Abstract
Masked hypertension (MHT), defined as nonelevated blood pressure (BP) in the clinic setting and elevated BP assessed by ambulatory monitoring, is associated with increased risk of target organ damage, cardiovascular disease, and mortality. Currently, no estimate of MHT prevalence exists for the general US population. After pooling data from the Masked Hypertension Study (n = 811), a cross-sectional clinical investigation of systematic differences between clinic BP and ambulatory BP (ABP) in a community sample of employed adults in the New York City metropolitan area (2005–2012), and the National Health and Nutrition Examination Survey (NHANES; 2005–2010; n = 9,316), an ongoing nationally representative US survey, we used multiple imputation to impute ABP-defined hypertension status for NHANES participants and estimate MHT prevalence among the 139 million US adults with nonelevated clinic BP, no history of overt cardiovascular disease, and no use of antihypertensive medication. The estimated US prevalence of MHT in 2005–2010 was 12.3% of the adult population (95% confidence interval: 10.0, 14.5)—approximately 17.1 million persons aged ≥21 years. Consistent with prior research, estimated MHT prevalence was higher among older persons, males, and those with prehypertension or diabetes. To our knowledge, this study provides the first estimate of US MHT prevalence—nearly 1 in 8 adults with nonelevated clinic BP—and suggests that millions of US adults may be misclassified as not having hypertension.
Keywords: ambulatory blood pressure, blood pressure, masked hypertension, multiple imputation, prevalence
In the United States, measurement of blood pressure (BP) in the clinic setting is the primary approach used for diagnosing hypertension (1, 2). Clinic BP (CBP) measurement relies on a small number of readings, ideally 3 but often fewer, taken in a medical office. Some years ago, the concept of “true BP” was introduced (3), defined as the mean level, over time, of a person's BP in his/her natural environment. Several studies have found that CBP provides a relatively poor estimate of “true BP,” while ambulatory blood pressure monitoring (ABPM), which measures out-of-clinic BP in a person's normal, everyday environment, provides the best available estimate (4).
It has long been known that people can have elevated CBP but nonelevated ambulatory BP (ABP) (5). This phenomenon, sometimes called “white-coat hypertension,” has typically been found to not be associated with increased risk of cardiovascular disease (CVD) (6–8). More recently, it has been recognized that people can also have nonelevated CBP but elevated ABP—that is, masked hypertension (MHT) (9). In contrast to white-coat hypertension, persons with MHT have an increased risk of CVD (7, 8, 10–14). The confluence of the failure to be diagnosed by the conventional approach of only measuring BP in the clinic setting and an increased CVD risk makes MHT a potentially significant public health concern. Many US and international guidelines recommend the use of ABPM to exclude white-coat hypertension in persons who have elevated CBP (1, 15–17). Although the US Preventive Services Task Force also recently recommended using ABPM in persons with elevated CBP to identify and avoid treating those with white-coat hypertension (18), the Task Force did not comment on using ABPM in persons with nonelevated CBP for the identification of MHT.
In 2 systematic reviews of population-based studies, the prevalence of MHT among persons with nonelevated CBP was 10%–30% (13, 14). However, the studies identified in those reviews were all conducted in Europe and Japan. Currently, there is no estimate of the prevalence of MHT for the US population, as ABPM has never been conducted in a US national-scale survey. In this study, we 1) externally validated a strategy for imputing ABP-defined hypertension status and 2) used this strategy to simulate/impute ABP-defined hypertension status for participants in the National Health and Nutrition Examination Survey (NHANES; 2005–2010 cycles) with nonelevated CBP in order to estimate the prevalence of MHT among US adults.
METHODS
Masked Hypertension Study
The Masked Hypertension Study (MHTS) includes 888 participants recruited between February 2005 and July 2012 with CBP and adequate 24-hour ABPM data. To be eligible, participants had to be ≥21 years of age, able to speak and read English, and employed at one of 2 universities, their affiliated hospitals, or a financial institution in the New York City metropolitan area (Manhattan and Stony Brook, New York). The study included a BP screening conducted prior to enrollment, followed by 3 study visits made 1 week apart and a 24-hour ABP recording. For safety reasons, persons who had systolic/diastolic BP (SBP/DBP) readings at or above 160/105 mmHg (average of second and third auscultatory readings) during the screening were referred to their physicians for management of their BP and were not included in the MHTS; nearly all of these individuals would have had a clinic BP reading of ≥140/90 mmHg and been excluded from the present analyses. Additionally, the MHTS excluded participants with a self-reported history of overt CVD (myocardial infarction, stroke, congestive heart failure, heart transplant, or major coronary surgery), chronic kidney disease, chronic liver disease, chronic adrenal disease, specific thyroid conditions (e.g., Hashimoto's or Grave's disease), or a current/recent diagnosis of cancer, unless they had been out of treatment and disease-free for at least 6 months. Persons taking medication for hypertension or any other cardiovascular condition (excluding statins) and women who were pregnant were also excluded. After applying these exclusion criteria, 1,011 participants provided written informed consent and were enrolled in the MHTS. The institutional review boards at the participating research centers—Stony Brook University and Columbia University—approved the conduct of the MHTS. Further details on the study design have been provided elsewhere (19).
