Abstract
The spread of Zika virus in the Americas has been associated with a surge in Guillain-Barré syndrome (GBS) cases. Given the severity of GBS, territories affected by Zika virus need to plan health-care resources to manage GBS patients. To inform such planning in Martinique, we analyzed Zika virus surveillance and GBS data from Martinique in real time with a modeling framework that captured dynamics of the Zika virus epidemic, the risk of GBS in Zika virus–infected persons, and the clinical management of GBS cases. We compared our estimates with those from the 2013–2014 Zika virus epidemic in French Polynesia. We were able to predict just a few weeks into the epidemic that, due to lower transmission potential and lower probability of developing GBS following infection in Martinique, the total number of GBS cases in Martinique would be substantially lower than suggested by simple extrapolations from French Polynesia. We correctly predicted that 8 intensive-care beds and 7 ventilators would be sufficient to treat GBS cases. This study showcased the contribution of modeling to inform local health-care planning during an outbreak. Timely studies that estimate the proportion of infected persons that seek care are needed to improve the predictive power of such approaches.
Keywords: epidemic forecasts, Guillain-Barré syndrome, health-care planning, Zika virus
An epidemic of Zika virus (ZIKV) infections is ongoing in the Americas. Intense local transmission is currently reported in large swaths of South America (1–3). As of December 2, 2016, 50 countries and territories have reported ZIKV cases (4). The main route of transmission to humans is through bites of infected Aedes mosquitoes (5). Human-to-human transmission may also occur via sexual contact (6) or blood transfusion (7).
ZIKV infection is often asymptomatic or may give rise to mild symptoms such as fever, rash, joint pain, and conjunctivitis (8). However, there is now strong evidence that ZIKV infection is associated with congenital malformations, such as microcephaly in infants born to mothers infected during pregnancy (9–12), and neuropathies such as Guillain-Barré syndrome (GBS) (13–15), a usually rare autoimmune disease that can result in near-total paralysis. In response to the increased reporting of such complications in the Americas, the World Health Organization declared ZIKV a public health emergency of international concern on February 1, 2016 (16).
GBS is a severe, acute paralytic neuropathy, which usually follows an infection or other immune stimulation (17). Clinical management of GBS patients includes intravenous administration of immunoglobulin or plasmapheresis according to severity. During the 2013–2014 ZIKV epidemic in French Polynesia, a group of islands with 270,000 inhabitants, 42 ZIKV-associated GBS cases (1.6 per 10,000 inhabitants) were observed, 16 (38%) needed intensive care, and 12 (29%) also required mechanical ventilation (14).
Given the severity of GBS, it is important that countries affected by ZIKV evaluate the resources they will need to manage GBS patients, in particular in terms of intensive-care bed capacity and mechanical ventilators. French authorities were confronted with these issues in December 2015, when a ZIKV epidemic started in Martinique, a French island of 380,000 inhabitants in the Caribbean Sea. What would be the expected number of GBS cases in Martinique? What would be the number of GBS cases requiring intensive care or mechanical ventilation during the epidemic? Prior to the outbreak, the intensive care wards in Martinique had a capacity of 8 ventilators. Was this capacity sufficient, or did it need to be increased?
To support health-care planning, we analyzed ZIKV surveillance and GBS data from Martinique in real time with a modeling framework that captured ZIKV epidemic dynamics, the risk of GBS in ZIKV-infected persons, and the clinical management of GBS cases. To reduce uncertainty about key epidemic parameters at the start of the epidemic in Martinique, our analysis was informed by prior knowledge acquired during the 2013–2014 ZIKV epidemic in French Polynesia. The framework was used for the real-time characterization and monitoring of the epidemic in Martinique and for the forecasting of needs in health care. Results of these analyses were shared with French authorities to inform planning (18). While a number of modeling studies have focused on improving the ability to characterize and predict epidemic trajectories in real time (19–23), our approach went a step further by explicitly modeling and predicting specific health-care requirements, such as bed capacity and ventilator availability, that are more directly relevant for health-care planning.
