Abstract
Evidence supports an association between maternal exposure to air pollution during pregnancy and children’s health outcomes. Recent interest has focused on identifying critical windows of vulnerability. An analysis based on a distributed lag model (DLM) can yield estimates of a critical window that are different from those from an analysis that regresses the outcome on each of the 3 trimester-average exposures (TAEs). Using a simulation study, we assessed bias in estimates of critical windows obtained using 3 regression approaches: 1) 3 separate models to estimate the association with each of the 3 TAEs; 2) a single model to jointly estimate the association between the outcome and all 3 TAEs; and 3) a DLM. We used weekly fine-particulate-matter exposure data for 238 births in a birth cohort in and around Boston, Massachusetts, and a simulated outcome and time-varying exposure effect. Estimates using separate models for each TAE were biased and identified incorrect windows. This bias arose from seasonal trends in particulate matter that induced correlation between TAEs. Including all TAEs in a single model reduced bias. DLM produced unbiased estimates and added flexibility to identify windows. Analysis of body mass index z score and fat mass in the same cohort highlighted inconsistent estimates from the 3 methods.
Keywords: air pollution, children’s health, confounding bias, critical windows, distributed lag models, seasonality
There is a growing body of epidemiologic and experimental evidence that supports an association between maternal exposures to air pollution and both birth and children’s health outcomes, including decreased birth weight and increased risk of preterm birth and childhood wheeze (1–8). Because prenatal development occurs through a multiple-event process starting in early gestation (9), it is hypothesized that the association between maternal exposure to air pollution and children’s health outcomes may vary throughout pregnancy. In particular, there may be critical windows during which there is an increased association between prenatal exposure and a future health outcome (10–12). However, different statistical approaches to estimate the association between exposure over the course of pregnancy and future health outcomes can result in substantially different estimates of the exposure effect and can identify different critical windows.
With regards to air pollution and child health, the most commonly used approach to estimate the association between maternal exposures during pregnancy and a future health outcome is to regress the outcome on each trimester average exposure (TAE) separately in 3 regression models. Several recent papers reviewed numerous studies that have taken this approach to estimate the association between maternal exposures to fine particulate matter (having an aerodynamic diameter of ≤2.5 μm (PM2.5)) and children’s health outcomes (3, 13, 14). The use of TAEs is particularly appealing because it is easy to implement and interpret. Further, it is convenient given that pregnancy is typically 9 months long and easily broken down into intervals of 3 months. However, biological changes do not cleanly follow 3-month intervals, and the potential for windows to span multiple trimesters or to exist in periods shorter or longer than 3 months must be considered.
Several recent studies have proposed data-driven methods, such as distributed lag models (DLMs), to estimate the association between maternal exposure to PM2.5 and children’s health outcomes (15–19). The DLM framework was originally developed for time-series analysis where an outcome observed on a given day is regressed on exposures over a previous time period to estimate how the effect of exposure on 1 day is distributed over the subsequent time period (20, 21). When applied to maternal exposure during pregnancy, the DLM regresses a child’s health outcome on exposure measured at regular intervals, such as daily or weekly, throughout the pregnancy. In this setting, the DLM estimates the time-varying association between a time-varying exposure and an outcome observed at a single time. DLMs are often constrained so that the exposure effect varies smoothly over time using a parametric model (such as a quadratic function of time), splines, Bayesian priors, or other penalization approach (21, 22), but they are not constrained to correspond with clinically defined trimesters. Hence the class of DLMs is a data-driven approach to identifying critical windows and is likely to identify critical windows that would be missed by traditional regression approaches.
This work was motivated by analyses of data from a Boston-area prebirth cohort designed to identify critical windows of air pollution exposures on multiple children’s health outcomes. Preliminary analyses of the relationship between weekly PM2.5 exposure and several children’s health outcomes showed that the results were sensitive to the analysis method used and different conclusions would be reached with different methods. In the present work, we compared statistical approaches to estimating the association between maternal exposure to air pollution during pregnancy and children’s health outcomes in both a simulation study and data analysis. The approaches are: 1) a model with a single TAE without controlling for exposures in the other 2 trimesters; 2) a model containing all TAEs in a single regression model; and 3) a DLM. The simulation study is designed to illustrate and quantify the bias in the estimated time-varying exposure effects using these methods under 6 realistic scenarios. We then illustrated the sensitivity of estimates obtained from the methods by estimating the relationship between PM2.5 and each of body mass index z score (BMIz) and fat mass in the same cohort as a case study.
METHODS
Data
We used data from the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project (23). ACCESS is a prospective, longitudinal study originally funded to recruit n = 500 mother-child pairs between August 2002 and January 2007. The women were at least 18 years of age, spoke English or Spanish, and received prenatal care at one of 2 hospitals in the Boston, Massachusetts, area or at affiliated community health centers.