During the first study visit, 3 manual (auscultatory) BP measurements were taken by a nurse/technician using a mercury sphygmomanometer, according to the recommendations of the American Heart Association (20). Each participant's CBP was calculated as the average of these 3 BP readings. Information on age, race/ethnicity, and sex was obtained during this first visit. During the fifth visit, made 4 weeks after the first, height and weight were measured following standardized procedures (used to calculate body mass index; weight (kg)/height (m)2); smoking status (never, past, or current smoking) was ascertained by interview; and diabetes status was assessed (self-report or fasting glucose concentration ≥126 mg/dL or hemoglobin A1c level ≥6.5%).
At the end of the third visit, participants were fitted with a 24-hour ABP monitor (model 90207; Spacelabs Healthcare, Snoqualmie, Washington) programmed to take readings at 28-minute intervals throughout the subsequent 24 hours. Sleep and awake periods were based on actigraphy data (Actiwatch; Philips Respironics, Murrayville, Pennsylvania), supplemented with self-reported sleep onset and wake-up times. Of the 1,011 enrolled individuals, 893 completed the 24-hour ABPM procedure. Following the approach adopted by the International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (21), we excluded 5 persons who had fewer than 10 valid awake readings from all analyses, which resulted in a final sample size of 888. The average percentage of valid readings was 93%, and 94% of participants had more than 80% valid readings; the average number of valid awake readings was 32 (standard deviation, 5). Consistent with all current guidelines (1, 15–17), ambulatory hypertension was defined as an average awake systolic ABP ≥135 mmHg or diastolic ABP ≥85 mmHg.
National Health and Nutrition Examination Survey
The NHANES is an ongoing cross-sectional survey that recruits participants using a multistage clustered sampling approach (22). Using this design, NHANES data can be weighted to generate disease prevalence estimates that are representative of the civilian, noninstitutionalized population in the United States. NHANES data are collected through in-person interviews and physical examinations performed in mobile examination centers. NHANES is conducted in 2-year cycles; we combined data from cycles 2005–2006, 2007–2008, and 2009–2010 to obtain data representative of a time period similar to that of the MHTS.
During the physical examination, 3 (auscultatory) blood pressure measurements were taken using a mercury column, as in the MHTS, and the average was used to define CBP. ABPM was not performed. Information on demographic factors, including age, race/ethnicity, sex, education, and smoking status, was collected in NHANES using standardized questionnaires. Additionally, height and weight were measured and used to calculate body mass index. Diabetes was defined as fasting glucose concentration ≥126 mg/dL, nonfasting glucose concentration ≥200 mg/dL, glycated hemoglobin level ≥6.5%, or self-reported history of diabetes with concurrent use of antidiabetic medication.
Inclusion/exclusion criteria
By definition, only persons with nonelevated CBP can have MHT. Therefore, we excluded MHTS and NHANES participants with a clinic SBP/DBP reading of ≥140/90 mmHg. For comparability with the MHTS, we also excluded from the NHANES sample those who were under the age of 21 years, had a history of CVD, or were taking antihypertensive medication. We further excluded the few NHANES participants with no valid CBP readings. After applying these exclusion criteria, we analyzed data from 811 MHTS participants and 9,316 NHANES participants.
Two-stage multiple imputation of ABP among NHANES participants
In logistic regression analyses of the MHTS participants, we determined that ambulatory hypertension status (i.e., mean awake ABP ≥135/85 mmHg) was best predicted by a combination of age, sex, race/ethnicity, body mass index, and clinic SBP and DBP; educational level and smoking status were not predictive. Next, because there were sporadic missing data on the demographic/clinical characteristics, we used the fully conditional specification method of multiple imputation (23, 24) to impute these values, separately for the MHTS and NHANES data sets. This step imputed missing data for smoking status, years of education, body mass index, and individual CBP readings for NHANES participants who had only 1 or 2 CBP readings. The procedure yielded 10 imputed data sets for each study. After pooling the data from the 2 studies, we excluded observations with elevated CBP (i.e., SBP/DBP ≥140/90 mmHg) and used the regression-based predicted mean matching fully conditional specification algorithm (24, 25) to impute ambulatory hypertension status—that is, MHT status—for all NHANES participants on the basis of their clinic SBPs and DBPs, the factors noted above, and diabetes status. For each of the 10 pooled MHTS-NHANES data sets with complete data on the predictors, 50 data sets with imputed data on MHT status were generated, yielding a total of 500 multiply imputed data sets. Finally, using software that accounts for NHANES’ multistage sampling design (the SURVEYMEANS procedure in SAS; SAS Institute, Inc., Cary, North Carolina), we estimated the prevalence of MHT and its standard error for all NHANES participants and several subgroups in each imputed data set, and the results were pooled across data sets using standard statistical procedures (23) (see Web Appendix 1, available at http://aje.oxfordjournals.org/); this yielded a point estimate and 95% confidence interval for the national prevalence of MHT.
Validation
To assess the validity of our overall approach, we conducted a simulation analysis using data from a third study, the Improving the Detection of Hypertension (IDH) Study, for which approximately 400 individuals, primarily from the northern Manhattan community surrounding Columbia University Medical Center, had 3 in-clinic auscultatory BP readings taken with a mercury column during their initial visit and then completed 24-hour ABPM. Comparable information on age, sex, race/ethnicity, education, smoking status, body mass index, and diabetes status was collected. Web Appendix 2 provides further details on the IDH Study, including descriptive characteristics of the sample (Web Table 1). We generated 200 bootstrap samples (with replacement) from both the MHTS and IDH samples, pooling them into 200 MHTS-IDH data sets. Ignoring the actual ABPM data in the IDH Study, we performed the identical multiple imputation process for the IDH participants as described above for NHANES; 500 imputed data sets were generated independently for each of the 200 bootstrap samples. We then compared the MHT prevalence estimate and 95% confidence interval in the IDH data, derived from the imputation approach, with the actual MHT prevalence in the IDH Study for each bootstrap sample, and summarized the results across the 200 samples.
