Abstract
Early menopause, which is the cessation of ovarian function before age 45 years, affects 5%–10% of Western women and is associated with an increased risk of adverse health outcomes. Literature suggests that high levels of vegetable protein intake may prolong female reproductive function. We evaluated the association of long-term intake of vegetable protein, animal protein, and specific protein-rich foods with incidence of early natural menopause in the Nurses’ Health Study II cohort. Women included in analyses (n = 85,682) were premenopausal at baseline (1991) and followed until 2011 for onset of natural menopause. Protein intake was assessed via food frequency questionnaire. In Cox proportional hazard models that were adjusted for age, smoking, body mass index, and other factors, women in the highest quintile of cumulatively averaged vegetable protein intake (median, 6.5% of calories) had a significant 16% lower risk of early menopause compared with women in the lowest quintile (3.9% of calories; 95% confidence interval: 0.73, 0.98; P for trend = 0.02). Intake of specific foods, including pasta, dark bread, and cold cereal, was also associated with lower risk (P < 0.05). Conversely, animal protein intake was unrelated to risk. High consumption of vegetable protein, equivalent to 3–4 servings per day of protein-rich foods, is associated with lower incidence of early menopause in US women.
Keywords: cohort studies, diet, dietary proteins, menopause, vegetable proteins, women
Early menopause, or the cessation of ovarian function before to age 45 years, affects 5%–10% of Western women and is associated with increased risk of adverse health outcomes, including premature mortality and cardiovascular disease (1–3). Additionally, women who experience early menopause may have reduced fertility as long as a decade before onset of menopause (1, 3). The causes of natural early menopause are not well understood, and most diagnoses are not attributable to genetic factors or autoimmune conditions (1, 2).
Results of recent studies suggest dietary protein sources may be associated with infertility, rate of ovarian aging, oocyte quality, and timing of menopause (4–12). In a study of diet composition and ovulatory infertility in the Nurses’ Health Study (NHS) II, high levels of animal protein intake were positively associated with infertility, whereas vegetable protein intake was associated with lower risk for infertility; replacing 5% of animal protein intake with vegetable protein was associated with 50% lower risk for infertility (P = 0.007) (8). Additionally, vegetable protein and animal protein have been shown to have different associations with ovarian function in animal models (6).
To our knowledge, the association between vegetable and animal protein intake and early menopause has not been directly evaluated. We prospectively investigated these associations among women enrolled in the NHS II, hypothesizing that intake of higher amounts of vegetable protein would be inversely associated with incidence of early menopause, whereas animal protein intake would be associated with higher incidence of early menopause.
METHODS
The NHS II began in 1989 when 116,686 US female registered nurses, aged 25–42 years, completed a mailed questionnaire and provided information on current and lifelong health, prescription medication use, and lifestyle factors. Participants have completed new questionnaires every 2 years since, and the follow-up for each questionnaire cycle has been at least 90%. The institutional review board at Brigham and Women’s Hospital (Boston, Massachusetts) approved the NHS II study protocol. The present analysis uses questionnaire data from 1991 through 2011.
Protein assessment
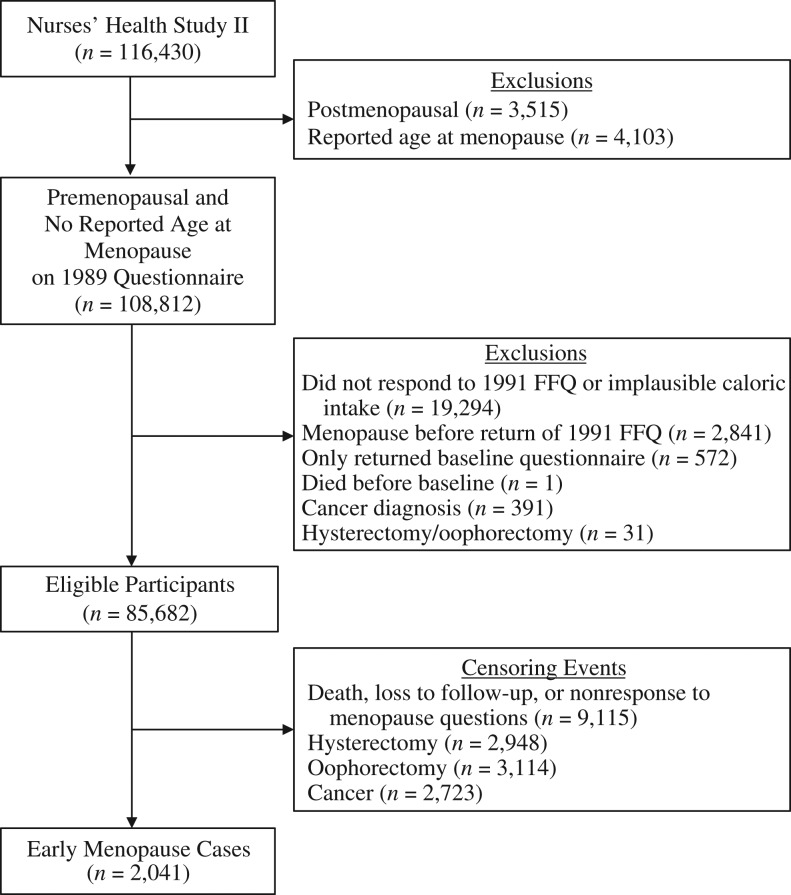
We assessed intake of total protein and protein from vegetable and animal sources in 1991 and every 4 years thereafter via the Harvard food frequency questionnaire (FFQ) (13). We asked participants to report how often they consumed a single serving of 131 foods, beverages, and dietary supplements over the previous year, with response options ranging from “never or less than once a month” to “6+ per day.” We excluded participants from the analysis if they reported implausible calorie intake (i.e., <600 or >3,500 kcal/day) (Figure 1).