Between 3 and 6 (mean = 3.95; standard deviation, 0.67) years of age, child weight and height were measured using a calibrated scale. BMIz was derived based on 2000 Centers for Disease Control and Prevention reference data (24), based on the sex of the child and age at measurement. Fat mass was determined based on bipolar bioelectrical impedance assessed using the BIM4 bioimpedance analyzer (Impedimed, Queensland, Australia) (25).
Maternal exposures to PM2.5 were estimated based on residential address using a hybrid land-use regression model that incorporated satellite-derived aerosol optical depth measures (26). Each mother was assigned an average PM2.5 exposure value for each week of pregnancy based on the predicted values at her address of residence. We limited our analysis to full-term births (at least 37 weeks’ gestation) and to exposures during the first 37 weeks of pregnancy, for which we had complete exposure data for all full-term births.
Statistical methods
We estimated the association between weekly PM2.5 exposure over the first 37 weeks of pregnancy and 2 outcomes, BMIz and fat mass, using linear regression. To compare the estimates under different modeling assumptions, we employed different regression approaches.
Separate TAE
The “separate TAE” approach uses separate regression models to estimate the association between each of the TAEs and the outcome. The model to estimate the association between the jth TAE (TAEj for j = 1,2,3) and the outcome (Y) for individual is
| (1) |
where is a vector of baseline covariates, , , and are unknown regression coefficients, and are independent and identically distributed mean zero residuals. We fitted this model separately for each TAE, j = 1,2,3. Each model estimates the association between 1 TAE and the outcome without controlling for the other 2 TAEs. The regression coefficient represents the cumulative effect over trimester j.
Joint TAE
The “joint TAE” approach estimates the association between all TAEs and the outcome in a single regression model. The joint TAE model is
| (2) |
Using this approach, represents the cumulative effect over trimester j adjusted for the other 2 TAEs.
Distributed lag model
The DLM assumes that the association between exposure and outcome varies smoothly over time. The DLM takes the form
| (3) |
where is the regression coefficient for week t. While any of several models for the functional form of as a function of t can be employed (17–19, 27), we modeled as a natural cubic spline. The model controls for the exposure in every other week but makes the assumption that the association between exposure and outcome varies smoothly across weeks. Fitting this model requires the specification of the degrees of freedom of the natural splines. We selected degrees of freedom by letting it range from 3 to 20 and selecting the best-fitting model using generalized cross-validation (28).
The DLM estimates the weekly association between exposure and outcome. The 2 TAE-based models estimate the cumulative exposure effect over each trimester. To make the models comparable, we divided the estimated regression coefficient from the joint and separate TAE approaches by the number of weeks in a given trimester. This results in an estimate of the weekly association between exposure and outcome.
Simulation study
We compared the methods in a simulation study designed to evaluate the bias in each method under 6 plausible patterns for the time-varying exposure effect. This included an exposure effect in 1 trimester only, exposure effect in multiple trimesters, constant exposure effect over the whole pregnancy, and no exposure effect.
For the simulation study we used the observed PM2.5 exposures from the 238 births in the ACCESS data for which there were complete exposure data (up to 37 weeks). Using the real exposure from the ACCESS data ensured realistic seasonal trends and autocorrelation among the weekly exposures.
We simulated the outcomes from a DLM following equation 3. The week-specific exposure effects were constructed to vary smoothly over time using B-splines, and the outcome is Gaussian with variance 100. This gave an average signal-to-noise ratio of about 1:5 across the 6 scenarios. The simulation assumed that no other covariates affect the outcome. In order to isolate the impact of the choice of model, and not any additional residual confounding, there were no seasonal trends in the simulation other than those that can be fully explained by the observed PM2.5 exposures. Additional details on the simulation are provided in Web Appendix 1 (available at https://academic.oup.com/aje).
We compared the performance of the regression approaches in 6 simulated scenarios. These scenarios are specified to have: exposure effect in a critical window that aligns with clinically defined trimesters (scenarios 1 and 2); exposure effect in a critical window that does not align with clinically defined trimesters (scenarios 3 and 4); a constant exposure effect across all 37 weeks (scenario 5); and no exposure effect (scenario 6). For each scenario, we simulated 1,000 data sets and analyzed each data set with all of the regression approaches.
Analysis of the association between PM2.5 and BMIz and fat mass
We applied the approaches to estimate the association between maternal exposure to PM2.5 during pregnancy and child BMIz and fat mass in the ACCESS cohort, both overall and stratified by sex.
We controlled for potential confounding variables: maternal age at enrollment, maternal prepregnancy body mass index, child age at measurement of BMIz and fat mass, an indicator for mother self-reported race (African-American, Hispanic, non-Hispanic White), and an indicator for mother’s highest level of education less than a high school diploma. In addition, we included indicators for missing race, educational level, maternal body mass index, season of birth, and child’s sex.