Statistical analysis
All analyses were performed using SAS 9.4. Characteristics of the MHTS and NHANES samples are presented as percentages for categorical measures and mean values and standard deviations for continuous measures. The NHANES estimates are based on SAS procedures that incorporate NHANES’ complex multistage sampling design (26). For those measures with missing data, the analysis was performed separately for each imputed data set, and the results were pooled across data sets using standard statistical procedures (23).
In addition to estimating the overall prevalence of MHT among US adults with nonelevated CBP, we estimated the US prevalence of MHT among subgroups categorized as having optimal clinic SBP/DBP (<120/80 mmHg), prehypertension at the lower range of clinic SBP/DBP (≥120/80 mmHg and <130/85 mmHg), or prehypertension at the upper range of clinic SBP/DBP (≥130/85 mmHg and <140/90 mmHg). Prevalence estimates were also calculated by sex, age group, race/ethnicity, and diabetes status. Locally weight scatterplot smoothing (LOESS) curves were fitted to prevalence estimates calculated for each age and CBP value (rounded to the nearest 1 mmHg). Because the MHTS recruited only employed adults, a sensitivity analysis was performed in which the imputation of ABP-defined hypertension status and resulting estimates of the prevalence of MHT were restricted to employed NHANES participants.
RESULTS
External validation of the method
The observed prevalence of MHT among the 347 IDH participants with nonelevated CBP was 16.4%. Across the 200 IDH bootstrap samples, the actual mean prevalence of observed MHT was also 16.4% (range, 11.0%–21.2%). The mean of the 200 estimated prevalence rates, based on multiple imputation of ABP-defined hypertension status, was 15.1% (range, 10.6%–21.3%). Thus, on average, the estimated prevalence rates were 1.24% below the actual prevalence rates, with the average standard error being 3.2%. Importantly, in 192 of the 200 bootstrap samples (96.0%), the actual prevalence of MHT among the IDH participants was within the 95% confidence interval of the prevalence estimate obtained using the multiple imputation procedure. Thus, although there is some indication that the estimates generated by the multiple imputation approach may be downwardly biased, the 95% confidence intervals are neither too conservative nor too liberal. This demonstrates that after pooling of the MHTS data with data from a markedly different population, multiple imputation can be used to generate a valid 95% confidence interval for the prevalence of MHT in the second study. Web Table 2 shows the results obtained from generating subgroup prevalence estimates for the 200 bootstrap samples and assessing how often their 95% confidence intervals include the actual subgroup prevalence.
Primary analysis
Demographic and clinical characteristics of the MHTS and NHANES samples are shown in Table 1. Compared with NHANES participants, MHTS participants were more likely to be female, be aged 45–64 years, have more than a high school education, and not smoke. Similar percentages of MHTS and NHANES participants were non-Hispanic white, but a higher percentage of NHANES participants were non-Hispanic black or Hispanic. The MHTS participants had slightly lower clinic SBP (113.8 mmHg vs. 115.2 mmHg) and higher clinic DBP (74.4 mmHg vs. 69.1 mmHg) than NHANES participants. In MHTS, the mean awake SBP/DBP ABP was 121.9/76.6 mmHg. The prevalence of MHT among persons with nonelevated CBP in the MHTS was 14.4% (95% confidence interval (CI): 12.0, 16.8). This estimate is based on a single visit to the clinic (mean of 3 blood pressure readings). In a previous publication (19), we reported that the prevalence of MHT among persons with nonelevated CBP was 15.7% when CBP was based on 3 clinic visits (3 readings/visit).
Table 1.
Characteristics of Participants With Nonelevated Clinic Blood Pressurea in the Masked Hypertension Study (2005–2012) and the National Health and Nutrition Examination Survey (2005–2010)
| Variable | MHTS (n = 811) | NHANES (n = 9,316) | Employed NHANES Respondents Only (n = 6,327) | |||
|---|---|---|---|---|---|---|
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | |
| Male sex | 38.7 | 47.7 | 52.7 | |||
| Age, years | 44.7 (10.5) | 41.0 (13.9) | 39.4 (11.8) | |||
| Age range, yearsb | ||||||
| 21–44 | 47.5 | 62.5 | 66.2 | |||
| 45–64 | 50.6 | 31.1 | 31.8 | |||
| ≥65 | 2.0 | 6.4 | 2.0 | |||
| Race/ethnicity | ||||||
| Non-Hispanic white | 69.8 | 68.2 | 68.7 | |||
| Non-Hispanic black | 7.0 | 9.9 | 9.6 | |||
| Hispanic | 12.1 | 15.1 | 15.0 | |||
| Other | 11.1 | 6.7 | 6.6 | |||
| Education | ||||||
| Less than high school | 0.7 | 16.7 | 14.1 | |||
| High school diploma | 11.3 | 22.9 | 21.9 | |||
| More than high school | 88.1 | 60.4 | 63.9 | |||
| Smoking status | ||||||
| Never smoked | 67.2 | 54.7 | 56.2 | |||
| Past smoker | 24.9 | 20.9 | 20.5 | |||
| Current smoker | 8.0 | 24.4 | 23.3 | |||
| BMIc | 27.4 (5.3) | 27.7 (6.0) | 27.6 (5.8) | |||
| Obesity (BMI ≥30) | 27.1 | 28.9 | 28.4 | |||
| Clinic blood pressured | ||||||
| Systolic | 113.8 (10.8) | 115.2 (10.9) | 115.2 (10.6) | |||
| Diastolic | 74.4 (7.4) | 69.1 (9.5) | 69.5 (9.4) | |||
| Ambulatory blood pressure | ||||||
| Systolic | 121.9 (9.7) | N/A | N/A | |||
| Diastolic | 76.6 (7.0) | |||||
Abbreviations: BMI, body mass index; MHTS, Masked Hypertension Study; N/A, not applicable; NHANES, National Health and Nutrition Examination Survey; SD, standard deviation.