METHODS
Data on the 2013–2014 epidemic in French Polynesia
The weekly number of patients who attended consultations for suspected ZIKV infection was estimated from the data provided by the local sentinel surveillance system during the ZIKV epidemic. The surveillance system included an average of approximately 50 sentinel sites, covering 30% of all general practitioners in the territory. From these data we extrapolated the total number of consultations. Patients with suspected infection were those who presented with rash, fever with temperature lower than 38.5°C, or both and with at least 2 of the following symptoms: conjunctivitis, joint pain with or without muscle pain, and limb edema. Laboratory confirmation of infection was obtained for a small proportion of the cases.
Forty-two GBS cases were diagnosed during the ZIKV epidemic (14). Thirty-seven (88%) of these patients also reported ZIKV-associated symptoms prior to the onset of GBS, and all had ZIKV-neutralizing antibodies. Sixteen cases (38%) needed intensive care, and 12 (29%) also required mechanical ventilation. Detailed clinical data were recorded at hospital admission and up to 3 months after discharge (14). For each GBS patient we used the dates of ZIKV-associated symptoms (when reported), GBS symptom onset, intensive care unit (ICU) admission and discharge (when applicable), and the start and end of the mechanical ventilation (when applicable).
A first serological survey, which was conducted on a subset of the general population (n = 196) recruited in February–March 2014, estimated the ZIKV seroprevalence at 49% (24). However, this survey was conducted before the end of the outbreak. To fit our model, we therefore relied on another serological survey conducted after the epidemic (May–June 2014), which found a seroprevalence of 66% in a sample of 476 schoolchildren (24). We assumed that seroprevalence in the general population would be similar to that in school-aged children.
Demographic data for the year 2014 were obtained from the French Polynesia Statistical Institute website (25).
Data on the epidemic in Martinique
We used the estimated weekly number of consultations for suspected ZIKV infection and data on hospitalized GBS cases—including the dates of hospitalization, ICU admission and discharge (when applicable), and start and end of mechanical ventilation (when applicable)—provided by the French Institute for Public Health Surveillance (26). The weekly number of consultations for suspected ZIKV infection was estimated from the sentinel surveillance data of a network comprising 20% of the general practitioners of Martinique. As of week 32 in 2016, 26 GBS cases were reported, of whom 14 (30%) needed intensive care and 11 (42%) also required mechanical ventilation.
Weeks 5, 6, 12, 13, 14, 18, 20, and 28 in 2016 had large uncertainties in the estimated number of consultations for ZIKV due to the closure of several sentinel sites for holidays. We therefore did not use these data points for model fitting. We did, however, use the weekly number of hospitalized GBS cases for those weeks, because hospital admissions were not affected by the holidays.
Demographic data for the year 2015 were taken from the French National Institute for Statistics and Economic Studies website (27).
Mathematical model
We developed a mathematical model that captured the ZIKV epidemic dynamics, the risk of GBS in ZIKV-infected persons, and the clinical management of GBS patients.
To characterize the dynamics of ZIKV infections, associated consultations, and GBS presentations, we used a simple, deterministic susceptible-infectious-recovered compartmental model with 6 parameters: 1) the reproduction number R0—the average number of secondary ZIKV cases generated by a single infectious individual in a completely susceptible population; 2) the average infectious period; 3) the number of infected persons I0 at the time the first consultations for suspected ZIKV infection were reported; 4) the proportion of infected persons who consulted practitioners for ZIKV-related symptoms (probability of seeking care ρ); 5) the probability pGBS of developing GBS once infected with ZIKV; and 6) an additional parameter δ to capture overdispersion in the observed number Ct of consultations for week t. We assumed that Ct had a negative binomial distribution with mean Et predicted by the model and overdispersion parameter kt = (Et)δ (more detail in Web Appendix 1, available at http://aje.oxfordjournals.org/).
We then simulated the trajectory of the clinical management of GBS cases, relying on estimates from French Polynesia for 1) the proportion of GBS cases requiring intensive care, 2) the proportion of GBS cases requiring ventilation, 3) the time from ZIKV symptoms to hospitalization for GBS, 4) the time from hospitalization to intensive care admission, 5) the time between admission to intensive care and the start of mechanical ventilation, 6) the duration of mechanical ventilation, 7) the time between the end of mechanical ventilation and the discharge from intensive care, and 8) the total duration of the stay in intensive care (Web Appendix 2, Web Figure 1, and Web Table 1). We documented the mean, upper bound (97.5% percentile), and lower bound (2.5% percentile) of the number of GBS patients requiring ICU admission and ventilation. The upper-bound value corresponds to the capacity needed to ensure continuity of care with high probability.