RESULTS
Correlation in the TAEs
There were 238 mother-child pairs for which there were complete data on exposure through 37 weeks’ gestation and BMIz. Complete information including fat mass was available for 224 of those children. Table 1 shows the average and standard deviation of the TAEs as well as the correlation between the TAEs for PM2.5 among the 238 births. The mean exposure was fairly constant across trimesters, ranging from 10.65 μg/m3 to 10.81 μg/m3. The standard deviations range from 1.52 to 1.87. The TAEs are correlated. TAE1 and TAE3 are positively correlated with each other (Pearson correlation, 0.67). On the other hand, TAE2 is negatively correlated with both TAE1 and TAE3 (correlations of −0.34 and −0.39, respectively).
Table 1.
Summary of Trimester Average Exposures for 238 Mother-Child Pairs Using Data From the Asthma Coalition on Community, Environment, and Social Stress in Boston, Massachusetts, 2002–2009a
| PM2.5 in μg/m3, Mean (SD) | Correlation With | |||
|---|---|---|---|---|
| TAE1 | TAE2 | TAE3 | ||
| TAE1 | 10.81 (1.87) | 1.00 | −0.34 | 0.67 |
| TAE2 | 10.69 (1.52) | −0.34 | 1.00 | −0.39 |
| TAE3 | 10.65 (1.83) | 0.67 | −0.39 | 1.00 |
Abbreviations: PM2.5, particulate matter having an aerodynamic diameter of ≤2.5 μm; SD, standard deviation; TAE, trimester average exposure.
a The table shows the mean values and standard deviations of the TAE for each trimester and the Pearson correlation between TAEs.
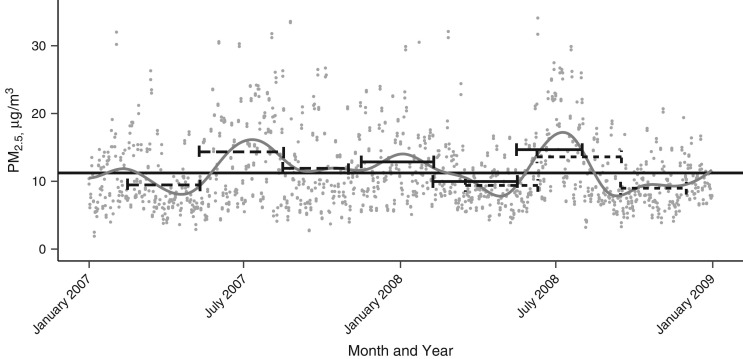
Figure 1 illustrates that this correlation structure arises from seasonal trends in PM2.5. The figure shows PM2.5 levels in Suffolk County, Massachusetts, in 2007 and 2008, as an example, with a smoothed trend line. The horizontal lines span the first, second, and third trimesters for hypothetical births. The height of the lines shows levels of the TAEs for each hypothetical birth. For each of the hypothetical births, the TAEs show an oscillating up-down-up or down-up-down pattern. This pattern results in the positive correlation between TAE1 and TAE3 and negative correlation between TAE2 and the other 2 TAEs.
Figure 1.
Illustration of how seasonal patterns in particulate matter having an aerodynamic diameter of ≤2.5 μm (PM2.5) result in the correlation between trimester average exposures (TAEs) in Suffolk County, Massachusetts, 2007 and 2009. The gray dots are PM2.5 values, and the gray line is the smoothed trend. The horizontal line segments illustrate the TAEs for hypothetical births (each line type—solid, long dash, and short dash—is a different birth). The horizontal line segments are vertically aligned to the TAE and each span 1 trimester.
Simulation study
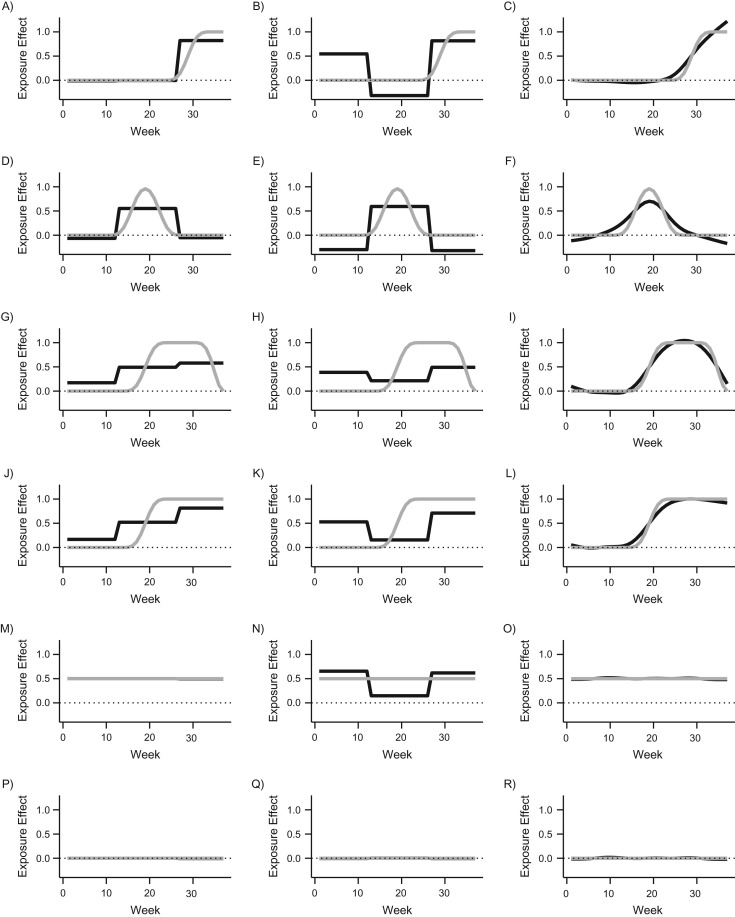
