factors (19, 20, 36). Collinearity occurs when 2 or more covariates are correlated with each other, and biased point estimates and measures of variability can result when these collinear covariates are included in a regression model together (36, 37). The combination of multiple markers of kidney function has been shown previously to improve the estimation of ESRD risk (38, 39). The use of multiple markers (in our study, those that represent the constructs of glycemia and kidney function) reduces the influence of measurement error and confounding due to any one marker. By improving the estimation of these underlying constructs using multiple markers, we were then better able to adjust for these latent variables and reduce the likelihood of residual confounding. In our study, 1,5-AG remained significantly associated with ESRD after adjusting for glycemia markers using traditional regression techniques (Poisson) but not when using structural equation modeling. Structural equation modeling may be preferable to more traditional regression techniques in order to more appropriately incorporate multiple measures of a given construct and to evaluate the independence of an association after more completely accounting for potential confounding factors.
There are a few limitations of the present study to consider when interpreting our findings. As with any observational study design, there is the potential for residual confounding to explain part of the observed associations, due to imprecise measurement or unknown confounding factors. In particular, urine albumin-to-creatinine ratio was not measured at baseline for this study. We were therefore unable to adjust for this important determinant of kidney disease. However, we were able to use serum measurements of several filtration markers—creatinine, cystatin C, and β2-microglobulin—to estimate the latent variable for baseline kidney function. In addition, the ARIC Study is a well-characterized cohort with thorough in-person examinations for the assessment of study participants’ health status and risk factors, allowing for rigorous adjustment of known risk factors and thereby decreasing the likelihood of residual confounding.
Our study also has several strengths. The length of follow-up and size of the study population was sufficient to observe several hundred new cases of ESRD in a community-based population. To define the outcome of incident ESRD, we linked the ARIC Study to the US Renal Data System registry, which is a complete list of recipients of renal replacement therapy (i.e., transplant or dialysis) (40, 41). Another strength is the use of structural equation modeling, which allowed us to test a complex set of pathways, to estimate and adjust for latent glycemia and latent kidney function using multiple markers of each construct, and to compare our results with a more common, regression modeling technique. The novelty of our study is also a major strength: To our knowledge, this is the first documentation of the association between 1,5-AG and ESRD.
In conclusion, structural equation modeling is a useful statistical technique for testing complex pathways and modeling latent constructs (in this case, kidney function and glycemia) based on multiple measures (creatinine, cystatin C, and β2-microglobulin for kidney function; glucose, HbA1c, fructosamine, glycated albumin, and diabetes for glycemia). In our community-based population, lower serum levels of 1,5-AG at baseline were strongly associated with increased risk of incident ESRD, independent of demographic characteristics, established risk factors for kidney disease, and baseline kidney function. Blood levels of 1,5-AG represent hyperglycemia and glucose variability, an important metabolic pathway that accelerates progression to ESRD.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Casey M. Rebholz, Morgan E. Grams, Alden L. Gross, Yingying Sang, Josef Coresh, Elizabeth Selvin); Welch Center for Prevention, Epidemiology, and Clinical Research, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Casey M. Rebholz, Morgan E. Grams, Alden L. Gross, Yingying Sang, Josef Coresh, Elizabeth Selvin); Division of Nephrology, Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Morgan E. Grams); Department of Biostatistics, Mailman School of Public Health, Columbia University, New York, New York (Yuan Chen); and Division of General Internal Medicine, Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Josef Coresh, Elizabeth Selvin).
The Atherosclerosis Risk in Communities study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Additional support was provided by National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107782 to C.M.R., R01 DK089174 and K24 DK106414 to E.S., and K08 DK092287 to M.E.G.).
Some of the data reported here have been supplied by the United States Renal Data System registry. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government. Reagents for the 1,5-anhydroglucitol assays were donated by GlycoMark Inc., reagents for the fructosamine and β2-microglobulin assays were donated by Roche Diagnostics, and reagents for the glycated albumin assays were donated by the Asahi Kasei Corporation. The funders did not have a role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Conflict of interest: none declared.
