Abstract
The prevalence of binge drinking in the United States is rising. While alcohol is a risk factor for breast cancer, less is known about the impact of episodic heavy drinking. In 2003–2009, women aged 35–74 years who were free of breast cancer were enrolled in the Sister Study (n = 50,884). Residents of the United States or Puerto Rico who had a sister with breast cancer were eligible. Multivariable Cox regression was used to estimate adjusted hazard ratios and 95% confidence intervals for breast cancer. During follow-up (mean = 6.4 years), 1,843 invasive breast cancers were diagnosed. Increased breast cancer risk was observed for higher lifetime alcohol intake (for ≥230 drinks/year vs. <60 drinks/year, hazard ratio (HR) = 1.35, 95% confidence interval (CI): 1.15, 1.58). Relative to low-level drinkers (<60 drinks/year), hazard ratios were increased for ever binge drinking (HR = 1.29, 95% CI: 1.15, 1.45) or blacking out (HR = 1.39, 95% CI: 1.17, 1.64). Compared with low-level drinkers who never binged, moderate drinkers (60–229 drinks/year) who binged had a higher risk (HR = 1.25, 95% CI: 1.08, 1.44). There was evidence of effect modification between moderate lifetime drinking and binging (relative excess risk due to interaction = 0.33, 95% CI: 0.10, 0.57). Our findings support the established association between lifetime alcohol intake and breast cancer and provide evidence for an increased risk associated with heavy episodic drinking, especially among moderate lifetime drinkers.
Keywords: alcohol, alcohol drinking, binge drinking, breast cancer
In recent years, the prevalences of heavy drinking and binge drinking in the United States have increased sharply (1). This increase has been particularly notable for women, with the prevalence of heavy drinking and binge drinking increasing 38.1% and 18.3%, respectively, from 2002 to 2012 (1). These changes have raised concern regarding the public health impact of these heavy drinking patterns (2). In general, higher levels of alcohol intake have been associated with a number of adverse health outcomes (3), including breast cancer (4). Alcohol has been classified as a Group 1 carcinogen by the International Agency for Research on Cancer (5, 6). However, less well understood is the impact of heavy episodic drinking, or binge drinking, on breast carcinogenesis (7).
For women, binge drinking is often defined as the consumption of 4 or more alcoholic drinks at one sitting, which results in a rapid increase in blood alcohol concentrations (8). A higher blood alcohol concentration can impact biochemical and metabolic processes in response to alcohol drinking (7). Additionally, experimental studies demonstrate that binge drinking increases inflammation levels as well as insulin resistance (9, 10). Inflammation and insulin resistance are hypothesized to be key biological mechanisms for the development of cancer, and thus binge drinking could be particularly relevant for breast cancer risk (11).
In this study, we aimed to estimate the association between lifetime alcohol intake and binge drinking behaviors and breast cancer risk and to evaluate potential effect-measure modification of the relationship between lifetime alcohol intake and breast cancer risk by binge drinking. We hypothesized that both lifetime alcohol intake and binge drinking behaviors would be associated with breast cancer risk and that there would be evidence of a synergistic relationship between lifetime alcohol intake and binge drinking, related to the occurrence of extreme elevations in blood alcohol levels. Better understanding of alcohol drinking patterns, a modifiable breast cancer risk factor, could inform public health strategies for deterring less favorable drinking behaviors.
METHODS
Study population
The Sister Study is a prospective cohort study that was designed to evaluate breast cancer risk factors. In 2003–2009, women with no personal history of breast cancer were recruited for the study through a volunteer network of breast cancer professionals and advocates, as well as a media campaign. Eligibility requirements for study participation included living in the United States or Puerto Rico, being aged 35–74 years, and having a sister who had been diagnosed with breast cancer. Participants completed an extensive telephone questionnaire at baseline, which assessed information on demographic factors, medical and family history, and lifestyle factors, including alcohol intake and lifetime drinking behaviors.
This research was approved by the institutional review boards of the National Institute of Environmental Health Sciences and the Copernicus Group. Written informed consent was obtained from all participants. In this study, we included breast cancer cases that were diagnosed prior to July 1, 2014 (Sister Study Data Release 4.1).
Participants also complete biennial surveys and annual health updates to provide current risk factor information and to notify the study of changes in health. Participation rates have been over 90% throughout follow-up (12).
Outcome assessment
Self-reported diagnoses are validated using medical records. Approximately 80% of medical records have been obtained, and the agreement between self-reported tumor characteristics and medical record-abstracted information is high (13). Thus, self-reported data were used in the absence of available medical records. We considered the estrogen receptor (ER) status of the tumor (ER-positive (ER+) vs. ER-negative (ER−)) and menopausal status at diagnosis (premenopausal vs. postmenopausal) as secondary outcomes.
Exposure and covariate assessment
Information on alcohol consumption was collected as part of the baseline questionnaire. Women were asked about their history of alcohol consumption, including beer and other malt beverages, wine and wine coolers, and liquor. Women were asked about the age at which they started drinking and/or quit drinking. They answered questions on frequency of alcohol intake (days per week, per month, or per year) and how many drinks they tended to have per day on each day they drank, both for current consumption (in the 12 months prior to baseline interview) and by decade of life. For each decade, women were asked what type(s) of alcohol they tended to consume. Former drinkers were defined as women who had not consumed alcohol during the 12 months prior to baseline. Average lifetime alcohol intake was derived by calculating the number of drinks per year for each decade of life and applying weights, where the weights were defined as the number of years spent drinking during that age interval.