a Defined as clinic systolic/diastolic blood pressure <140/90 mmHg (average of 3 readings taken at a single clinic visit). Persons with a history of cardiovascular disease, use of antihypertensive medication, or age <21 years were excluded.
b Because of rounding, the percentages within a block do not always sum to exactly 100.0%.
c Weight (kg)/height (m)2.
d Clinic blood pressure was based on the average of 3 readings taken at a single clinic visit.
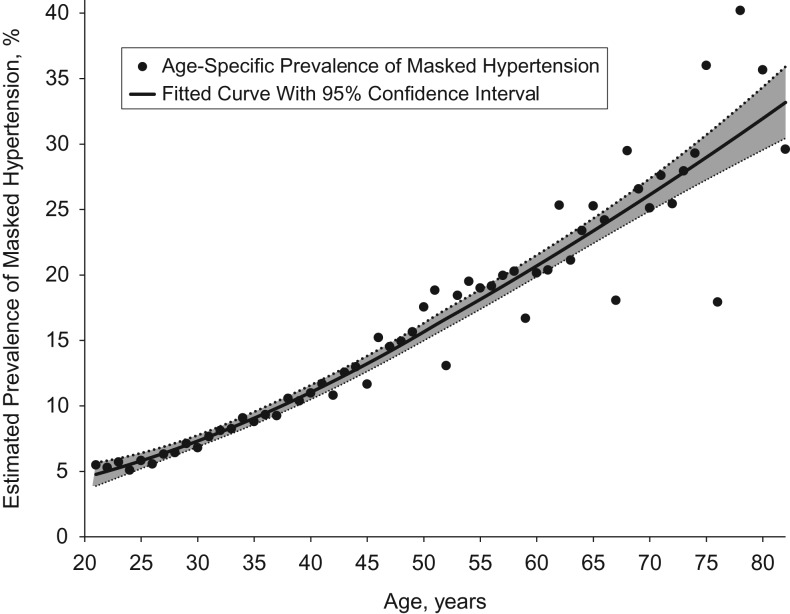
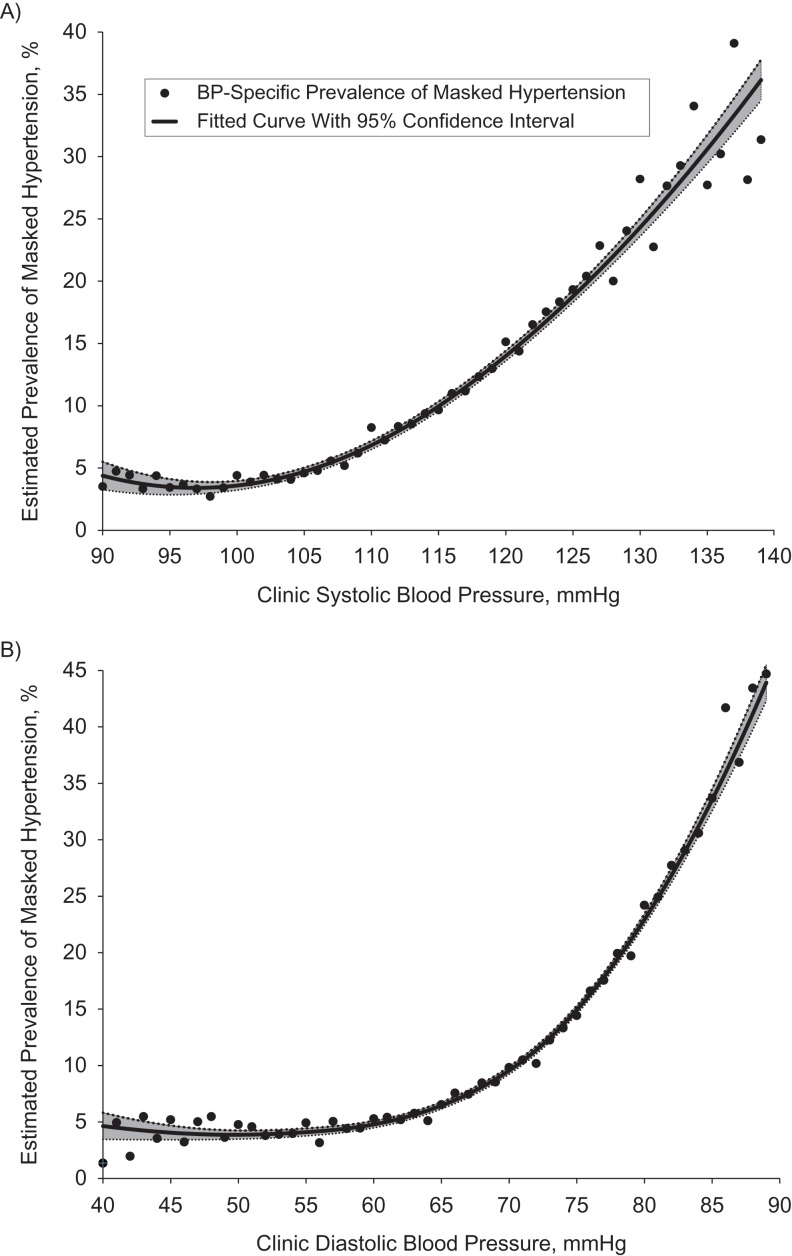
The estimated prevalence of MHT among the approximately 139.3 million US adults with nonelevated CBP who were not taking antihypertensive medication and did not have a history of overt CVD was 12.3% (95% CI: 10.0, 14.5). This corresponds to approximately 17.1 million (95% CI: 14.0, 20.3) US adults. The estimated prevalence of MHT was 6.6% (95% CI: 4.4, 8.8) among persons with optimal CBP (<120/80 mmHg), 17.3% (95% CI: 13.6, 21.0) among those with prehypertension at the lower range (CBP ≥120/80 mmHg and <130/85 mmHg), and 29.7% (95% CI: 24.1, 35.3) among those with prehypertension at the upper range (CBP ≥130/85 mmHg and <140/90 mmHg) (see Table 2). This translates into an estimated 5.6 million (95% CI: 3.7, 7.5), 6.3 million (95% CI: 4.9, 7.6), and 5.2 million (95% CI: 4.3, 6.7) persons with MHT in these 3 groups, respectively. The estimated prevalence of MHT was more than twice as great in men as in women (18.1% vs. 7.0%) and among persons aged 45 years or older as compared with those aged 21–44 years (17.2% vs. 8.2%). MHT was more common in non-Hispanic blacks and persons with diabetes mellitus, but the confidence intervals for these groups were wide. Figures 1 and 2 show the estimated prevalences of MHT across the continuous spectra of age, clinic SBP, and clinic DBP.
Table 2.
Estimated Prevalence of Masked Hypertension Among US Adults With Nonelevated Clinic Blood Pressurea, National Health and Nutrition Examination Survey, 2005–2010