Figure 2 shows results from the simulation study. In scenarios 1 and 2, we simulated situations where there was an exposure effect in 1 trimester only (in trimester 3 for scenario 1 and in trimester 2 for scenario 2). For the trimester where there was a true exposure effect, all methods yield an unbiased effect estimate. Additionally, joint TAE and DLM both resulted in unbiased estimates in the trimesters where there was no exposure effect. However, estimates from the separate TAE approach were biased in the trimesters where there was no true exposure effect. Specifically, estimates in scenario 1 from the separate TAE approach were positively biased in TAE1 and negatively biased in TAE2. In scenario 2, the estimates using the separate TAE approach were negatively biased for both TAE1 and TAE3. Hence, when there was a true exposure effect in only 1 trimester, both joint TAE model and DLM were unbiased, but the separate TAE approach was biased.
Figure 2.
Results from the simulation study comparing the joint trimester average exposure (TAE), separate TAE, and distributed lag model (DLM) approaches. A) Joint TAE and scenario 1; B) separate TAE and scenario 1; C) DLM and scenario 1; D) joint TAE and scenario 2; E) separate TAE and scenario 2; F) DLM and scenario 2; G) joint TAE and scenario 3; H) separate TAE and scenario 3; I) DLM and scenario 3; J) joint TAE and scenario 4; K) separate TAE and scenario 4; L) DLM and scenario 4; M) joint TAE and scenario 5; N) separate TAE and scenario 5; O) DLM and scenario 5; P) joint TAE and scenario 6; Q) separate TAE and scenario 6; and R) DLM and scenario 6. The estimated DLM functions were constructed with natural splines with the degrees of freedom selected with generalized cross-validation. The gray lines show the simulated true exposure effects for each week. The black lines are the mean estimated weekly exposure effects over 1,000 simulated data sets.
In scenarios 3 and 4, the critical windows did not align with clinically defined trimesters. For scenario 3, the window spanned parts of the second and third trimester while for scenario 4 the window was the entire second half of pregnancy. In both scenarios 3 and 4, the separate TAE approach was biased. The estimated exposure effect in the first trimester was larger than the exposure effect in the second trimester, despite a true exposure effect in the second trimester but no true exposure effect in the first trimester.
For both scenarios 3 and 4, the DLM model resulted in unbiased estimates. The joint TAE model correctly identified that the largest exposure effect was in the third trimester and the smallest was in the first trimester, but the estimates were slightly biased for each trimester.
In scenario 5, there was a constant exposure effect over the entire 37 weeks of gestation. Both the DLM and the joint TAE model were unbiased. The separate TAE model was again biased, showing positively biased estimates in the first and third trimester and a negative bias in the second trimester.
Finally, in scenario 6, there was no exposure effect. All methods were unbiased. This is the only scenario for which the separate TAE model was unbiased. Additional results for the cumulative effect are shown in Web Table 1.
Analysis of the association between PM2.5 and BMIz
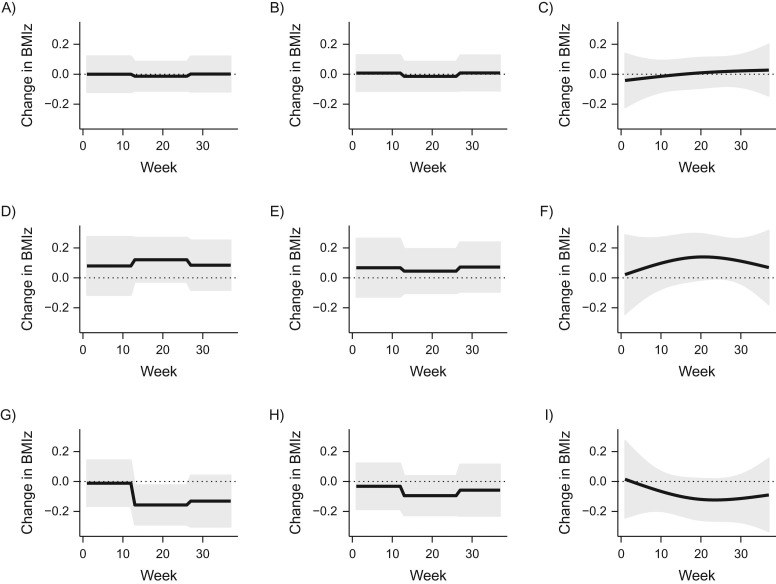
Figure 3 shows the estimated association between maternal PM2.5 exposure and BMIz, both overall and stratified by sex. The analysis includes 238 births (108 girls and 130 boys). All regression models in the data analysis adjusted for the potential confounders previously described.