Binge drinking was defined as drinking 4 or more alcohol beverages in a row at one sitting and was assessed by decade of life and over the past year. Women who reported binging at any point were further asked how many times in their lifetime they had woken up on the morning after drinking and couldn't remember where they had been or what had happened (i.e., “blacking out”). The questionnaire also included a question on whether a health professional had ever told them that drinking was hurting their health.
Data on covariates of interest, including demographic factors, reproductive history, pack-years of smoking, and use of postmenopausal hormones and oral contraceptives, were obtained from the interview. Height and weight at baseline were measured at a home visit by a trained examiner and used to calculate body mass index (BMI).
Statistical analysis
To evaluate the association between alcohol intake and risk of invasive breast cancer, we used multivariable Cox proportional hazards models to estimate hazard ratios and 95% confidence intervals. The time scale for the Cox model was age, with person-time accruing from age at study enrollment to age at invasive breast cancer diagnosis or censoring at the age of last follow-up or age of diagnosis with in situ disease.
Cox models using restricted cubic splines with 4 knots (at the 5th, 35th, 65th, and 95th percentiles) were used to determine the most appropriate cutpoints for average lifetime alcohol intake (14). Using the spline analysis (see Web Figure 1, available at https://academic.oup.com/aje), low average lifetime alcohol intake was defined as fewer than 60 drinks/year, moderate lifetime alcohol intake was defined as 60–229 drinks/year, and high lifetime alcohol intake was defined as ≥230 drinks/year, on average. The quantity 60 drinks/year represents approximately 1 drink/week; 230 drinks/year represents approximately 4.5 drinks/week. Low average lifetime alcohol intake was used as the referent group when considering the association of individual binge drinking behaviors with breast cancer. Nondrinkers were included in the low-intake category, as their hazard ratios for breast cancer were the same. P values for trend associations were estimated using a χ2 test for the ordinal characterization of the variable. We also evaluated the associations of lifetime alcohol intake and binge drinking with ductal carcinoma in situ, breast cancer hormone receptor status (ER+, ER−), and menopausal status at diagnosis (premenopausal, postmenopausal). For ER-specific analyses, cases without the outcome of interest were censored at the time of diagnosis. A case-case analysis was used to test differences in association by ER status of the tumor (15). When considering premenopausal breast cancer as an outcome, we censored women who became postmenopausal during the follow-up period at their age of menopause. The women who reached menopause without developing breast cancer then contributed person-time to the postmenopause-specific analyses.
The proportional hazards assumption was assessed using an interaction term with the survival time in the regression model using α = 0.05 as well as visually using log-log survival plots. We found no evidence of time-variant associations.
Effect-measure modification of the relationship between breast cancer and lifetime alcohol intake by binge drinking (ever, never) was evaluated on both the additive and multiplicative scales. Additive interaction was tested by calculation of the relative excess risk due to interaction (RERI) (16). A cross-product term and likelihood ratio test were used to evaluate interaction on the multiplicative scale. Additionally, birth cohort, age, postmenopausal hormone use, tobacco use, BMI, and number of first-degree relatives with breast cancer were also considered as potential effect-measure modifiers. Confounders were identified using the prior literature and a directed acyclic graph (17). Multivariable-adjusted models included race/ethnicity (non-Hispanic white, other), education (high school diploma/equivalent or less, some college, 4-year degree or higher), age at menarche (years; continuous), age at first birth (nulliparous or <21, 21–24, 25–28, 29–31, or ≥32 years), parity (nulliparous or 1, 2–3, or ≥4 births), use of oral contraceptives (ever, never), use of hormone replacement therapy at enrollment (none, estrogen only, estrogen + progesterone combined or both estrogen and estrogen + progesterone combined), age at menopause (based on enrollment information; premenopausal or <40, 40–50, 51–55, or >55 years), pack-years of smoking at enrollment (nonsmoker, smoker with <20 pack-years, smoker with ≥20 pack-years), and BMI (weight (kg)/height (m)2; <18.5, 18.5–24.9, 25.0–29.9, or ≥30). Two-sided χ2 tests were used with a P value of 0.05 to evaluate statistical significance. All analyses were performed using SAS software, version 9.3 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Our study population primarily included low-level and moderate lifetime drinkers, averaging fewer than 60 drinks/year or 60–229 drinks/year, respectively (Table 1). Approximately 10% were defined as having higher lifetime alcohol consumption (≥230 drinks/year); these women were more likely to report greater pack-years of tobacco use and to be non-Hispanic white. Study participants with low lifetime alcohol consumption had a slightly higher BMI, had lower educational attainment, and less frequently reported using exogenous hormones.
Table 1.