| Subgroup | Prevalence, % | No. of Persons, millions | ||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
| Total | 12.3 | 10.0, 14.5 | 17.1 | 14.0, 20.3 |
| Clinic blood pressure, mmHg | ||||
| <120/80 | 6.6 | 4.4, 8.8 | 5.6 | 3.7, 7.5 |
| ≥120/80–<130/85 | 17.3 | 13.6, 21.0 | 6.3 | 4.9, 7.6 |
| ≥130/85–<140/90 | 29.7 | 24.1, 35.3 | 5.2 | 4.3, 6.2 |
| Sex | ||||
| Male | 18.1 | 13.9, 22.3 | 12.1 | 9.3, 14.9 |
| Female | 7.0 | 4.8, 9.1 | 5.1 | 3.5, 6.7 |
| Age group, years | ||||
| 21–44 | 8.2 | 5.6, 10.8 | 7.2 | 4.9, 9.4 |
| 45–64 | 17.2 | 13.6, 20.7 | 7.4 | 5.9, 9.0 |
| ≥65 | 28.0 | 15.2, 40.8 | 2.5 | 1.4, 3.7 |
| Race/ethnicity | ||||
| Non-Hispanic white | 12.4 | 9.7, 15.0 | 11.7 | 9.2, 14.2 |
| Non-Hispanic black | 15.7 | 3.5, 28.0 | 2.2 | 0.5, 3.9 |
| Hispanic | 10.8 | 4.5, 17.2 | 2.3 | 0.9, 3.6 |
| Other | 9.8 | 3.0, 16.7 | 0.9 | 0.3, 1.6 |
| Diabetes | ||||
| No | 12.1 | 9.9, 14.3 | 16.1 | 13.1, 19.1 |
| Yes | 16.6 | 7.0, 26.2 | 1.0 | 0.4, 1.6 |
Abbreviation: CI, confidence interval.
a Defined as clinic systolic/diastolic blood pressure < 140/90 mmHg (average of 3 readings taken at a single clinic visit). Persons with a history of cardiovascular disease, use of antihypertensive medication, or age <21 years were excluded.
Figure 1.
Estimated prevalence of masked hypertension in the United States, by age, 2005–2010. Estimates were based on multiple imputation (500 data sets) of hypertension status as defined by ambulatory blood pressure for 9,316 adult participants in the National Health and Nutrition Examination Survey (2005–2010) with nonelevated clinic blood pressure, no history of overt cardiovascular disease, and no use of antihypertensive medication. A locally weight scatterplot smoothing (LOESS) curve using second-degree polynomials (black line) was fitted to the 65 age-specific estimates (black circles), with weights proportional to the inverse of each estimate's squared standard error; the smoothing parameter (1.00) was selected to optimize the generalized cross-validation criterion (36). Gray area, 95% confidence interval.
Figure 2.