For both French Polynesia and Martinique, we made the assumption that the entire population was susceptible to ZIKV at the beginning of the outbreak, which was consistent with serological data for French Polynesia (28). We also assumed that all GBS cases presented for hospitalization, because of the severity of the syndrome.
Model parameters were estimated via Markov chain Monte Carlo sampling (29). For the analysis of the epidemic in French Polynesia, we used uniform priors for all parameters and evaluated the sensitivity of results to the choice of priors. For Martinique, where less data were available—especially at the beginning—we fixed the average infectious period at the value obtained for French Polynesia (11 days) and used Gaussian priors centered at the posterior mean estimated for French Polynesia for all the other parameters.
In a series of sensitivity analyses we explored the robustness of our findings to assumptions about priors (Web Appendix 3, Web Table 2), the average infectious period (Web Appendix 4, Web Figures 2 and 3, Web Appendix 5, Web Tables 3 and 4), and the structure of the compartmental model (Web Appendix 6, Web Table 5, Web Figure 4).
Retrospective and real-time analyses
The analysis was performed retrospectively for French Polynesia. For Martinique, estimates and predictions were revised whenever more data became available or past weeks’ data were updated.
Model validation
While this article was under review, estimates of seroprevalence in blood donors became available for 2 different time points (30). We therefore compared our model predictions for Martinique with these independent estimates. We assumed that it takes 2 weeks for infected individuals to seroconvert.
RESULTS
The ZIKV epidemic in French Polynesia
In French Polynesia, the Zika virus outbreak began in October 2013, peaked in December 2013, and was over in April 2014 (Figure 1A). By the end of the outbreak, public health officials had recorded more than 8,000 suspected cases of ZIKV infection. It was estimated that approximately 32,000 patients consulted practitioners for suspected ZIKV infection during this outbreak (31, 32) (Figure 1A).
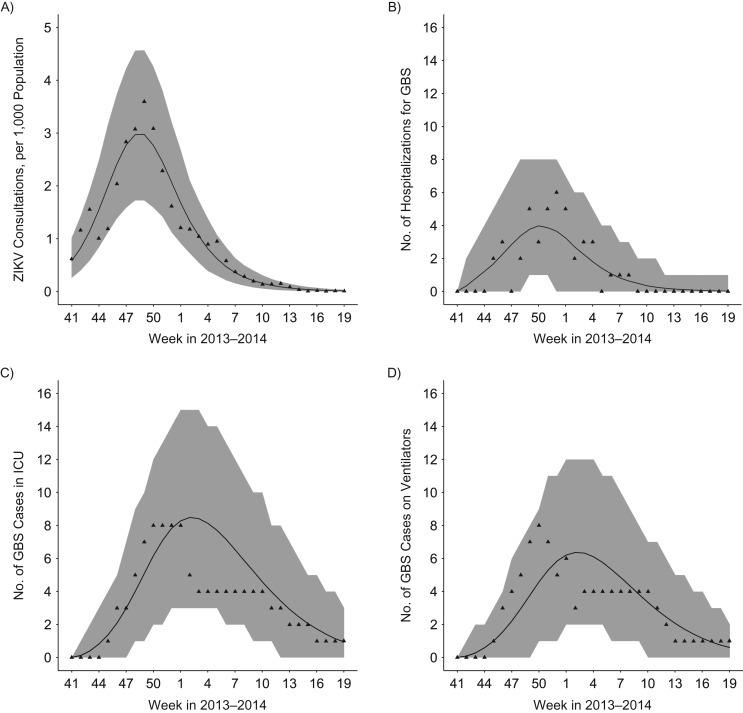
Figure 1.
Epidemic curves and model fit for French Polynesia, 2013–2014. A) Weekly number of consultations for suspected Zika virus (ZIKV) infection. B) Weekly number of Guillain-Barré syndrome (GBS) cases by hospitalization date. C) Weekly number of GBS cases in the intensive care unit (ICU). D) Weekly number of mechanically ventilated GBS cases. Triangles denote the data. Shaded area: 95% credible intervals.