Characteristics of the Study Population at Baseline, According to Lifetime Alcohol Intake, Sister Study, 2003–2009
| Population Characteristic | Average Lifetime Alcohol Consumption | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (<60 Drinks/Year) (n = 20,671) |
Medium (60–229 Drinks/Year) (n = 16,060) |
High (≥230 Drinks/Year) (n = 5,139) |
|||||||
| Mean (SD) | No. of Women | % | Mean (SD) | No. of Women | % | Mean (SD) | No. of Women | % | |
| Age at baseline, years | 55.7 (8.9) | 54.9 (8.9) | 55.3 (8.8) | ||||||
| Age at menarche, years | 12.6 (1.5) | 12.7 (1.5) | 12.7 (1.5) | ||||||
| Age at first birth, yearsa | 24.5 (5.1) | 25.4 (5.4) | 25.3 (5.9) | ||||||
| Paritya | 2.5 (1.1) | 2.3 (1.0) | 2.2 (1.0) | ||||||
| Age at menopause, years | 48.3 (6.4) | 48.5 (6.2) | 48.4 (6.3) | ||||||
| Pack-years of smokingb | 13.5 (14.8) | 14.0 (14.8) | 18.4 (16.8) | ||||||
| Body mass indexc | 28.2 (6.4) | 26.9 (5.8) | 26.9 (5.8) | ||||||
| Race/ethnicity | |||||||||
| Non-Hispanic white | 17,181 | 83.1 | 14,314 | 89.1 | 4,682 | 91.1 | |||
| Other | 3,488 | 16.9 | 1,744 | 10.9 | 455 | 8.9 | |||
| Education | |||||||||
| High school diploma/equivalent or less | 3,106 | 15.0 | 1,989 | 12.4 | 762 | 14.8 | |||
| Some college | 7,050 | 34.1 | 5,156 | 32.1 | 1,729 | 33.7 | |||
| 4-year college degree or more | 10,512 | 50.9 | 8,914 | 55.5 | 2,647 | 51.5 | |||
| Use of oral contraceptives | |||||||||
| Never | 3,542 | 17.2d | 2,024 | 12.6 | 620 | 12.1 | |||
| Ever | 17,110 | 82.9 | 14,027 | 87.4 | 4,517 | 87.9 | |||
| Postmenopausal hormone usee | |||||||||
| None | 5,632 | 40.7 | 3,987 | 38.9d | 1,369 | 40.2d | |||
| Estrogen only | 3,913 | 28.3 | 2,614 | 25.5 | 831 | 24.4 | |||
| E+P or both estrogen and E+P | 4,284 | 31.0 | 3,638 | 35.5 | 1,210 | 35.5 | |||
Abbreviations: E+P, estrogen plus progesterone; SD, standard deviation.
a Limited to parous women (n = 33,933).
b Limited to ever smokers (n = 19,799).
c Weight (kg)/height (m)2.
d Categories do not sum to 100 due to rounding.
e Limited to those who were postmenopausal at baseline (n = 27,564).
Women with both moderate and high average lifetime alcohol intake had an increased risk of developing breast cancer (for 60–229 drinks/year, hazard ratio (HR) = 1.19, 95% confidence interval (CI): 1.06, 1.33; for ≥230 drinks/year, HR = 1.35, 95% CI: 1.15, 1.58) relative to low average intake (<60 drinks/year) (Table 2). This association remained after further adjustment for ever binging (data not shown). Positive, but less precise, associations were observed for both heavy current drinkers and heavy former drinkers, with point estimates being similar in magnitude to that of the lifetime average measure (for current intake of ≥2 drinks/day vs. never drinking, HR = 1.22, 95% CI: 0.89, 1.68; for former intake of ≥2 drinks/day vs. former intake of <1 drink/day, HR = 1.26, 95% CI: 0.81, 1.95). There were no appreciable differences by duration (years) of drinking or age at first drinking. Similarly, time since last drinking was not related to risk among former drinkers.
Table 2.
Risk of Invasive Breast Cancer According to History of Alcohol Consumption, Sister Study, 2003–2014
| Alcohol Consumption History | Person-Years of Follow-up | No. of Breast Cancer Cases (n = 1,843) | Age-Adjusted | Multivariable-Adjusteda | P for Trendb | ||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| All Participants | |||||||
| Current consumption status | |||||||
| Never drinker | 11,928 | 65 | 1.00 | Referent | 1.00 | Referent | 0.17 |
| Former drinker | 48,125 | 277 | 1.09 | 0.84, 1.43 | 1.04 | 0.79, 1.37 | |
| Current drinker | |||||||
| <1 drink/day | 221,035 | 1,219 | 1.12 | 0.87, 1.43 | 1.06 | 0.82, 1.36 | |
| 1–1.9 drinks/day | 28,552 | 170 | 1.17 | 0.87, 1.55 | 1.10 | 0.82, 1.48 | |
| ≥2 drinks/day | 16,043 | 110 | 1.30 | 0.95, 1.76 | 1.22 | 0.89, 1.68 | |
| Average lifetime consumption, drinks/yearc | |||||||
| <60 | 132,999 | 669 | 1.00 | Referent | 1.00 | Referent | <0.01 |
| 60–229 | 104,747 | 622 | 1.20 | 1.08, 1.34 | 1.19 | 1.06, 1.33 | |
| ≥230 | 33,026 | 229 | 1.38 | 1.19, 1.61 | 1.35 | 1.15, 1.58 | |
| Duration of drinking, years | |||||||
| Never drinker | 11,928 | 65 | 1.00 | Referent | 1.00 | Referent | 0.24 |
| <20 | 25,496 | 113 | 1.01 | 0.74, 1.37 | 0.94 | 0.68, 1.28 | |
| 20–39 | 154,258 | 776 | 1.12 | 0.86, 1.45 | 1.02 | 0.78, 1.33 | |
| ≥40 | 79,192 | 567 | 1.17 | 0.90, 1.52 | 1.09 | 0.83, 1.42 | |
| Age at starting to drink, years | |||||||
| Never drinker | 11,928 | 65 | 1.00 | Referent | 1.00 | Referent | 0.92 |
| <15 | 28,843 | 161 | 1.27 | 0.94, 1.69 | 1.16 | 0.86, 1.57 | |
| 15–19 | 197,085 | 1,070 | 1.11 | 0.87, 1.43 | 1.05 | 0.81, 1.36 | |