Estimated prevalence of masked hypertension according to clinic blood pressure (BP) in the United States, 2005–2010. A) Systolic BP; B) diastolic BP. Estimates were based on multiple imputation (500 data sets) of hypertension status as defined by ambulatory blood pressure for 9,316 adult participants in the National Health and Nutrition Examination Survey (2005–2010) with nonelevated clinic BP, no history of overt cardiovascular disease, and no use of antihypertensive medication. A locally weight scatterplot smoothing (LOESS) curve using second-degree polynomials (black line) was fitted to the 50 BP-specific estimates (black circles), with weights proportional to the inverse of each estimate's squared standard error; smoothing parameters (1.00 for part A, 0.73 for part B) were selected to optimize the generalized cross-validation criterion (36). Gray area, 95% confidence interval.
Projections restricted to employed adults in NHANES
The estimated prevalence of MHT among employed NHANES participants (n = 6,327) was 11.8% (95% CI: 9.0, 14.7), slightly lower than in the overall population (Table 3). This corresponds to an estimated 12.2 million employed adults (95% CI: 9.3, 15.2) with MHT. Patterns in the prevalence of MHT across subgroups of employed US adults were similar to those observed in the overall population (Table 3).
Table 3.
Estimated Prevalence of Masked Hypertension Among Employed US Adults With Nonelevated Clinic Blood Pressurea, National Health and Nutrition Examination Survey, 2005–2010
| Subgroup | Prevalence, % | No. of Persons, millions | ||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
| Total | 11.8 | 9.0, 14.7 | 12.2 | 9.3, 15.2 |
| Clinic blood pressure, mmHg | ||||
| <120/80 | 6.4 | 3.7, 9.0 | 4.0 | 2.3, 5.7 |
| ≥120/80–<130/85 | 16.5 | 12.0, 21.0 | 4.5 | 3.3, 5.8 |
| ≥130/85–<140/90 | 29.5 | 22.4, 36.5 | 3.7 | 2.8, 4.5 |
| Sex | ||||
| Male | 15.7 | 11.2, 20.2 | 8.5 | 6.1, 11.0 |
| Female | 7.6 | 4.6, 10.4 | 3.7 | 2.2, 5.1 |
| Age group, years | ||||
| 21–44 | 8.6 | 5.3, 11.9 | 5.9 | 3.6, 8.2 |
| 45–64 | 17.3 | 13.1, 21.5 | 5.7 | 4.3, 7.1 |
| ≥65 | 32.5 | 16.4, 48.5 | 0.7 | 0.3, 1.0 |
| Race/ethnicity | ||||
| Non-Hispanic white | 11.8 | 8.7, 14.8 | 8.4 | 6.2, 10.5 |
| Non-Hispanic black | 15.7 | 6.2, 25.2 | 1.6 | 0.6, 2.5 |
| Hispanic | 10.6 | 2.2, 19.0 | 1.6 | 0.3, 3.0 |
| Other | 9.9 | 2.7, 17.0 | 0.7 | 0.2, 1.2 |
| Diabetes | ||||
| No | 11.7 | 8.8, 14.6 | 11.6 | 8.7, 14.5 |
| Yes | 15.8 | 5.0, 26.6 | 0.6 | 0.2, 1.0 |
Abbreviation: CI, confidence interval.
a Defined as clinic systolic/diastolic blood pressure <140/90 mmHg (average of 3 readings taken at a single clinic visit). Persons with a history of cardiovascular disease, use of antihypertensive medication, or age <21 years were excluded.
DISCUSSION
Conceptually, MHT represents the failure to diagnose hypertension in the clinic setting. To our knowledge, this study provides the first empirically based estimate of the national prevalence of MHT among US adults with a nonelevated CBP who are not taking antihypertensive medication—that is, the population that is routinely screened for hypertension in primary-care settings. Although several cohort studies have indicated that MHT is associated with an increased risk of CVD events and mortality (7, 8, 11–14), ABPM has not been assessed in any US national health survey; hence, no direct estimate of prevalence is currently available (27). In this study, we combined data from a moderate-sized community sample with data from a large, nationally representative sample (NHANES) to address this evidence gap. Using multiple imputation to simulate the ABP-defined hypertension status of NHANES participants, we estimate that the national prevalence of MHT among the 139 million US adults with a nonelevated CBP is 12.3%. This represents more than 17 million adults who may not currently be recognized by their physicians as having an increased risk of hypertension-related CVD events and mortality, and who might benefit from pharmacological or nonpharmacological treatment to lower their blood pressure.
Although there is no estimate of the prevalence of white-coat hypertension among US adults based on a nationally representative sample, a common estimate is that 20% of persons meeting criteria for hypertension (CBP ≥140/90 mmHg or use of antihypertensive medication) have white-coat hypertension. Using Egan et al.’s estimate, based on NHANES data, that 65 million US adults aged ≥18 years meet this definition of hypertension (28), a rough estimate of the number of persons with white-coat hypertension would be 13 million. Based on our estimates, it is very likely that the number of adults with MHT exceeds the number with white-coat hypertension.
Who should wear a 24-hour ABP monitor?
Several studies have shown that ABP is a better predictor of cardiovascular risk than CBP (29–31). Plausible reasons for ABP's superior predictive power over CBP include the greater number of readings (i.e., increased reliability) and a better estimate of a person's average blood pressure during normal everyday experiences (i.e., increased ecological validity). In 2011, the United Kingdom National Health Service revised the National Institute for Health and Care Excellence blood pressure screening guidelines to include the routine use of ABPM to confirm a diagnosis of hypertension in persons with elevated CBP (15). This guideline estimated a potential savings of £10 million ($16 million) over 4–5 years, mainly from the identification of white-coat hypertension and subsequent reduction in treatment costs (32). In a review of the literature on the economic value of adding secondary diagnostic modalities to CBP (33), we found that augmenting an initial diagnosis of elevated BP in the clinic with out-of-clinic BP measurement such as ABPM is cost-effective. Recent guidelines issued by the US Preventive Services Task Force (18) also recommend that ABPM be used to confirm a diagnosis of hypertension in persons with elevated CBP to avoid overtreatment of persons with white-coat hypertension.