The posterior means and the 95% credible intervals for model parameters are reported in Web Table 6. The basic reproduction number was estimated at 1.61 (95% credible interval (CI): 1.53, 1.69), resulting in an attack rate of 65% (95% CI: 61, 70), consistent with the serological study that informed inference (24). We also estimated an average infectious period of 11.0 (95% CI: 9.5, 12.6) days. We found that 18% (95% CI: 16, 21) of persons infected with ZIKV sought medical care for ZIKV symptoms. The risk of developing GBS was estimated at 2.48 (95% CI: 1.81, 3.26) per 10,000 ZIKV infections, close to what was previously reported (14). The average stay in ICU was 38 days (range, 2–127) (n = 16), and the average duration of mechanical ventilation was 49 days (range, 27–101) (n = 12) (see Web Appendix 2, Web Figure 1).
The model satisfyingly captured the observed dynamics of ZIKV consultations (Figure 1A), GBS hospitalizations (Figure 1B), need for intensive care beds (Figure 1C), and use of mechanical ventilators (Figure 1D).
The ZIKV epidemic in Martinique
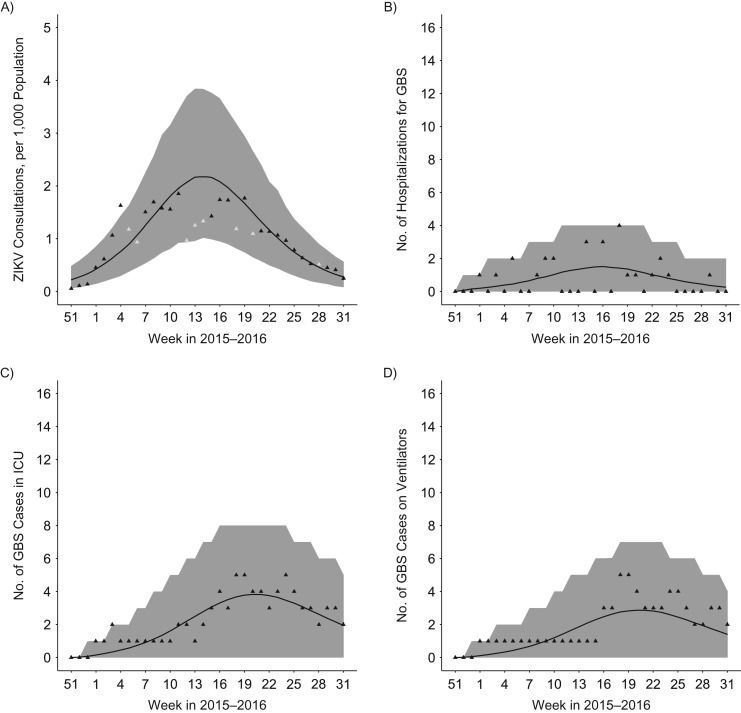
The outbreak in Martinique began in December 2015. As of August 11, 2016 (week 32 in 2016), the number of consultations for suspected ZIKV infection was estimated at approximately 35,000. Twenty-six GBS cases were reported, 25 of whom had confirmed ZIKV infection.
The basic reproduction number was lower in Martinique (1.36; 95% CI: 1.30, 1.42) than in French Polynesia (1.61; 95% CI: 1.53, 1.69), resulting in a lower predicted attack rate of 48% (95% CI: 43, 53). We found that 22% (95% CI: 20, 25) of persons infected with ZIKV attended consultations for symptoms in Martinique, roughly the same proportion as in French Polynesia (18%; 95% CI: 16, 21).
The risk of developing GBS following ZIKV infection was 1.58 per 10,000 ZIKV infections (95% CI: 1.04, 2.22). It was lower than in French Polynesia, although the difference was borderline significant (risk ratio = 0.65; 95% CI: 0.39, 1.00).
The model provided a satisfactory fit for the observed dynamics of ZIKV consultations (Figure 2A), GBS hospitalizations (Figure 2B), need for intensive care beds (Figure 2C), and use mechanical ventilators (Figure 2D).
Figure 2.