| ≥20 | 88,178 | 546 | 1.12 | 0.87, 1.45 | 1.06 | 0.82, 1.38 | |
| Former Drinkers | |||||||
| Former consumption level, drinks/day | |||||||
| <1 | 34,878 | 196 | 1.00 | Referent | 1.00 | Referent | 0.36 |
| 1–1.9 | 2,851 | 17 | 1.13 | 0.69, 1.86 | 0.97 | 0.58, 1.64 | |
| ≥2 | 3,448 | 29 | 1.56 | 1.04, 2.32 | 1.26 | 0.81, 1.95 | |
| Years since last alcoholic beverage | |||||||
| ≤5 | 15,330 | 89 | 1.00 | Referent | 1.00 | Referent | 0.66 |
| 6–14 | 9,992 | 43 | 0.76 | 0.53, 1.10 | 0.72 | 0.49, 1.04 | |
| ≥15 | 22,221 | 139 | 1.05 | 0.80, 1.38 | 1.03 | 0.79, 1.36 | |
| Years since being a regular drinker | |||||||
| ≤5 | 14,131 | 81 | 1.00 | Referent | 1.00 | Referent | 0.65 |
| 6–14 | 10,023 | 45 | 0.81 | 0.56, 1.16 | 0.73 | 0.50, 1.07 | |
| ≥15 | 23,441 | 146 | 1.06 | 0.80, 1.39 | 1.03 | 0.78, 1.36 | |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Adjusted for age, race/ethnicity, education, age at menarche, age at first birth, parity, use of hormonal birth control, pack-years of smoking, use of postmenopausal hormones, age at menopause and menopausal status, and body mass index.
bP value for trend was calculated with Wald's χ2 test.
c A consumption level of 60 drinks/year represents approximately 1 drink/week; a level of 230 drinks/year represents approximately 4.5 drinks/week.
Measures of heavy episodic or binge drinking behaviors were also associated with an elevated risk when compared with low-average drinking (Table 3). Breast cancer risk was associated with both ever binging (HR = 1.29, 95% CI: 1.15, 1.45) and current binging (HR = 1.38, 95% CI: 1.19, 1.61), as well as drinking to the point of hurting one's health (HR = 1.54, 95% CI: 1.12, 2.11) and blacking out while drinking (HR = 1.39, 95% CI: 1.17, 1.64). Results were similar regardless of whether or not low-average lifetime drinkers who also reported binge drinking behaviors were excluded (data not shown). Hazard ratios for binge drinking behaviors were elevated with increasing frequency of binge drinking over the life course (for 25–249 binge drinking episodes, HR = 1.23, 95% CI: 1.05, 1.45; for ≥250 binge drinking episodes, HR = 1.47, 95% CI: 1.25, 1.72) and for reporting more instances of blacking out (≥3 times: HR = 1.46, 95% CI: 1.19, 1.80). We also considered binge drinking by decade of life but found that associations with breast cancer remained similar across the life span (data not shown).
Table 3.
Risk of Invasive Breast Cancer Among Women With Binge Drinking Behaviors as Compared With Low-Average Lifetime Drinkers (<60 Drinks/Year), Sister Study, 2003–2014
| Binge Drinking Behaviora | Person-Years of Follow-up | No. of Breast Cancer Cases (n = 1,843) | Age-Adjusted | Multivariable-Adjustedb | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Ever binge drinking | ||||||
| Low-level drinker | 132,999 | 669 | 1.00 | Referent | 1.00 | Referent |
| Yes | 107,502 | 673 | 1.32 | 1.18, 1.47 | 1.29 | 1.15, 1.45 |
| 1–24 times | 34,644 | 207 | 1.22 | 1.04, 1.42 | 1.18 | 1.00, 1.38 |
| 25–249 times | 36,216 | 213 | 1.26 | 1.08, 1.47 | 1.23 | 1.05, 1.45 |
| ≥250 times | 35,281 | 237 | 1.45 | 1.25, 1.69 | 1.47 | 1.25, 1.72 |
| Current binge drinking (in the past 12 months) | ||||||
| Low-level drinker | 132,999 | 669 | 1.00 | Referent | 1.00 | Referent |
| Yes | 44,466 | 278 | 1.39 | 1.20, 1.60 | 1.38 | 1.19, 1.61 |
| 1–4 times | 20,971 | 135 | 1.44 | 1.19, 1.73 | 1.43 | 1.18, 1.73 |
| ≥5 times | 24,192 | 145 | 1.34 | 1.11, 1.60 | 1.34 | 1.11, 1.62 |
| “Blacking out” while drinkingc | ||||||
| Low-level drinker | 132,999 | 669 | 1.00 | Referent | 1.00 | Referent |
| Yes | 33,639 | 216 | 1.41 | 1.21, 1.65 | 1.39 | 1.17, 1.64 |
| 1–2 times | 14,384 | 89 | 1.33 | 1.06, 1.66 | 1.32 | 1.05, 1.66 |
| ≥3 times | 18,744 | 124 | 1.49 | 1.22, 1.81 | 1.46 | 1.19, 1.80 |
| Drinking that hurt one's healthd | ||||||
| Low-level drinker | 132,999 | 669 | 1.00 | Referent | 1.00 | Referent |
| Yes | 5,995 | 45 | 1.48 | 1.09, 2.01 | 1.54 | 1.12, 2.11 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Binge drinking was defined as consuming 4 or more alcoholic beverages in a row at one sitting. Low-average lifetime drinkers who reported binge drinking behaviors were included in the “low-level drinker” referent group.
b Adjusted for age, race/ethnicity, education, age at menarche, age at first birth, parity, use of hormonal birth control, pack-years of smoking, use of postmenopausal hormones, age at menopause and menopausal status, and body mass index.
c Questions on history and frequency of blacking out were only posed to women who reported ever binge drinking.
d Ever being told by a health professional that drinking was hurting one's health.