None of the clinical guidelines recommend ABPM as an adjunct to CBP for the purpose of diagnosing MHT. We believe this is a serious omission, given the increased risk of CVD events and mortality associated with MHT. However, the use of ABPM in all persons with nonelevated CBP would not be cost-effective. Our study confirms findings from previous analyses of community and patient samples showing that the prevalence of MHT is higher among men, those aged ≥45 years, those with diabetes, and (especially) those with prehypertension. Therefore, it may be prudent to perform ABPM in persons with an elevated risk of having MHT. The health benefits and net costs of treating persons with MHT have yet to be evaluated, and future research is needed to identify which individuals/patients with nonelevated CBP should be screened for MHT.
Limitations of this study
Our results should be considered in the context of some study limitations. First, this study should be viewed as providing an interim estimate of the national prevalence of MHT. If and when a national health survey, such as NHANES, includes ABPM, we will have a more definitive estimate. More specifically, the 95% confidence interval for our prevalence estimate is 2.35 times as wide as would have been obtained if NHANES included ABPM. Second, our application of multiple imputation relies on the assumption that the multivariate relationship of ABP-defined hypertension status to CBP and the other prespecified predictors in the MHTS is consistent with what it would have been in NHANES had the latter study collected ABP data. Importantly, for this approach to be valid, it is not necessary that the MHTS sample be representative of the US adult population, only that the relationship of ABP-defined hypertension to clinic BP and other predictors be generalizable to the larger population. While untestable, this assumption is certainly plausible as a first-order approximation.
Third, because MHTS enrolled employed persons in the New York City metropolitan area, the possibility that employment status modifies the relationship of ambulatory hypertension status to CBP cannot be ruled out. However, our approach was validated in an independent cohort study, the IDH Study, which enrolled both employed and unemployed community participants. Further, the prevalence estimates for all US adults and for only employed US adults were quite similar. Fourth, by design, persons taking antihypertensive medication were excluded from the MHTS. Therefore, our prevalence estimates cannot be extrapolated to persons taking antihypertensive medication who appear, based on CBP, to have their hypertension controlled but might have “masked uncontrolled hypertension.” There is evidence to suggest that the prevalence of masked uncontrolled hypertension in treated individuals is quite high (34, 35). Finally, this study defined MHT as the combination of a nonelevated CBP and elevated awake ABP; had we also included ABP-defined nocturnal hypertension in the definition, the estimated US prevalence of MHT would necessarily have been higher.
While we would have liked to estimate the prevalence of white-coat hypertension among US adults with elevated CBP, the MHTS includes only 77 persons with untreated elevated CBP (i.e., ≥140/90 mmHg). With so few individuals, multiple imputation would have yielded a 95% confidence interval that was too wide to be informative.
Conclusion
In conclusion, we estimate that approximately 17.1 million US adults (almost 1 in 8) with nonelevated CBP and no use of antihypertensive medication have MHT. Consistent with previous research, the prevalence of MHT is higher in older adults, males, those with diabetes, and (especially) those with prehypertension. Given that MHT cannot be diagnosed in the clinic setting but is associated with an increased risk of CVD events and mortality, our results suggest that current BP screening strategies in the United States that rely only on CBP assessment will misdiagnose a substantial number of adults as not having hypertension. Additional studies should 1) identify cost-effective strategies for diagnosing MHT in adults with nonelevated CBP, especially among subgroups with a higher risk of MHT and CVD, and 2) identify appropriate treatment modalities to reduce the health risks associated with MHT. Given the high prevalence of MHT among US adults, incorporating ABPM into clinic-based hypertension screening could substantially impact the cardiovascular health of the US population.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Health Policy and Management, Columbia Mailman School of Public Health, Columbia University, New York, New York (Y. Claire Wang); Center for Cardiovascular Behavioral Medicine, Department of Medicine, Columbia University Medical Center, New York, New York (Daichi Shimbo, Joseph E. Schwartz); Department of Epidemiology, School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama (Paul Muntner); Division of General Medicine, Department of Medicine, Columbia University Medical Center, New York, New York (Andrew E. Moran); Cardiovascular Institute, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, New York (Lawrence R. Krakoff); and Applied Behavioral Medicine Research Institute, Department of Psychiatry, School of Medicine, Stony Brook University, Stony Brook, New York (Joseph E. Schwartz).
This study was supported by a program project grant from the National Heart, Lung, and Blood Institute (grant P01-HL047540) and received additional support from the National Center for Advancing Translational Sciences (formerly the National Center for Research Resources), National Institutes of Health, through grants MO1-RR10710 (Stony Brook University) and UL1-TR000040 (formerly UL1-RR024156; Columbia University). P.M. was supported by grant 15SFRN2390002 from the American Heart Association.