Epidemic curves and model fit for Martinique, France, as of week 32 in 2016. A) Weekly number of consultations for suspected Zika virus (ZIKV) infection. B) Weekly number of Guillain-Barré syndrome (GBS) cases by hospitalization date. C) Weekly number of GBS cases in the intensive care unit (ICU). D) Weekly number of mechanically ventilated GBS cases. Triangles denote the data; grey triangles in panel A indicate data points—not used for model fitting—with large uncertainties due to holidays. Shaded area: 95% credible intervals.
Real-time analyses in Martinique
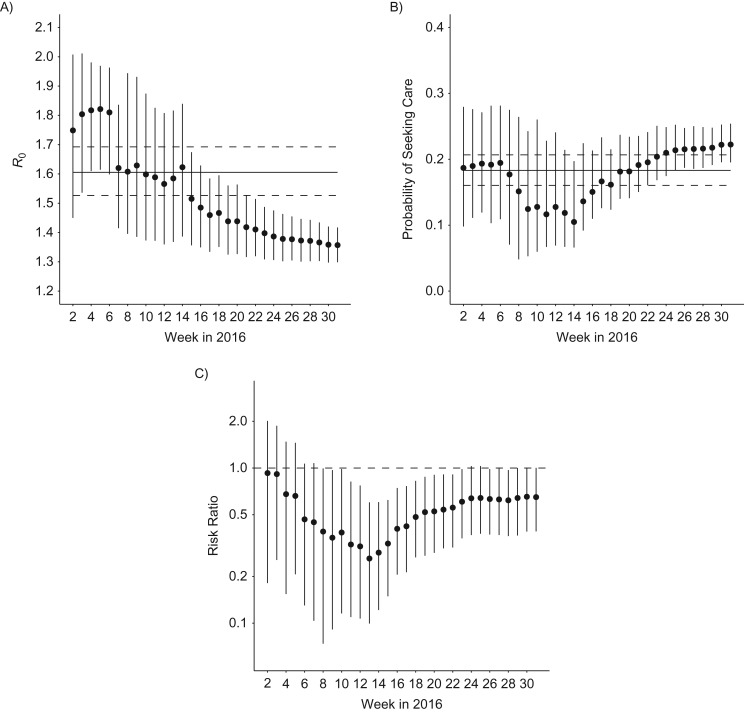
Figure 3 shows real-time estimates of model parameters starting from week 2 in 2016. Estimates of the reproduction number declined following a slowing down of the epidemic growth around week 6 in 2016 (Figures 2A and 3A). Uncertainty about the probability of seeking care remained high until relatively late in the epidemic (Figure 3B). The model predicted a borderline significant difference between Martinique and French Polynesia regarding the probability of GBS following ZIKV infection from week 8 onward (Figure 3C).
Figure 3.
Real-time estimates of key model parameters for Martinique, France, 2015–2016. A) Reproduction number R0. B) Probability of seeking care for individuals infected with Zika virus. Solid and dashed lines in panels A and B denote the posterior means and 95% credible intervals obtained for French Polynesia. C) Risk ratio for Guillain-Barré syndrome in Martinique relative to French Polynesia. Dots and bars denote the posterior means and 95% credible intervals obtained for Martinique.