Figure 1.
Exclusion and censoring criteria based on data from the Nurses’ Health Study II, 1991–2011. FFQ, food frequency questionnaire.
Protein intake was calculated by multiplying the protein content of a single serving of each food item by its frequency of consumption and then summing across all items. Intakes of vegetable and animal protein were derived similarly, as were intakes of other macro- and micronutrients. We then calculated total, vegetable, and animal protein intake as percentages of total calories by multiplying intake of each protein type in grams by 4 kcal/g, dividing by the total number of calories consumed, and multiplying by 100. Intakes of micronutrients were adjusted for total energy intake using the residual method (13).
The validity and reproducibility of the FFQ were evaluated in the original NHS, a similar cohort to NHS II (14, 15). The correlation coefficient for total protein assessed by FFQ versus from 4 weeks of daily food records was 0.47.
Assessment of early menopause
Menopausal status and timing have been assessed biennially in the NHS II starting in 1989. Participants were asked if their menstrual periods had ceased; response options were 1) no: premenopausal; 2) yes: no menstrual periods; 3) yes: had menopause but now have periods induced by hormones; and 4) not sure (e.g., started hormones before cessation of periods). Women who reported that their periods had ceased were asked to report the age at which their periods ceased (open response) and for which of the following reasons their periods ceased: 1) surgery, 2) radiation or chemotherapy, or 3) natural cessation. Women were subsequently asked if they had had a hysterectomy, an oophorectomy (bilateral or single), or used replacement sex hormones. Women were followed for incidence of menopause until 2011. We defined cases of early menopause as women who reported natural menopause between return of their baseline questionnaire (1991) and before age 45 years.
Self-assessment of menopause has been validated in the NHS. In the NHS, 82% of women experiencing natural menopause repeatedly recalled the same age at menopause within 1 year on repeated health questionnaires, whereas 99% of women whose age at menopause was confirmed via medical records accurately reported their age at menopause within 1 year (16).
Covariate assessment
Age at menarche and race/ethnicity were reported in 1989. Oral contraception (OC) use and parity were assessed biennially. Months of breastfeeding were self-reported biennially from 1989 until 2003, and information was updated in 2009. Physical activity was assessed every 4 years and used to calculate metabolic equivalent of task hours per week (17, 18). Body mass index (measured as weight in kilograms divided by the square of height in meters) was calculated using height reported at baseline and weight reported biennially. Smoking status and quantity were assessed biennially and used to calculate pack-years. Dietary factors, including intake of alcohol, carbohydrates, fats, vitamin D, and calcium, were assessed via FFQ, as described earlier in the Methods.
Statistical analyses
The present analysis was limited to NHS II participants who were premenopausal in 1991, returned a valid FFQ in 1991, and returned at least 1 more survey after 1991. Women were further excluded if they had had an oophorectomy, a hysterectomy, or had been diagnosed with a cancer other than nonmelanoma skin cancer before 1991 (Figure 1).
To assess covariates for inclusion in statistical models, we first divided participants into quintiles by vegetable protein intake in 1991 based on the distribution within the cohort. We then compared the distribution of covariates measured at baseline with vegetable protein intake in age-adjusted general linear models.