Figure 3.
Results from the analysis of the association between particulate matter having an aerodynamic diameter of ≤2.5 μm (PM2.5) and body mass index z score (BMIz) in data from the Asthma Coalition on Community, Environment, and Social Stress in the area of Boston, Massachusetts, 2002–2009. The estimates are for all children (n = 238), boys only (n = 130), and girls only (n = 108), each using methods from among joint trimester average exposure (TAE), separate TAE, and distributed lag model (DLM). A) Joint TAE; B) separate TAE; C) DLM; D) joint TAE for boys; E) separate TAE for boys; F) DLM for boys; G) joint TAE for girls; H) separate TAE for girls; I) DLM for girls. For each estimate using DLM, there were 3 degrees of freedom. All estimates were adjusted for maternal race, maternal age, maternal prepregnancy body mass index, maternal educational level, and age of the child when BMIz was measured. The overall estimate was adjusted for child sex. The thick lines show the estimated values, and the gray ribbons show the 95% pointwise confidence intervals.
In the overall analysis, the methods all estimated only a small association between time-varying exposure and outcome. None of the methods identified a critical window.
For the boy-specific analysis, the joint TAE and DLM approaches intensified the largest association between exposure and outcome during the second trimester. However, the separate TAE model estimated the smallest association during the second trimester. Hence, while the joint TAE and DLM suggested that there may be a critical window around the second trimester, the separate TAE provided the contradictory result that there was a decreased association between exposure and outcome during that same period.
For the girl-specific analysis, the methods all identified a negative association between exposure in the second and third trimesters and the outcome. The separate TAE model estimated a smaller association than the other 2 methods, but the general pattern was consistent across all methods.
Analysis of the association between PM2.5 and fat mass
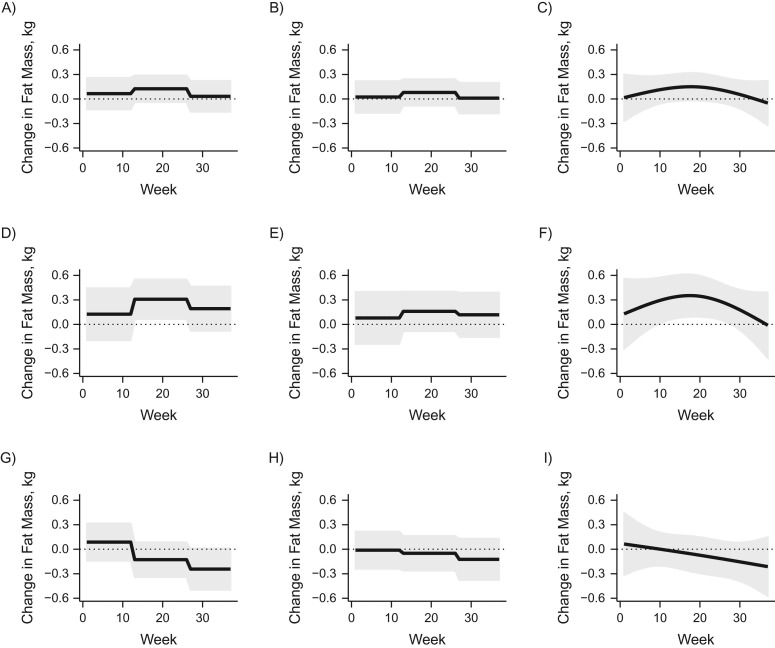
Figure 4 shows the overall and sex-specific estimates of the association between maternal PM2.5 exposure and fat mass as well as 95% pointwise confidence bands. The analysis included 224 births (94 girls and 130 boys). All estimates were adjusted for the potential confounders previously described.
Figure 4.
Results from the analysis of the association between fine particulate matter (PM2.5) and fat mass (kg) in data from the Asthma Coalition on Community, Environment, and Social Stress in the area of Boston, Massachusetts, 2002–2009. The estimates are for all children (n = 224), boys only (n = 130), and girls only (n = 94), each using methods from among joint trimester average exposure (TAE), separate TAE, and distributed lag model (DLM). A) Joint TAE; B) separate TAE; C) DLM; D) joint TAE for boys; E) separate TAE for boys; F) DLM for boys; G) joint TAE for girls; H) separate TAE for girls; I) DLM for girls. For each estimate using DLM, there were 3 degrees of freedom. All estimates were adjusted for maternal race, maternal age, maternal prepregnancy body mass index, maternal educational level, and age of the child when fat mass was measured. The overall estimate was adjusted for child sex. The thick lines show the estimated values, and the gray ribbons show the 95% pointwise confidence intervals.