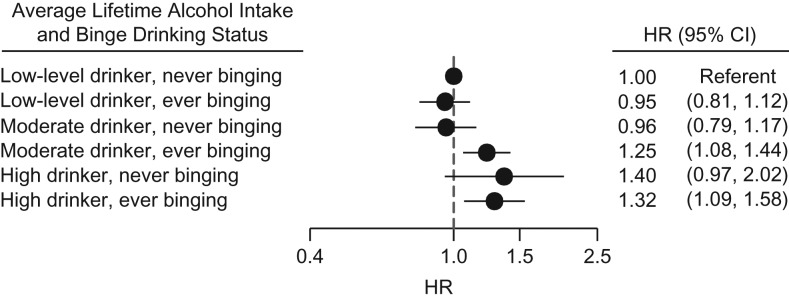
Relative to low-average drinkers who reported no binging, moderate drinkers (60–229 drinks/year) who also reported binging were at an elevated risk of breast cancer (HR = 1.25, 95% CI: 1.08, 1.44). In contrast, there was no evidence for an increase in risk for being a low-level drinker and ever binging (HR = 0.95, 95% CI: 0.81, 1.12), nor was there an increase in risk for moderate drinking without binging (HR = 0.96, 95% CI: 0.79, 1.17) (Figure 1). The interaction between low/moderate lifetime drinking and ever/never binging was significant on the additive scale (RERI = 0.33, 95% CI: 0.10, 0.57). However, no added risk for binging was observed at the highest level of lifetime drinking (≥230 drinks/year). Estimates were similar when we considered interaction between current binging and moderate lifetime alcohol intake, but the measure of additive interaction was not statistically significant (RERI = 0.28, 95% CI: −0.07, 0.63).
Figure 1.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for the joint association of lifetime alcohol consumption (low (<60 drinks/year), moderate (60–229 drinks/year), or high (≥230 drinks/year)) and ever binge drinking (≥4 alcoholic beverages at one sitting) with incident invasive breast cancer, Sister Study, 2003–2014. The interaction between low/moderate drinking and ever/never binging was significant on the additive scale (relative excess risk due to interaction = 0.33, 95% CI: 0.10, 0.57). Bars, 95% CIs.
We also evaluated effect-measure modification of lifetime alcohol intake by binging on the multiplicative scale (Table 4). Although we found no effect-measure modification on the multiplicative scale, we observed that among women who were in the moderate category of lifetime drinking (60–229 drinks/year), ever binge drinkers had a 30% higher breast cancer risk (HR = 1.30, 95% CI: 1.06, 1.59). Similarly, elevated estimates were observed for blacking out while drinking (HR = 1.13, 95% CI: 0.92, 1.39) and current binging (HR = 1.17, 95% CI: 0.98, 1.41) in women with a moderate lifetime alcohol intake. However, none of the binge drinking characterizations (current or ever binging, drinking that hurt one's health, or blacking out) resulted in an increased risk among women with either low or high lifetime average intake.
Table 4.
Risk of Invasive Breast Cancer According to Alcohol Consumption History and Binge Drinking Status, Sister Study, 2003–2014a
| Binge Drinking Statusb | Average Lifetime Alcohol Consumption | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (<60 Drinks/Year) | Medium (60–229 Drinks/Year) | High (≥230 Drinks/Year) | |||||||
| No. of Women | HR | 95% CI | No. of Women | HR | 95% CI | No. of Women | HR | 95% CI | |
| Ever “blacking out” while drinking | |||||||||
| No | 235 | 1.00 | Referent | 346 | 1.00 | Referent | 111 | 1.00 | Referent |
| Yes | 41 | 0.94 | 0.67, 1.31 | 131 | 1.13 | 0.92, 1.39 | 85 | 1.03 | 0.77, 1.38 |
| Ever binging | |||||||||
| No | 393 | 1.00 | Referent | 145 | 1.00 | Referent | 33 | 1.00 | Referent |
| Yes | 276 | 0.96 | 0.81, 1.14 | 477 | 1.30 | 1.06, 1.59 | 196 | 0.95 | 0.63, 1.42 |
| Current binging | |||||||||
| No | 628 | 1.00 | Referent | 447 | 1.00 | Referent | 125 | 1.00 | Referent |
| Yes | 41 | 0.95 | 0.69, 1.32 | 175 | 1.17 | 0.98, 1.41 | 103 | 1.09 | 0.83, 1.44 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a HRs were adjusted for age, race/ethnicity, education, age at menarche, age at first birth, parity, use of hormonal birth control, pack-years of smoking, use of postmenopausal hormones, age at menopause and menopausal status, and body mass index.
b Binge drinking was defined as consuming 4 or more alcoholic beverages in a row at one sitting.