REFERENCES
- 1. Yamanouchi T, Tachibana Y, Akanuma H, et al. . Origin and disposal of 1,5-anhydroglucitol, a major polyol in the human body. Am J Physiol. 1992;263(2 Pt 1):E268–E273. [DOI] [PubMed] [Google Scholar]
- 2. Yamanouchi T, Shinohara T, Ogata N, et al. . Common reabsorption system of 1,5-anhydro-D-glucitol, fructose, and mannose in rat renal tubule. Biochim Biophys Acta. 1996;1291(1):89–95. [DOI] [PubMed] [Google Scholar]
- 3. Akanuma Y, Morita M, Fukuzawa N, et al. . Urinary excretion of 1,5-anhydro-D-glucitol accompanying glucose excretion in diabetic patients. Diabetologia. 1988;31(11):831–835. [DOI] [PubMed] [Google Scholar]
- 4. Parrinello CM, Selvin E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep. 2014;14(11):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Juraschek SP, Steffes MW, Miller ER 3rd, et al. . Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care. 2012;35(11):2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin A(1c) and fasting glucose. Clin Chem. 2012;58(12):1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buse JB, Freeman JL, Edelman SV, et al. . Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol Ther. 2003;5(3):355–363. [DOI] [PubMed] [Google Scholar]
- 8. Stettler C, Stahl M, Allemann S, et al. . Association of 1,5-anhydroglucitol and 2-h postprandial blood glucose in type 2 diabetic patients. Diabetes Care. 2008;31(8):1534–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dungan KM. 1,5-anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn. 2008;8(1):9–19. [DOI] [PubMed] [Google Scholar]
- 10. Wang C, Song J, Ma Z, et al. . Fluctuation between fasting and 2-H postload glucose state is associated with chronic kidney disease in previously diagnosed type 2 diabetes patients with HbA1c ≥ 7%. PLoS One. 2014;9(7):e102941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garg R, Williams ME. Diabetes management in the kidney patient. Med Clin North Am. 2013;97(1):135–156. [DOI] [PubMed] [Google Scholar]
- 12. Chiu PF, Wu CL, Huang CH, et al. . Lower blood glucose and variability are associated with earlier recovery from renal injury caused by episodic urinary tract infection in advanced type 2 diabetic chronic kidney disease. PLoS One. 2014;9(9):e108531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Selvin E, Rawlings AM, Grams M, et al. . Association of 1,5-anhydroglucitol with diabetes and microvascular conditions. Clin Chem. 2014;60(11):1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu B, Zheng Y, Nettleton JA, et al. . Serum metabolomic profiling and incident CKD among African Americans. Clin J Am Soc Nephrol. 2014;9(8):1410–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tangri N, Stevens LA, Schmid CH, et al. . Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int. 2011;79(4):471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stevens LA, Coresh J, Greene T, et al. . Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–2483. [DOI] [PubMed] [Google Scholar]
- 17. Inker LA, Schmid CH, Tighiouart H, et al. . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beran TN, Violato C. Structural equation modeling in medical research: a primer. BMC Res Notes. 2010;3:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gross AL, Power MC, Albert MS, et al. . Application of latent variable methods to the study of cognitive decline when tests change over time. Epidemiology. 2015;26(6):878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gross AL, Sherva R, Mukherjee S, et al. . Calibrating longitudinal cognition in Alzheimer's disease across diverse test batteries and datasets. Neuroepidemiology. 2014;43(3–4):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 22. Inker LA, Tighiouart H, Coresh J, et al. . GFR estimation using β-trace protein and β2-microglobulin in CKD. Am J Kidney Dis. 2016;67(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Selvin E, Rawlings A, Lutsey P, et al. . Association of 1,5-anhydroglucitol with cardiovascular disease and mortality. Diabetes. 2016;65(1):201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. [DOI] [PubMed] [Google Scholar]
- 25. Pencina MJ, D'Agostino RB, Pencina KM, et al. . Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176(6):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Diabetes Association Postprandial blood glucose. American Diabetes Association. Diabetes Care. 2001;24(4):775–778. [DOI] [PubMed] [Google Scholar]
- 27. Ceriello A, Taboga C, Tonutti L, et al. . Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation. 2002;106(10):1211–1218. [DOI] [PubMed] [Google Scholar]
- 28. Ceriello A, Esposito K, Piconi L, et al. . Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–1354. [DOI] [PubMed] [Google Scholar]
- 29. Wascher TC, Schmoelzer I, Wiegratz A, et al. . Reduction of postchallenge hyperglycaemia prevents acute endothelial dysfunction in subjects with impaired glucose tolerance. Eur J Clin Invest. 2005;35(9):551–557. [DOI] [PubMed] [Google Scholar]
- 30. Moriel P, Abdalla DS. Nitrotyrosine bound to beta-VLDL-apoproteins: a biomarker of peroxynitrite formation in experimental atherosclerosis. Biochem Biophys Res Commun. 1997;232(2):332–335. [DOI] [PubMed] [Google Scholar]
- 31. Beckman JS, Beckman TW, Chen J, et al. . Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87(4):1620–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azuma K, Kawamori R, Toyofuku Y, et al. . Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arterioscler Thromb Vasc Biol. 2006;26(10):2275–2280. [DOI] [PubMed] [Google Scholar]
- 33. Otsuka A, Azuma K, Iesaki T, et al. . Temporary hyperglycaemia provokes monocyte adhesion to endothelial cells in rat thoracic aorta. Diabetologia. 2005;48(12):2667–2674. [DOI] [PubMed] [Google Scholar]
- 34. Quagliaro L, Piconi L, Assaloni R, et al. . Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis. 2005;183(2):259–267. [DOI] [PubMed] [Google Scholar]
- 35. Quagliaro L, Piconi L, Assaloni R, et al. . Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52(11):2795–2804. [DOI] [PubMed] [Google Scholar]
- 36. Slinker BK, Glantz SA. Multiple regression for physiological data analysis: the problem of multicollinearity. Am J Physiol. 1985;249(1 Pt 2):R1–R12. [DOI] [PubMed] [Google Scholar]
- 37. Vatcheva KP, Lee M, McCormick JB, et al. . Multicollinearity in regression analyses conducted in epidemiologic studies. Epidemiology (Sunnyvale). 2016;6(2):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peralta CA, Shlipak MG, Judd S, et al. . Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305(15):1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rebholz CM, Grams ME, Matsushita K, et al. . Change in novel filtration markers and risk of ESRD. Am J Kidney Dis. 2015;66(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collins AJ, Foley RN, Gilbertson DT, et al. . United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl (2011). 2015;5(1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Foley RN, Collins AJ. The USRDS: what you need to know about what it can and can't tell us about ESRD. Clin J Am Soc Nephrol. 2013;8(5):845–851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.