The methods estimated similar overall trends. For both the overall analysis and the boy-specific analysis, the methods all estimated the that largest association was in the second trimester. For the girl-specific analysis, the methods all estimated a decreasing trend with the largest association in the first trimester and a negative association in the third trimester. However, the methods differed in terms of magnitude and significance. Only the joint TAE and the DLM approaches found a significant association for boys in the second trimester. In large part, the lack of significance in the separate TAE model was due to a smaller point estimate. Web Table 2 shows the cumulative and trimester-specific estimates using each method.
DISCUSSION
Many studies have estimated the association between maternal exposure to PM2.5 during pregnancy and outcomes at birth or in children’s health. Ambient air pollution exposure data can be observed daily. The potential to leverage the high temporal resolution of exposure data to define biologically relevant critical windows is only beginning to be appreciated. Use of TAEs to estimate the exposure effect, with or without controlling for exposure in other trimesters, is a popular method of choice (3, 13, 14). Several other recent studies have estimated the association using DLMs (15–19, 29). We have shown through a simulation study and in a real-data analysis that these approaches can result in substantially different estimates of the association between exposure and outcome, particularly with respect to the ranking of the trimester-specific effect sizes.
The results of the simulation study highlighted that estimating the effect of an exposure during a given window without controlling for exposures in other time windows (the separate TAE approach) can result in biased estimates. Adjusting for exposure during other time periods (joint TAE or DLM) reduced this bias. In several cases (simulation scenarios 3 and 4), the estimates with the separate TAE approach failed to rank the trimesters correctly by the strength of their association with the outcome (Figure 2H and 2K). We demonstrated that this bias could be eliminated when employing a DLM.
Bias in the trimester-specific effect estimates arose due to seasonal trends in air pollution exposures that result in correlation between TAEs (Table 1 and Figure 1). When one estimates the association between the TAEs and the outcome without controlling for other TAEs, the other TAEs act as unmeasured confounders if they are also associated with the outcome. For example, in simulation scenario 1, TAE3 was positively associated with both the outcome and TAE1. When estimating the association between TAE1 and the outcome without controlling for TAE3, the estimated exposure effect of TAE1 was positively biased. In reality, season and other factors that are predictive of seasonal trends in air pollution are the confounding factors.
When the true critical window is 1 or more trimesters (simulation scenarios 1 and 2), the joint TAE and DLM approaches were unbiased. Hence, controlling for exposure during the other trimesters eliminates this bias. However, when the critical window did not align with the trimester boundaries (simulation scenarios 3 and 4), both the joint TAE model and the separate TAE model were misspecified. In this situation, the DLM was the only unbiased approach tested here.
The analysis of the ACCESS cohort further highlights the sensitivity of results to the method of analysis. For the analysis of BMIz, the joint TAE and DLM analysis found the greatest association between PM2.5 and BMIz in the second trimester for boys. However, the separate TAE approach found the weakest association during that same time period (Figure 3E). For fat mass, the joint TAE and DLM found a statistically significant window for boys during the second trimester. However, the separate TAE approach found a weaker association during that period and no statistical significance (Figure 4E). In both analyses, correlation between TAEs resulted in estimates from the separate TAE approach that were compressed toward the null relative to the estimates obtained with the other methods. Sex differences may result for a number of reasons. The prenatal developmental process is not homogeneous across sexes, and sensitive stages of development during which the neonate is vulnerable may occur during different time periods for boys and girls. This may result in a different pattern and strength of association between exposure and outcome between the sexes.
In light of these results, we recommend using the DLM approach when the air pollution exposures are measured at a sufficiently fine temporal resolution to apply the method (e.g., daily or weekly). Both the joint TAE and DLM approaches adjust for exposure during different periods of gestation and reduce the bias incurred by the separate TAE approach. The joint TAE model was unbiased when the exposure was associated with the outcome only in critical windows that align with clinically defined trimesters or is constant. When the critical window did not align with trimesters, the joint TAE estimates were slightly biased but showed a pattern generally consistent with the true exposure effect.
The DLM model is the most flexible model tested here. In all simulation scenarios, the DLM model performed well. DLM has the added advantage of not prespecifying a critical window, which itself can introduce bias. The flexibility of the DLM is better able to capture peaks in the time-varying exposure effect and can evaluate the effect of acute changes in exposure that would be muted by averaging over a 3-month window. Furthermore, the DLM was the only method with unbiased estimates of the cumulative effect (Web Table 1). The results also showed that none of the methods will identify an association when there is not a true association at any time during pregnancy.
The simulated data contained no residual seasonal effects that were not explained by the exposure. Hence, further adjusting for seasonality will not sufficiently control for confounding in the separate TAE model. However, it is important to note that in real-data analyses, seasonal trends may confound the exposure effect through other pathways. It is therefore important to adjust for seasonal trends in practice even when controlling for exposures over other time periods.