Higher lifetime alcohol consumption was also associated with breast cancer when we limited outcomes to ER+ tumors (for 60–229 drinks/year, HR = 1.18, 95% CI: 1.04, 1.35; for ≥230 drinks/year, HR = 1.42, 95% CI: 1.19, 1.70), as was ever binging (HR = 1.32, 95% CI: 1.16, 1.51), although estimates were not statistically different from estimates for ER− breast cancer (Web Table 1). Neither the association between breast cancer and lifetime alcohol consumption nor the association between breast cancer and ever binging depended on menopausal status at diagnosis (Web Table 2). We observed no association of either lifetime alcohol intake or binge drinking with ductal carcinoma in situ.
The association between breast cancer and lifetime alcohol intake did not vary by degree of family history of breast cancer (1 relative vs. ≥2 relatives), birth cohort, smoking status, BMI, age, or use of hormone replacement therapy (data not shown).
DISCUSSION
In a large, prospective cohort study of mostly light drinkers (averaging <1 drink/day), we confirmed previously established findings of an association between breast cancer risk and higher lifetime alcohol intake (18–21) and found evidence to support a synergistic relationship between moderate lifetime alcohol intake and heavy episodic drinking. Heavy drinking behaviors, including binging, blacking out, and drinking to the point of harming one's health, were also each associated with breast cancer risk.
Binge drinking behaviors were associated with up to a 50% increase in breast cancer risk relative to low-average drinking. However, when considered in conjunction with lifetime alcohol intake, binge drinking increased breast cancer risk only in moderate lifetime drinkers. It is plausible that binge drinking in low-average drinkers is rare and does not substantially increase risk, while persons in the highest category of lifetime alcohol intake may have already reached a threshold beyond which binge drinking does not increase risk. In moderate drinkers, the association for binge drinking appeared to be the most evident. Previous studies have suggested that alcohol may increase breast cancer risk even at low levels of consumption (4); however, these studies have often not been able to consider drinking patterns. In contrast, we found no elevated breast cancer risk for moderate drinkers who did not exhibit binge drinking behaviors.
The few studies that have previously evaluated binge drinking have reported positive associations between current binging and breast cancer, with binging being defined as consuming more drinks over the weekend (22) and as ever drinking ≥6 drinks/day in a typical month (21). One case-control study also found effect-measure modification of current alcohol consumption by current binge drinking status, with elevated risk limited to the highest category of drinkers (≥91 g/week) who also reported binging (defined as 5 or more drinks in one sitting) (23). However, that specific analysis was limited to current alcohol consumption and current binging, which may not reflect the entirety of the relevant time period.
Lifetime alcohol intake was associated with breast cancer when data were limited to ER+ tumors, and estimates of association were similar for pre- and postmenopausal breast cancer. These findings are consistent with previous meta-analyses (4, 24). However, we did not find evidence to support that either early age at first drinking or years of drinking was associated with breast cancer risk. Similarly, the associations did not vary by smoking status or BMI, which is consistent with the results of 2 pooled analyses (4, 25). We also did not find evidence that the association varied by age, birth cohort, or use of hormone replacement therapy.
Alcohol is hypothesized to act via multiple mechanisms to influence breast cancer risk; alcohol may increase circulating sex hormone levels (26) and stimulate proliferation of ER+ cells (27, 28). The metabolism of alcohol may also result in carcinogenic products and reactive oxygen species (29, 30); thus, alcohol may act as a weak carcinogen (7). Recent studies suggest that aberrant DNA methylation patterns (31, 32) and interference with epithelium-stroma interactions may also play important roles (33) in alcohol-induced carcinogenesis. The association observed with binge or heavy drinking behaviors also suggests other potential biological mechanisms, including increased inflammation and insulin resistance (9, 10). Metabolic processes that eliminate alcohol from the body via the enzymes alcohol dehydrogenase and aldehyde dehydrogenase may not be sufficient during periods of heavy drinking (34). As such, after consuming multiple drinks in one sitting, a woman's blood alcohol concentration is high enough to induce the activity of an additional enzyme, cytochrome P-450 2E1 (35); the metabolism of alcohol by cytochrome P-450 2E1 can result in the formation of mutagenic DNA adducts and reactive oxygen species (34, 36).
The inclusion of both lifetime alcohol consumption information and questions about binge drinking behaviors is an important strength of this study, as simply considering measures of lifetime alcohol intake cannot differentiate between someone who binge drinks twice a week and someone who consumes about 1 drink each day. This study also evaluated other measures of heavy episodic drinking besides binge drinking, including whether the participant had been told her drinking was hurting her health and whether she drank to the point of blacking out. Due to the large Sister Study sample size and the low correlations between lifetime alcohol intake and binge drinking variables (r = 0.1–0.3), we were able to consider potential modification between cumulative lifetime alcohol intake and specific binge drinking experiences.
The information included in this study on alcohol consumption was self-reported. Self-reported information on alcohol intake has been demonstrated to be reasonably valid, but nonetheless there may have been some exposure misclassification (37). The potential for misclassification may be most relevant for high-risk behaviors, such as binging, because of social desirability bias (38). The alcohol exposure information was collected prior to breast cancer diagnosis and thus would not have been influenced by case status.