We evaluated the associations of total, vegetable, and animal protein intakes with early menopause by using Cox proportional hazards models to calculate hazard ratios and 95% confidence intervals stratified by age in months and questionnaire cycle. To test for linear trend across quintiles, we modeled the median value of each quintile as a continuous variable. Participants contributed follow-up time from the date of return of the 1991 questionnaire until the onset of menopause, age 45 years, hysterectomy, oophorectomy, diagnosis of cancer other than nonmelanoma skin cancer, death, loss to follow-up, or the end of follow-up in June 2011, whichever came first. In a sensitivity analysis, we further censored at first report of hormone therapy use and excluded women who had used hormone therapy before baseline.
We assessed long-term protein intake in several ways. We first evaluated risk associated with intake only at baseline (1991). We then repeated our analysis using the cumulative average of intake across follow-up, updating exposure information every 4 years. Results from these analyses were similar; thus, only the cumulative average models are emphasized because they may best reflect the impact of long-term diet (19).
Our initial model (model 1) was adjusted only for age and total, vegetable, or animal protein. In model 2, we mutually adjusted vegetable and animal protein intake (both as percentage of calories from protein) for one another and included calories per day, because these variables were considered a priori. In model 3, we additionally adjusted for lifestyle variables (i.e., body mass index, smoking, physical activity), reproductive variables (i.e., parity, age at menarche, breast feeding, OC use, number of pregnancies), and dairy protein. These factors were also chosen a priori; none of these factors met statistical criteria for confounding in our regression models (>10% change in the hazard ratio for protein intake). Race/ethnicity and additional dietary factors (i.e., alternative healthy eating index diet score, carbohydrates, saturated fat, ω-3 fatty acids intake, vitamin D, calcium, vitamin B6, and alcohol) were also tested for inclusion, but these factors had minimal impact on estimates when added to regression models and were not included in final models. To address residual confounding, we repeated our analyses in subgroups of never smokers and women who never reported OC use.
Finally, we examined if specific foods rich in protein were related to risk of early menopause. We tested how each 1-serving-per-day increase in the following foods and food groups was associated with early menopause risk: red meats and poultry, red meats, processed meats, chicken/turkey, seafood, eggs, soy/tofu, peas and lima beans, beans and lentils, peanuts, other nuts, peanut butter, pasta, dark bread, and cold cereal. Statistical analyses were conducted with SAS version 9.3 software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Distributions of baseline covariates by quintile of vegetable protein consumption in 1991 are presented in Table 1. Women with higher vegetable protein intake were older and consumed less animal protein than women with lower intake. Higher vegetable protein intake was associated with lower body mass index, fewer pack-years of smoking, and less OC use, but longer duration of breastfeeding and higher physical activity levels (P < 0.05 for all comparisons). Foods explaining the greatest amount of variation in vegetable protein intake at baseline included pasta (7.85%), dark bread (6.33%), and cold cereal (6.11%). Soy intake explained 0.55% of the variation.
Table 1.
Age-Standardized Baseline Characteristicsa, According to Quintile of Percentage Calories From Vegetable Protein (n = 85,682), Nurses’ Health Study II, 1991–2011