Dr. William B. White of the University of Connecticut School of Medicine (Farmington, Connecticut) provided valuable feedback and suggestions on an earlier draft of the manuscript. Dr. Peter Kaufmann of the National Heart, Lung, and Blood Institute (Bethesda, Maryland) and Dr. Kenneth Kleinman of Harvard Medical School (Boston, Massachusetts) provided valuable suggestions for both the primary analysis and the design of the external validation study during discussions with the senior author. We thank each of them for their contribution.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. [DOI] [PubMed] [Google Scholar]
- 3. Pickering TG. The ninth Sir George Pickering memorial lecture. Ambulatory monitoring and the definition of hypertension [editorial]. J Hyptertens. 1992;10(5):401–409. [DOI] [PubMed] [Google Scholar]
- 4. Pickering TG, Gerin W, Schwartz JE, et al. Franz Volhard lecture: should doctors still measure blood pressure? The missing patients with masked hypertension. J Hypertens. 2008;26(12):2259–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pickering TG, James GD, Boddie C, et al. How common is white coat hypertension. JAMA. 1988;259(2):225–228. [PubMed] [Google Scholar]
- 6. Verdecchia P, Reboldi GP, Angeli F, et al. Short- and long-term incidence of stroke in white-coat hypertension. Hypertension. 2005;45(2):203–208. [DOI] [PubMed] [Google Scholar]
- 7. Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25(11):2193–2198. [DOI] [PubMed] [Google Scholar]
- 8. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens. 2011;24(1):52–58. [DOI] [PubMed] [Google Scholar]
- 9. Pickering TG, Davidson K, Gerin W, et al. Masked hypertension. Hypertension. 2002;40(6):795–796. [DOI] [PubMed] [Google Scholar]
- 10. Liu JE, Roman MJ, Pini R, et al. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med. 1999;131(8):564–572. [DOI] [PubMed] [Google Scholar]
- 11. Björklund K, Lind L, Zethelius B, et al. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation. 2003;107(9):1297–1302. [DOI] [PubMed] [Google Scholar]
- 12. Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21(9):969–975. [DOI] [PubMed] [Google Scholar]
- 13. Bobrie G, Clerson P, Ménard J, et al. Masked hypertension: a systematic review. J Hypertens 2008;26(9):1715–1725. [DOI] [PubMed] [Google Scholar]
- 14. Peacock J, Diaz KM, Viera AJ, et al. Unmasking masked hypertension: prevalence, clinical implications, diagnosis, correlates and future directions. J Hum Hypertens. 2014;28(9):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Institute for Health and Care Excellence Hypertension: Clinical Management of Primary Hypertension in Adults. (NICE clinical guideline 127). London, United Kingdom: National Institute for Health and Care Excellence; 2011. [Google Scholar]
- 16. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32(1):3–15. [DOI] [PubMed] [Google Scholar]
- 17. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–1357. [DOI] [PubMed] [Google Scholar]
- 18. Siu AL; US Preventive Services Task Force . Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778–786. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz JE, Burg MM, Shimbo D, et al. Clinic blood pressure underestimates ambulatory blood pressure in an untreated employer-based US population: results from the Masked Hypertension Study. Circulation. 2016;134(23):1794–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals. Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–161. [DOI] [PubMed] [Google Scholar]
- 21. Thijs L, Hansen TW, Kikuya M, et al. The International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12(4):255–262. [DOI] [PubMed] [Google Scholar]
- 22. Curtin LR, Mohadjer LK, Dohrmann SM, et al. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital Health Stat 2. 2013;(160):1–23. [PubMed] [Google Scholar]
- 23. Little RJ, Rubin DB. Statistical Analysis With Missing Data. Hoboken, NJ: John Wiley & Sons, Inc.; 2002. [Google Scholar]
- 24. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. [DOI] [PubMed] [Google Scholar]
- 25. Schenker N, Taylor JMG. Partially parametric techniques for multiple imputation. Computat Stat Data Anal. 1996;22(4):425–446. [Google Scholar]
- 26. Johnson CL, Paulose-Ram R, Ogden CL, et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. Vital Health Stat 2. 2013;(161):1–24. [PubMed] [Google Scholar]
- 27. Giles TD, Black HR, Messerli F, et al. Ambulatory blood pressure monitoring should be included in the National Health and Nutritional Examination Survey (NHANES). J Am Soc Hypertens. 2012;6(5):364–366. [DOI] [PubMed] [Google Scholar]
- 28. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–2050. [DOI] [PubMed] [Google Scholar]
- 29. Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16(7):971–975. [DOI] [PubMed] [Google Scholar]
- 30. Hansen TW, Kikuya M, Thijs L, et al. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007;25(8):1554–1564. [DOI] [PubMed] [Google Scholar]
- 31. Pickering T, Schwartz J, Verdecchia P, et al. Prediction of strokes versus cardiac events by ambulatory monitoring of blood pressure: results from an international database. Blood Press Monit. 2007;12(6):397–399. [DOI] [PubMed] [Google Scholar]
- 32. Lovibond K, Jowett S, Barton P, et al. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet. 2011;378(9798):1219–1230. [DOI] [PubMed] [Google Scholar]
- 33. Wang YC, Koval AM, Nakamura M, et al. Cost-effectiveness of secondary screening modalities for hypertension. Blood Press Monit. 2013;18(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Franklin SS, Thijs L, Li Y, et al. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013;61(5):964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Banegas JR, Ruilope LM, de la Sierra A, et al. High prevalence of masked uncontrolled hypertension in people with treated hypertension. Eur Heart J. 2014;35(46):3304–3312. [DOI] [PubMed] [Google Scholar]
- 36. Craven P, Wahba G. Smoothing noisy data with spline functions. Numer Math. 1979;31(4):377–403. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.