Because of the low levels of alcohol consumption in the Sister Study, the average lifetime alcohol cutpoints chosen via splines were lower than the cutpoints used in some previous studies (18–20) but comparable to those of others (39, 40). Despite this, the current alcohol drinking seen in our study population was similar to alcohol consumption generally observed in the United States, with a median of <1 drink/day (41). It is important to note that women in our study population were at a higher risk of developing breast cancer due to their family history of the disease. There was no evidence that the relative risk of breast cancer associated with alcohol consumption differed for women who had 2 or more first-degree relatives with breast cancer versus women with only 1—findings similar to a previous report on the subject (42). We were unable to consider breast cancer gene (BRCA) mutation status to evaluate women who might be at the highest risk, although we would expect the proportion testing positive to be low, and this should not have biased our estimates (43).
In conclusion, this study confirms previously established associations of breast cancer with overall alcohol intake and also supports a role for binge drinking in breast carcinogenesis, particularly in women with moderate-level alcohol intake. Although it is currently estimated that approximately 5% of breast cancer cases can be attributed to alcohol (44), very few women may be aware of the association between alcohol and breast cancer (45). In light of the increasing frequency of binge drinking in the United States (1), the impact of binge drinking on health may increase and will continue to be a topic of concern. In terms of public health messaging, any increase in risk associated with alcohol intake must be balanced against the decreased cardiovascular disease risk observed with moderate levels of drinking (46), although this long-held belief has recently been drawn into question (47). Regardless, it does not appear that there is a cardiovascular benefit for binge drinkers (48). Thus, these findings support existing public health recommendations encouraging women to avoid binge drinking and to consume alcohol in moderation.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Alexandra J. White, Lisa A. DeRoo, Dale P. Sandler); Biostatistics and Computational Biology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Clarice R. Weinberg); and Department of Global Public Health and Primary Care, Faculty of Medicine and Dentistry, University of Bergen, Bergen, Norway (Lisa A. DeRoo).
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (project Z01-ES044005).
Conflict of interest: none declared.
REFERENCES
- 1. Dwyer-Lindgren L, Flaxman AD, Ng M, et al. Drinking patterns in US counties from 2002 to 2012. Am J Public Health. 2015;105(6):1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blazer DG, Wu LT. The epidemiology of at-risk and binge drinking among middle-aged and elderly community adults: National Survey on Drug Use and Health. Am J Psychiatry. 2009;166(10):1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365(9458):519–530. [DOI] [PubMed] [Google Scholar]
- 4. Hamajima N, Hirose K, Tajima K, et al. Alcohol, tobacco and breast cancer—collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87(11):1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baan R, Straif K, Grosse Y, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8(4):292–293. [DOI] [PubMed] [Google Scholar]
- 6. International Agency for Research on Cancer Alcohol Consumption and Ethyl Carbamate (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 96). Lyon, France: International Agency for Research on Cancer; 2010. [PMC free article] [PubMed]
- 7. Brooks PJ, Zakhari S. Moderate alcohol consumption and breast cancer in women: from epidemiology to mechanisms and interventions. Alcohol Clin Exp Res. 2013;37(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wechsler H, Austin SB. Binge drinking: the five/four measure. J Stud Alcohol. 1998;59(1):122–124. [DOI] [PubMed] [Google Scholar]
- 9. Ward RJ, Colivicchi MA, Allen R, et al. Neuro‐inflammation induced in the hippocampus of “binge drinking” rats may be mediated by elevated extracellular glutamate content. J Neurochem. 2009;111(5):1119–1128. [DOI] [PubMed] [Google Scholar]
- 10. Lindtner C, Scherer T, Zielinski E, et al. Binge drinking induces whole-body insulin resistance by impairing hypothalamic insulin action. Sci Transl Med. 2013;5(170):170ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. [DOI] [PubMed] [Google Scholar]
- 12. National Institute of Environmental Health Sciences Sister Study Response Rates for Annual and Detailed Follow-up As of February 8, 2016 Research Triangle Park, NC: National Institute of Environmental Health Sciences; 2016. https://sisterstudy.niehs.nih.gov/English/images/SIS-RespRatesFollowUps-website-20160208-508.pdf. Accessed January 8, 2016.
- 13. National Institute of Environmental Health Sciences The Sister Study. Breast cancer validation. http://sisterstudy.niehs.nih.gov/English/brca-validation.htm. Accessed December 1, 2015.