| Characteristic | Vegetable Protein Intake (% of Calories per Day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quintile 1 (Median, 3.7%) | Quintile 2 (Median, 4.4%) | Quintile 3 (Median, 4.9%) | Quintile 4 (Median, 5.4%) | Quintile 5 (Median, 6.3%) | ||||||
| Mean (SE) | % | Mean (SE) | % | Mean (SE) | % | Mean (SE) | % | Mean (SE) | % | |
| Age, yearsb | 35.2 (4.7) | 35.6 (4.6) | 35.8 (4.6) | 36.1 (4.5) | 36.3 (4.5) | |||||
| Animal protein intake, % calories per day | 15.8 (0.03) | 15.1 (0.03) | 14.6 (0.03) | 13.9 (0.03) | 12.2 (0.03) | |||||
| Dairy protein intake, % calories per day | 4.6 (0.01) | 4.3 (0.01) | 4.2 (0.01) | 3.9 (0.01) | 3.6 (0.01) | |||||
| Total calorie intake | 1,808 (4.2) | 1,821 (4.2) | 1,813 (4.2) | 1,795 (4.2) | 1,736 (4.2) | |||||
| Body mass indexc | 25.2 (0.04) | 24.8 (0.04) | 24.6 (0.04) | 24.3 (0.04) | 23.7 (0.04) | |||||
| Pack-years of smokingd | 13.1 (0.10) | 11.8 (0.10) | 11.2 (0.11) | 10.9 (0.11) | 10.5 (0.10) | |||||
| Oral contraceptive use, monthse | 60 (0.4) | 58 (0.4) | 56 (0.4) | 55 (0.4) | 53 (0.4) | |||||
| Parous | 74.0 | 76.3 | 76.7 | 75.0 | 68.4 | |||||
| Parity (pregnancies ≥6 months)f | 2.1 (0.01) | 2.2 (0.01) | 2.1 (0.01) | 2.1 (0.01) | 2.1 (0.01) | |||||
| Breastfeeding, monthsf | 10.9 (0.1) | 12.9 (0.1) | 13.4 (0.1) | 14.2 (0.1) | 15.6 (0.1) | |||||
| Age at menarche, years | 12.5 (0.01) | 12.4 (0.01) | 12.4 (0.01) | 12.4 (0.01) | 12.4 (0.01) | |||||
| Physical activity, MET-hours per week | 21.2 (0.5) | 23.0 (0.5) | 23.0 (0.5) | 24.9 (0.5) | 29.3 (0.5) | |||||
| Smoking status | ||||||||||
| Never | 63.4 | 66.0 | 67.0 | 67.2 | 66.1 | |||||
| Former | 21.6 | 23.5 | 24.0 | 24.8 | 27.1 | |||||
| Current | 14.8 | 10.5 | 8.9 | 8.0 | 6.7 | |||||
| Oral contraceptive use | ||||||||||
| Never | 15.0 | 15.5 | 15.6 | 16.2 | 17.7 | |||||
| Former | 73.2 | 73.0 | 72.6 | 72.4 | 71.3 | |||||
| Current | 11.8 | 11.4 | 11.8 | 11.3 | 10.9 | |||||
Abbreviations: MET, metabolic equivalent of task; SE, standard error.
a Values are standardized to the age distribution of the study population in 1991. All variables are significant at the P < 0.05 level.
b Values are expressed as unadjusted means and standard deviations.
c Weight (kg) divided by the square of the height (m2).
d Includes current and former smokers only.
e Includes current and former oral contraceptive users only.
f Includes parous women only.
Between 1991 and 2011, 2,041 women in the analytic cohort experienced onset of early natural menopause. The analytic cohort (n = 85,682) contributed 1,126,100 years of person-time. In the age-adjusted model (model 1), women in the highest quintile of vegetable protein intake (median, 6.5%) had a 17% lower risk of early menopause as compared with women in the lowest quintile (median, 3.9%; 95% confidence interval (CI): 0.72, 0.95; P for trend = 0.005; Table 2). Addition of covariates only slightly attenuated results. After adjustment for all covariates (model 3), women in the highest quintile of vegetable protein intake had a significant 16% lower risk of experiencing early menopause as compared with women in the lowest quintile (95% CI: 0.73, 0.98; P for trend = 0.02). Each 1% increase in vegetable protein intake was associated with a significant 6% lower risk of early menopause (95% CI: 0.90, 0.99). Though relatively few women in our study consumed very high levels of vegetable protein and our power for analyses of more extreme intake levels was limited, the hazard ratio for early menopause in women who consumed at least 9% of their calories from vegetable protein was 0.41 (95% CI: 0.19, 0.88) compared with those who consumed less than 4% of calories from vegetable protein (results not shown). In contrast, neither animal protein intake (P for trend = 0.74) nor total protein intake (P for trend = 0.52) was associated with risk of early menopause.
Table 2.