We are not the first to note that correlation between TAEs can bias trimester-specific effect estimates. Motivated by correlation between TAEs, Slama et al. (30) presented results for both the separate TAE and joint TAE models to estimate the effect of PM2.5 and nitrogen dioxide (NO2) on birth weight. Several results were statistically significant with the separate TAE model but not with the joint TAE model. Bell et al. (31) also addressed this issue by removing the correlation between TAEs via regression (32–33). This approach uses exposure variables: 1) TAE1; 2) residuals from regressing TAE2 on TAE1; and 3) residuals from regressing TAE3 on TAE1 and TAE2 (which is then repeated after permuting the order of the TAEs). The effect of the first method is the same as the TAE1 effect estimated with the separate TAE approach. The estimate of third method is the same as the estimated TAE3 effect in the joint TAE approach. Hence, this can be viewed as an alternative version of the separate TAE and joint TAE approaches. Another approach suggested within a causal inference framework is to use an imperfect negative control such postbirth exposure (34).
A notable limitation of this work is that we investigated the correlation structure among trimester-specific exposures to PM2.5 in the Boston area of Massachusetts. Seasonal patterns and the resulting correlation between TAEs may be different for different air pollutants and weather exposures or for other locations. Another limitation is that the DLM model requires selection of a tuning parameter. Here, we used generalized cross-validation, which can result in oversmoothed DLMs when the signal-to-noise ratio is small (see simulation scenario 2). However, the correct window was still identified. When there is more data or a larger signal-to-noise ratio, the tuning is more accurate. The natural spline basis used to constrain the DLM in this work is one of several formulations of the DLM. Other approaches include parametric models, Bayesian priors, other penalization approaches, or a cross-basis for nonlinear effects (35–37). Although they are not explored in this paper, it is important to note that these other formulations also properly adjust for exposure throughout pregnancy and can be expected to eliminate the bias observed with TAE models in this paper. Finally, the simulation study presented here focused solely on bias due to seasonal trends in exposure and isolated this from other potential sources of bias, such as from additional residual confounding. We did not investigate whether any of the methods are more or less prone to confounding by other factors. That issue deserves separate investigation.
When interest focuses on the association between maternal exposure to air pollution during pregnancy and birth or children’s health outcomes, the separate TAE model, arguably the most commonly used approach, can result in biased estimates of the exposure effect. The DLM yields unbiased estimates and adds flexibility to identify windows that do not correspond to a clinically defined trimester. When one uses TAEs, the joint TAE model should be used. DLM is preferred to using TAEs when possible.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Statistics, Colorado State University, Fort Collins, Colorado (Ander Wilson); Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, New York (Yueh-Hsiu Mathilda Chiu, Hsiao-Hsien Leon Hsu, Robert O. Wright, Rosalind J. Wright); Kravis Children’s Hospital, Department of Pediatrics, Icahn School of Medicine at Mount Sinai, New York, New York (Yueh-Hsiu Mathilda Chiu, Rosalind J. Wright); Institute for Exposomic Research, Icahn School of Medicine at Mount Sinai, New York, New York (Robert O. Wright, Rosalind J. Wright); and Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Brent A. Coull).
This work was supported by the US Environmental Protection Agency (grant 834798) and National Institutes of Health (grants ES020871, ES007142, CA134294, ES000002, ES023515, ES013744, OD023337, OD023286, and UG3 OD023337). The Asthma Coalition on Community, Environment, and Social Stress study has been supported by the National Institutes of Health (grants R01 ES010932, U01 HL072494, and R01 HL080674).
This publication’s contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US Environmental Protection Agency.
Conflict of interest: none declared.