- 14. Howe CJ, Cole SR, Westreich DJ, et al. Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22(6):874–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Begg CB, Zhang ZF. Statistical analysis of molecular epidemiology studies employing case-series. Cancer Epidemiol Biomarkers Prev. 1994;3(2):173–175. [PubMed] [Google Scholar]
- 16. Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17(3):227–236. [DOI] [PubMed] [Google Scholar]
- 17. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 18. Longnecker MP, Newcomb PA, Mittendorf R, et al. Risk of breast cancer in relation to lifetime alcohol consumption. J Natl Cancer Inst. 1995;87(12):923–929. [DOI] [PubMed] [Google Scholar]
- 19. Terry MB, Zhang FF, Kabat G, et al. Lifetime alcohol intake and breast cancer risk. Ann Epidemiol. 2006;16(3):230–240. [DOI] [PubMed] [Google Scholar]
- 20. Longnecker MP, Paganini-Hill A, Ross RK. Lifetime alcohol consumption and breast cancer risk among postmenopausal women in Los Angeles. Cancer Epidemiol Biomarkers Prev. 1995;4(7):721–725. [PubMed] [Google Scholar]
- 21. Chen WY, Rosner B, Hankinson SE, et al. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mørch LS, Johansen D, Thygesen LC, et al. Alcohol drinking, consumption patterns and breast cancer among Danish nurses: a cohort study. Eur J Public Health. 2007;17(6):624–629. [DOI] [PubMed] [Google Scholar]
- 23. Kinney AY, Millikan RC, Lin YH, et al. Alcohol consumption and breast cancer among black and white women in North Carolina (United States). Cancer Causes Control. 2000;11(4):345–357. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki R, Orsini N, Mignone L, et al. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status—a meta‐analysis of epidemiological studies. Int J Cancer. 2008;122(8):1832–1841. [DOI] [PubMed] [Google Scholar]
- 25. Smith-Warner SA, Spiegelman D, Yaun SS, et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA. 1998;279(7):535–540. [DOI] [PubMed] [Google Scholar]
- 26. Mendelson JH, Lukas SE, Mello NK, et al. Acute alcohol effects on plasma estradiol levels in women. Psychopharmacology (Berl). 1988;94(4):464–467. [DOI] [PubMed] [Google Scholar]
- 27. Fan S, Meng Q, Gao B, et al. Alcohol stimulates estrogen receptor signaling in human breast cancer cell lines. Cancer Res. 2000;60(20):5635–5639. [PubMed] [Google Scholar]
- 28. Singletary KW, Frey RS, Yan W. Effect of ethanol on proliferation and estrogen receptor-α expression in human breast cancer cells. Cancer Lett. 2001;165(2):131–137. [DOI] [PubMed] [Google Scholar]
- 29. Terry MB, Gammon MD, Zhang FF, et al. ADH3 genotype, alcohol intake and breast cancer risk. Carcinogenesis. 2006;27(4):840–847. [DOI] [PubMed] [Google Scholar]
- 30. Lorenti Garcia C, Mechilli M, Proietti De Santis L, et al. Relationship between DNA lesions, DNA repair and chromosomal damage induced by acetaldehyde. Mutat Res. 2009;662(1-2):3–9. [DOI] [PubMed] [Google Scholar]
- 31. Tao MH, Marian C, Shields PG, et al. Alcohol consumption in relation to aberrant DNA methylation in breast tumors. Alcohol. 2011;45(7):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Christensen BC, Kelsey KT, Zheng S, et al. Breast cancer DNA methylation profiles are associated with tumor size and alcohol and folate intake. PLoS Genet. 2010;6(7):e1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Y, Nguyen N, Colditz GA. Links between alcohol consumption and breast cancer: a look at the evidence. Womens Health (Lond). 2015;11(1):65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health. 2007;30(1):38–41. [PMC free article] [PubMed] [Google Scholar]
- 35. Salaspuro M, Lieber C. Non-uniformity of blood ethanol elimination: its exaggeration after chronic consumption. Ann Clin Res. 1978;10(5):294–297. [PubMed] [Google Scholar]
- 36. Millonig G, Wang Y, Homann N, et al. Ethanol‐mediated carcinogenesis in the human esophagus implicates CYP2E1 induction and the generation of carcinogenic DNA‐lesions. Int J Cancer. 2011;128(3):533–540. [DOI] [PubMed] [Google Scholar]
- 37. Del Boca FK, Darkes J. The validity of self‐reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98(suppl 2):1–12. [DOI] [PubMed] [Google Scholar]
- 38. Davis CG, Thake J, Vilhena N. Social desirability biases in self-reported alcohol consumption and harms. Addict Behav. 2010;35(4):302–311. [DOI] [PubMed] [Google Scholar]
- 39. Garland M, Hunter DJ, Colditz GA, et al. Alcohol consumption in relation to breast cancer risk in a cohort of United States women 25–42 years of age. Cancer Epidemiol Biomarkers Prev. 1999;8(11):1017–1021. [PubMed] [Google Scholar]
- 40. Romieu I, Scoccianti C, Chajes V, et al. Alcohol intake and breast cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2015;137(8):1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gallup, Inc Alcohol and drinking. http://www.gallup.com/poll/1582/alcohol-drinking.aspx. Published 2015. Accessed April 19, 2016.
- 42. Egan KM, Stampfer MJ, Rosner BA, et al. Risk factors for breast cancer in women with a breast cancer family history. Cancer Epidemiol Biomarkers Prev. 1998;7(5):359–364. [PubMed] [Google Scholar]
- 43. Weinberg CR, Shore DL, Umbach DM, et al. Using risk-based sampling to enrich cohorts for endpoints, genes, and exposures. Am J Epidemiol. 2007;166(4):447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boffetta P, Hashibe M, La Vecchia C, et al. The burden of cancer attributable to alcohol drinking. Int J Cancer. 2006;119(4):884–887. [DOI] [PubMed] [Google Scholar]
- 45. Spector D, Mishel M, Skinner CS, et al. Breast cancer risk perception and lifestyle behaviors among white and black women with a family history of the disease. Cancer Nurs. 2009;32(4):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rimm EB, Williams P, Fosher K, et al. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319(7224):1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chikritzhs T, Stockwell T, Naimi T, et al. Has the leaning tower of presumed health benefits from “moderate” alcohol use finally collapsed. Addiction. 2015;110(5):726–727. [DOI] [PubMed] [Google Scholar]
- 48. Murray RP, Connett JE, Tyas SL, et al. Alcohol volume, drinking pattern, and cardiovascular disease morbidity and mortality: is there a U-shaped function. Am J Epidemiol. 2002;155(3):242–248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.