Hazard Ratios for the Association of Protein Intake and Incidence of Early Menopause Among Women (n = 85,682), Nurses’ Health Study II, 1991–2011
| Protein Category and Intake Quintile | Median, % | No. of Cases | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Vegetable protein (% calories per day) | ||||||||
| 1 | 3.9 | 445 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2 | 4.6 | 403 | 0.85 | 0.74, 0.98 | 0.85 | 0.74, 0.97 | 0.88 | 0.77, 1.01 |
| 3 | 5.1 | 422 | 0.88 | 0.77, 1.01 | 0.88 | 0.76, 1.00 | 0.92 | 0.80, 1.05 |
| 4 | 5.6 | 378 | 0.79 | 0.69, 0.91 | 0.78 | 0.68, 0.90 | 0.82 | 0.71, 0.95 |
| 5 | 6.5 | 393 | 0.83 | 0.72, 0.95 | 0.80 | 0.70, 0.93 | 0.84 | 0.73, 0.98 |
| P for trend | 0.005 | 0.002 | 0.02 | |||||
| Per 1% increase in calories per day | 0.94 | 0.90, 0.99 | 0.93 | 0.89, 0.97 | 0.94 | 0.90, 0.99 | ||
| Animal protein (% calories per day) | ||||||||
| 1 | 10.0 | 413 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2 | 12.2 | 412 | 0.97 | 0.85, 1.12 | 0.97 | 0.84, 1.11 | 1.01 | 0.88, 1.16 |
| 3 | 13.8 | 408 | 0.97 | 0.85, 1.12 | 0.95 | 0.83, 1.10 | 1.01 | 0.88, 1.17 |
| 4 | 15.4 | 414 | 1.01 | 0.88, 1.16 | 0.97 | 0.84, 1.12 | 1.05 | 0.90, 1.21 |
| 5 | 18.0 | 394 | 1.01 | 0.88, 1.16 | 0.92 | 0.80, 1.07 | 1.02 | 0.87, 1.18 |
| P for trend | 0.75 | 0.31 | 0.74 | |||||
| Per 1% increase in calories per day | 1.01 | 0.99, 1.02 | 1.00 | 0.98, 1.01 | 1.01 | 0.99, 1.02 | ||
| Total protein (% calories per day) | ||||||||
| 1 | 15.3 | 411 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2 | 17.5 | 426 | 1.01 | 0.88, 1.16 | 1.01 | 0.88, 1.16 | 1.06 | 0.93, 1.22 |
| 3 | 18.9 | 403 | 0.97 | 0.84, 1.11 | 0.96 | 0.83, 1.10 | 1.03 | 0.89, 1.18 |
| 4 | 20.4 | 408 | 0.99 | 0.86, 1.14 | 0.98 | 0.85, 1.12 | 1.06 | 0.92, 1.22 |
| 5 | 22.8 | 393 | 1.01 | 0.87, 1.15 | 0.97 | 0.84, 1.11 | 1.06 | 0.91, 1.22 |
| P for trend | 0.94 | 0.53 | 0.52 | |||||
| Per 1% increase in calories per day | 1.00 | 0.99, 1.01 | 1.00 | 0.98, 1.01 | 1.01 | 0.99, 1.02 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; Q, quintile.
a Adjusted for age (continuous).
b Adjusted for age and total caloric intake (quintiles). Vegetable protein adjusted for animal protein (quintiles) and vice versa.
c Adjusted for model 2 covariates, pack-years of smoking (never, <20 years, or ≥20 years), body mass index (weight (kg)/height (m)2; <18.5, 18.5–24.9, 25–29.9, or ≥30.0), age at menarche (≤11, 12, 13–15, or ≥16 years), total duration of breastfeeding (never, ≤2 years, or >2 years), oral contraceptive use (never, former, or current), number of pregnancies ≥6 months (0, 1–2, or ≥3), dairy protein (quintiles), and physical activity level (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, 27.0–41.2, or ≥42.0 metabolic equivalent of task hours per week). Vegetable protein was adjusted for animal protein (quintiles) and vice versa.
Results from all other analyses were similar. In analyses considering vegetable protein intake at baseline only, women with the highest vegetable protein intake had a nonsignificant 12% lower risk of early menopause (model 3, 95% CI: 0.77, 1.02; data not shown). In a sensitivity analysis censoring follow-up at first hormone therapy use and excluding those with hormone therapy use before 1991 (n = 83,885 women; n = 1,759 events), the association of vegetable protein and early menopause risk was slightly stronger; in fully adjusted models, women in the fifth quintile of vegetable protein consumption had a 19% lower risk as compared with women in the first quintile (95% CI: 0.69, 0.95; P for trend = 0.01; Appendix Table 1). In analyses limited to never smokers (n = 55,942 women; n = 1,215 events), results were slightly attenuated (model 3: comparing quintile 5 vs. quintile 1, HR = 0.86, 95% CI: 0.71, 1.04; data not shown). In women who never used OC (n = 8,699 women; n = 224 events), women in the fifth quintile had a nonsignificant 23% lower risk compared with those in the first quintile (model 3: 95% CI: 0.49, 1.21; data not shown).
Finally, we assessed how specific protein-rich foods had associations with early menopause (Table 3). Intake of pasta, dark bread, and cold cereal were each associated with lower risk. For example, each 1 serving per day of pasta intake was associated with a 36% lower risk of early menopause, after adjusting for all covariates (95% CI: 0.49, 0.83). In contrast, red meat intake was positively associated with risk, such that each 1 serving per day was associated with a 12% higher risk (95% CI: 1.01, 1.23).