Abbreviations
- ACCESS
Asthma Coalition on Community, Environment, and Social Stress
- BMIz
body mass index z score
- DLM
distributed lag model
- PM2.5
particulate matter having an aerodynamic diameter of ≤2.5 μm
- TAE
trimester average exposure
REFERENCES
- 1. Edwards S, Maxson P, Sandberg N, et al. Air pollution and pregnancy outcomes In: Nadadur SS, Hollingsworth JW, eds. Air Pollution and Health Effects. London, United Kingdom: Springer-Verlag; 2015:51–91. [Google Scholar]
- 2. Stieb DM, Chen L, Eshoul M, et al. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–111. [DOI] [PubMed] [Google Scholar]
- 3. Shah PS, Balkhair T. Air pollution and birth outcomes: a systematic review. Environ Int. 2011;37(2):498–516. [DOI] [PubMed] [Google Scholar]
- 4. Srám RJ, Binková B, Dejmek J, et al. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113(4):375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allen JL, Oberdorster G, Morris-Schaffer K, et al. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2017;59:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giovanoli S, Engler H, Engler A, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339(6123):1095–1099. [DOI] [PubMed] [Google Scholar]
- 7. Cowell WJ, Bellinger DC, Coull BA, et al. Associations between prenatal exposure to black carbon and memory domains in urban children: modification by sex and prenatal stress. PLoS One. 2015;10(11):e0142492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiu YH, Coull BA, Sternthal MJ, et al. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol. 2014;133(3):713.e4–722.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kajekar R. Environmental factors and developmental outcomes in the lung. Pharmacol Ther. 2007;114(2):129–145. [DOI] [PubMed] [Google Scholar]
- 10. Barr M Jr, DeSesso JM, Lau CS, et al. Workshop to identify critical windows of exposure for children’s health: cardiovascular and endocrine work group summary. Environ Health Perspect. 2000;108(suppl 3):569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. West LJ. Defining critical windows in the development of the human immune system. Hum Exp Toxicol. 2002;21(9):499–505. [DOI] [PubMed] [Google Scholar]
- 12. Sánchez BN, Hu H, Litman HJ, et al. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect. 2011;119(3):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. US Environmental Protection Agency Integrated Science Assessment for Particulate Matter (Final Report, December 2009) Washington, DC: US Environmental Protection Agency; 2009. [PubMed] [Google Scholar]
- 14. Dadvand P, Parker J, Bell ML, et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;121(3):367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang HH, Warren JL, Darrow LA, et al. Assessment of critical exposure and outcome windows in time-to-event analysis with application to air pollution and preterm birth study. Biostatistics. 2015;16(3):509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu HH, Chiu YH, Coull BA, et al. Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. Am J Respir Crit Care Med. 2015;192(9):1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiu YH, Hsu HH, Coull BA, et al. Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ Int. 2016;87:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Warren J, Fuentes M, Herring AH, et al. Spatial-temporal modeling of the association between air pollution exposure and preterm birth: identifying critical windows of exposure. Biometrics. 2012;68(4):1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Darrow LA, Klein M, Strickland MJ, et al. Ambient air pollution and birth weight in full-term infants in Atlanta, 1994–2004. Environ Health Perspect. 2010;119(5):731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11(3):320–326. [DOI] [PubMed] [Google Scholar]
- 21. Zanobetti A, Wand MP, Schwartz J, et al. Generalized additive distributed lag models: quantifying mortality displacement. Biostatistics. 2000;1(3):279–292. [DOI] [PubMed] [Google Scholar]
- 22. Peng RD, Dominici F, Welty LJ. A Bayesian hierarchical distributed lag model for estimating the time course of risk of hospitalization associated with particulate matter air pollution. J R Stat Soc Ser C. 2009;58(1):3–24. [Google Scholar]
- 23. Wright RJ, Suglia SF, Levy J, et al. Transdisciplinary research strategies for understanding socially patterned disease: the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project as a case study. Cien Saude Colet. 2008;13(6):1729–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Center for Health Statistics CDC Growth Charts: United States US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2000. [Google Scholar]
- 25. Rush EC, Bristow S, Plank LD, et al. Bioimpedance prediction of fat-free mass from dual-energy X-ray absorptiometry in a multi-ethnic group of 2-year-old children. Eur J Clin Nutr. 2013;67(2):214–217. [DOI] [PubMed] [Google Scholar]
- 26. Kloog I, Koutrakis P, Coull BA, et al. Assessing temporally and spatially resolved PM2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmos Environ. 2011;45(35):6267–6275. [Google Scholar]
- 27. Warren JL, Stingone JA, Herring AH, et al. Bayesian multinomial probit modeling of daily windows of susceptibility for maternal PM2.5 exposure and congenital heart defects. Stat Med. 2016; 35(16): 2786–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Golub GH, Heath M, Wahba G. Generalized cross-validation as a method for choosing a good ridge parameter. Technometrics. 1979;21(2):215–223. [Google Scholar]
- 29. Warren J, Fuentes M, Herring A, et al. Bayesian spatial-temporal model for cardiac congenital anomalies and ambient air pollution risk assessment. Environmetrics. 2012;23(8):673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slama R, Morgenstern V, Cyrys J, et al. Traffic-related atmospheric pollutants levels during pregnancy and offspring’s term birth weight a study relying on a land-use regression exposure model. Environ Health Perspect. 2007;115(9):1283–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115(7):1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ebisu K, Belanger K, Bell ML. The Association between airborne PM2.5 chemical constituents and birth weight-implication of buffer exposure assignment. Environ Res Lett. 2014;9(8):084007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hyder A, Lee HJ, Ebisu K, et al. PM2.5 exposure and birth outcomes: use of satellite- and monitor-based data. Epidemiology. 2014;25(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weisskopf MG, Tchetgen Tchetgen EJ, Raz R. Commentary: on the use of imperfect negative control exposures in epidemiologic studies. Epidemiology. 2016;27(3):365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gasparrini A, Armstrong B, Kenward MG. Distributed lag non-linear models. Stat Med. 2010;29(21):2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gasparrini A. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat Med. 2014;33(5):881–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Welty LJ, Peng RD, Zeger SL, et al. Bayesian distributed lag models: estimating effects of particulate matter air pollution on daily mortality. Biometrics. 2009;65(1):282–291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.