Table 3.
Hazard Ratios for Early Menopause for Each 1 Serving-per-Day Increase in Intake of Specific Protein-Rich Foods Among Women (n = 85,682), Nurses’ Health Study II, 1991–2011
| Food | Model 1a | Model 2b | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| All meat | 0.99 | 0.92, 1.07 | 1.08 | 0.99, 1.18 |
| Red meat | 1.02 | 0.94, 1.12 | 1.12 | 1.01, 1.23 |
| Processed meat | 1.07 | 0.86, 1.33 | 1.21 | 0.96, 1.51 |
| Chicken/turkey | 0.91 | 0.77, 1.06 | 0.98 | 0.83, 1.15 |
| Seafood | 0.92 | 0.75, 1.14 | 1.00 | 0.81, 1.23 |
| Eggs | 1.06 | 0.84, 1.34 | 1.19 | 0.94, 1.51 |
| Soy/tofu | 0.59 | 0.30, 1.17 | 0.61 | 0.31, 1.20 |
| Beans/lentils | 0.81 | 0.57, 1.15 | 0.89 | 0.62, 1.27 |
| Peanuts | 0.81 | 0.44, 1.49 | 0.97 | 0.54, 1.77 |
| Peas/lima beans | 0.83 | 0.58, 1.19 | 0.96 | 0.66, 1.39 |
| Other nuts | 1.07 | 0.65, 1.75 | 1.19 | 0.74, 1.91 |
| Peanut butter | 1.04 | 0.88, 1.23 | 1.12 | 0.95, 1.32 |
| Pasta | 0.59 | 0.46, 0.75 | 0.64 | 0.49, 0.83 |
| Dark bread | 0.91 | 0.85, 0.97 | 0.93 | 0.87, 0.99 |
| Cold cereal | 0.79 | 0.70, 0.90 | 0.82 | 0.72, 0.94 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Adjusted for age (continuous).
b Adjusted for age, calories (continuous), pack-years of smoking (continuous), and body mass index (weight (kg)/height (m)2; <25 vs. ≥25).
DISCUSSION
In this prospective study, we found that higher vegetable protein intake was associated with lower likelihood of early menopause. Women consuming approximately 6.5% of their daily calories as vegetable protein had a significant 16% lower risk of early menopause than women consuming approximately 4% of their calories intake as vegetable protein. For a woman consuming a 2,000-calorie diet, this is equivalent to 32.5 g of vegetable protein per day, or 3–4 servings of protein-rich foods such as enriched pasta or breakfast cereal, tofu, and nuts. In contrast, high levels of animal protein intake were not associated with early menopause.
Few studies have evaluated how protein intake is associated with timing of menopause and, to our knowledge, no studies have specifically evaluated risk of early menopause. In a prospective analysis of 1,130 premenopausal Japanese women, Nagata et al. (10) assessed how protein source was related to timing of menopause over 6 years. Likelihood of experiencing menopause during follow-up did not differ between women with the highest vegetable protein intake (median, 45.8 g/day, energy-adjusted) and lowest intake (median, 35.7 g/day; HR = 0.99) but was higher among women with moderate intake (median, 40.3 g/day; HR = 1.51, 95% CI: 1.13, 2.02). In contrast, neither soy protein intake nor animal protein intake was associated with menopause onset (10). The ability to generalize between the findings of these studies is unclear, because average vegetable protein intake was considerably higher in the Japanese population, whereas animal protein intake was lower. In addition, a greater proportion of vegetable protein was from soy-based sources than in our US-based study, where the majority of vegetable protein intake was from wheat- and nut-based sources.
Nagel et al. (5) prospectively assessed how intake of specific foods and nutrients was associated with likelihood of menopause over 6 years among 5,110 German women (EPIC-Heidelberg). High levels of protein intake were associated with a nonsignificantly lower risk of menopause (HR = 0.78, 95% CI: 0.59, 1.04; P for trend = 0.09). Although the authors did not directly compare protein sources, they reported significantly lower risk of menopause onset with higher meat intake and no association with soy product intake. Additionally, in a retrospective analysis in the Shanghai Women’s Health Study, high levels of total protein intake were associated with slightly later mean age at menopause (48.9 vs. 49.3 years comparing lowest and highest; P for trend = 0.02) (4). Vegetable and animal protein sources were not directly evaluated, but the authors reported no association with either meat or soy food intake.
Each of the previous studies considered associations of protein intake with overall age at menopause, which may differ from associations with risk of early menopause. Study participants in these evaluations were substantially older at baseline than in our study, precluding the ability to specifically evaluate risk for early menopause. For example, in the Nagata et al. study (10), participants were 35–54 years old at baseline (mean age = 42.7 years) and the observed age at menopause ranged from 43–57 years. In the German study, participants were 35–65 years old at baseline and mean age at menopause was 51.3 years (5). Factors contributing to menopause timing among women who remain premenopausal into their 50s are likely to be substantially different from those associated with menopause before age 45 years.
Results from experimental studies in animal models provide physiologic evidence for a association between vegetable protein and rate of ovarian aging. Appt et al. (6) randomly assigned 61 female cynomolgus macaques to receive either a vegetable protein–based diet (soy with isoflavones) or an animal protein–based diet (casein and lactalbumin). After 32 months of treatment, ovaries were removed for analyses of follicle counts. Macaques randomly assigned to the animal protein diet had significantly fewer primordial, primary, and secondary follicles than those assigned to the soy-based diet. These changes were accompanied by changes in plasma lipid levels. Macaques fed the animal protein diet had significantly higher total and non–high density lipoprotein cholesterol levels and lower high density lipoprotein cholesterol levels than did monkeys fed the soy diet. The extent of iliac artery atherosclerosis, as assessed by artery plaque size, was also greater in the casein-and-lactalbumin group than the soy group (6).
The observations of Appt et al. (6) are consistent with their hypothesis that an atherogenic diet adversely affects the size of the follicle pool and decreases ovarian reserve, perhaps through promoting atherosclerosis in ovarian arteries and reducing blood flow. Alternatively, Appt et al. proposed that soy-based diets are protective of ovarian function and the slow rate of follicle depletion through estrogen-related pathways or by reducing inflammation and/or oxidative stress (6). Although it remains unknown whether these findings can directly translate to premenopausal women, they suggest a potentially important mechanism linking protein source with early reproductive decline and menopause onset in humans. Studies are warranted to directly evaluate how diet protein source may correlate with follicle count, anti-Müllerian hormone levels, and rate of decline of ovarian reserve.
Our study has several potential limitations. Cumulative vegetable protein intake was self-reported by FFQ. Although this technique is common and well validated, some misclassification of intake is possible. Similarly, age at menopause was self-reported; this has been shown to be highly accurate within a similar population, however (16). Importantly, in our prospective study, we would expect any misclassification of protein intake or menopause timing to be nondifferential; therefore, the associations we observed would be most likely be underestimated rather than overestimated.
In summary, we observed a lower risk of early onset of natural menopause among women who consumed high levels of protein from vegetable sources, equivalent to 3–4 servings per day of protein-rich foods, but no similar association with animal protein intake. Additional prospective evaluations of this association are warranted, including studies addressing potential underlying mechanisms involving both soy and non–soy-based vegetable proteins. A better understanding of how dietary vegetable protein intake is associated with ovarian aging may identify ways for women to modify their risk of early onset of menopause and associated health conditions.
ACKNOWLEDGMENTS
Authors’ affiliations: Department of Biostatistics & Epidemiology, School of Public Health and Health Sciences, University of Massachusetts Amherst, Amherst, Massachusetts (Maegan E. Boutot, Alexandra Purdue-Smithe, Brian W. Whitcomb, Kathleen L. Szegda, Susan E. Hankinson, Elizabeth R. Bertone-Johnson); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (JoAnn E. Manson, Susan E. Hankinson); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (JoAnn E. Manson, Susan E. Hankinson); Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (JoAnn E. Manson); and Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Bernard A. Rosner).
This project was supported by grants UM1CA176726 and R01HD078517 from the US Department of Health and Human Services, National Institutes of Health.
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- FFQ
food frequency questionnaire
- HR
hazard ratio
- OC
oral contraception
- NHS
Nurses’ Health Study
Appendix
Appendix Table 1.
Hazard Ratios for the Associations of Protein Intake and Incidence of Early Menopause With Censoring at Hormone Replacement Therapy Use Among Women (n = 83,885), Nurses’ Health Study II, 1991–2011
| Protein Category and Intake Quintile | Median, %a | No. of Cases | HRb | 95% CI |
|---|---|---|---|---|
| Calories from vegetable protein | ||||
| 1 | 3.9 | 391 | 1.00 | Referent |
| 2 | 4.6 | 342 | 0.85 | 0.73, 0.98 |
| 3 | 5.1 | 352 | 0.87 | 0.75, 1.01 |
| 4 | 5.6 | 337 | 0.83 | 0.71, 0.97 |
| 5 | 6.4 | 337 | 0.81 | 0.69, 0.95 |
| P for trend | 0.01 | |||
| Calories from animal protein | ||||
| 1 | 10.0 | 363 | 1.00 | Referent |
| 2 | 12.2 | 350 | 0.98 | 0.84, 1.14 |
| 3 | 13.8 | 351 | 1.00 | 0.85, 1.16 |
| 4 | 15.4 | 361 | 1.05 | 0.89, 1.22 |
| 5 | 18.0 | 334 | 0.98 | 0.83, 1.16 |
| P for trend | 0.92 | |||
| Calories from total protein | ||||
| 1 | 15.3 | 359 | 1.00 | Referent |
| 2 | 17.5 | 357 | 1.02 | 0.88, 1.19 |
| 3 | 18.9 | 355 | 1.04 | 0.89, 1.21 |
| 4 | 20.5 | 347 | 1.04 | 0.89, 1.22 |
| 5 | 22.9 | 341 | 1.06 | 0.91, 1.24 |
| P for trend | 0.46 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Median percentage of total calories from protein source.
b Model 1 was adjusted for age, total caloric intake (quintiles), pack-years of smoking (never, <20 years, or ≥20 years), body mass index (weight (kg)/height (m)2; <18.5, 18.5–24.9, 25–29.9, or ≥30.0), age at menarche (≤11, 12, 13–15, or ≥16 years), total duration of breastfeeding (never, ≤2 years, or >2 years), oral contraceptive use (never, former, or current), number of pregnancies ≥6 months (0, 1–2, or ≥3), dairy protein (quintiles), and physical activity level (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, 27.0–41.2, or ≥42.0 metabolic equivalent of task hours per week). Vegetable protein was adjusted for animal protein (quintiles) and vice versa.
REFERENCES
- 1. Torrealday S, Pal L. Premature menopause. Endocrinol Metab Clin North Am. 2015;44(3):543–557. [DOI] [PubMed] [Google Scholar]
- 2. Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30(5):465–493. [DOI] [PubMed] [Google Scholar]
- 3. Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65(2):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dorjgochoo T, Kallianpur A, Gao YT, et al. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women’s Health Study. Menopause. 2008;15(5):924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nagel G, Altenburg HP, Nieters A, et al. Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas. 2005;52(3–4):337–347. [DOI] [PubMed] [Google Scholar]
- 6. Appt SE, Chen H, Goode AK, et al. The effect of diet and cardiovascular risk on ovarian aging in cynomolgus monkeys (Macaca fascicularis). Menopause. 2010;17(4):741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carwile JL, Willett WC, Michels KB. Consumption of low-fat dairy products may delay natural menopause. J Nutr. 2013;143(10):1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chavarro JE, Rich-Edwards JW, Rosner BA, et al. Protein intake and ovulatory infertility. Am J Obstet Gynecol. 2008;198(2):210.e1–210.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris DH, Jones ME, Schoemaker MJ, et al. Body mass index, exercise, and other lifestyle factors in relation to age at natural menopause: analyses from the breakthrough generations study. Am J Epidemiol. 2012;175(10):998–1005. [DOI] [PubMed] [Google Scholar]
- 10. Nagata C, Takatsuka N, Kawakami N, et al. Association of diet with the onset of menopause in Japanese women. Am J Epidemiol. 2000;152(9):863–867. [DOI] [PubMed] [Google Scholar]
- 11. Nehra D, Le HD, Fallon EM, et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell. 2012;11(6):1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang H, Chen H, Qin Y, et al. Risks associated with premature ovarian failure in Han Chinese women. Reprod Biomed Online. 2015;30(4):401–407. [DOI] [PubMed] [Google Scholar]
- 13. Willett W. Nutritional Epidemiology. 3rd ed New York, NY: Oxford University Press; 2012. [Google Scholar]
- 14. Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–867. [DOI] [PubMed] [Google Scholar]
- 15. Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 16. Colditz GA, Stampfer MJ, Willett WC, et al. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987;126(2):319–325. [DOI] [PubMed] [Google Scholar]
- 17. Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. [DOI] [PubMed] [Google Scholar]
- 18. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl): S498–S516. [DOI] [PubMed] [Google Scholar]
- 19. Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–540. [DOI] [PubMed] [Google Scholar]