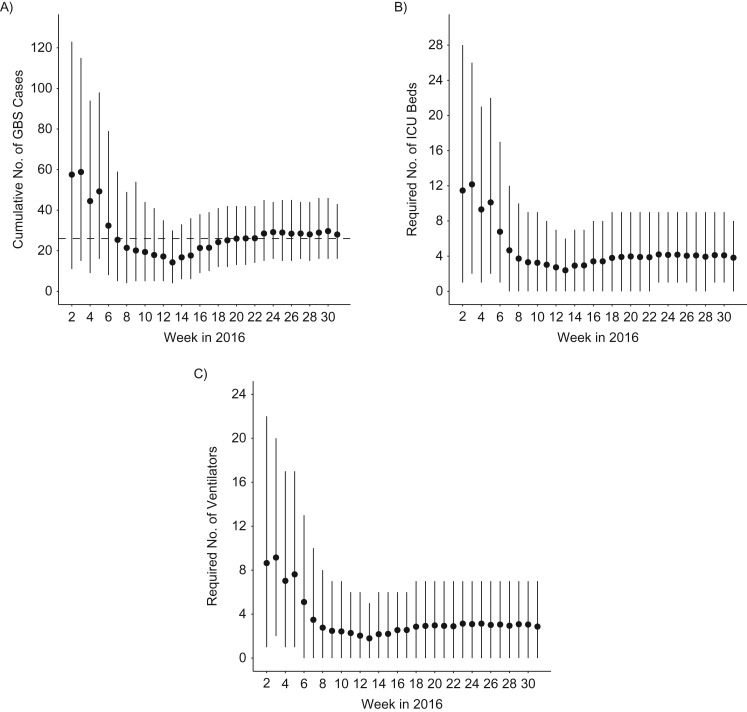
In the first week of our assessment (week 2 in 2016), when results were driven by the prior belief that the epidemic in Martinique would behave like the one in French Polynesia, we expected a total of approximately 60 GBS cases (Figure 4A), requiring on average 12 ICU beds (Figure 4B) and 9 ventilators (Figure 4C). However, the model quickly noted substantial differences between the epidemics in Martinique and in French Polynesia. By week 7 in 2016 our model was able to correctly predict that the total number of GBS cases for Martinique would actually be around 28 (Figure 4A), with an average of 4 intensive care beds (Figure 4B) and 3 ventilators (Figure 4C) required to manage them (upper bounds: 8 intensive care beds and 7 ventilators). It also correctly projected that these resources would be required between weeks 14 and 20 (Web Figure 5).
Figure 4.
Real-time estimates of the resources needed to manage Guillain-Barré syndrome (GBS) cases in Martinique, France, 2015–2016. A) Cumulative number of GBS cases. The dashed line corresponds to the total number of hospitalized GBS cases in Martinique as of week 32 in 2016 (26 cases). B) Required number of intensive care unit (ICU) beds. C) Required number of ventilators. Dots and bars denote the posterior means and 95% credible intervals.
Model validation
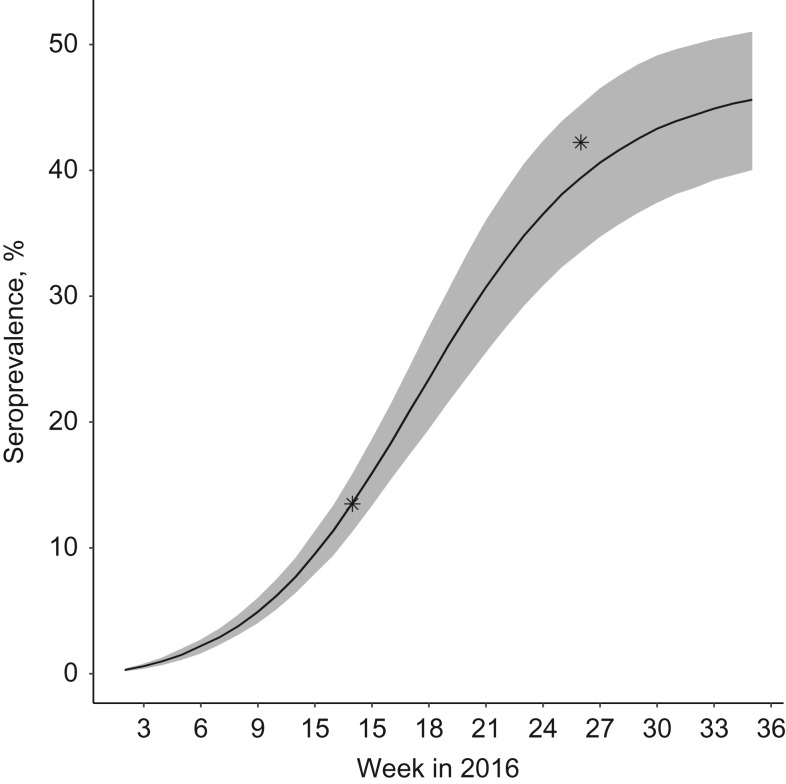
Figure 5 shows how seroprevalence was expected to change over time according to the model. It was very consistent with the results of recent serological studies conducted in blood donors on the island (13.5% seropositive among 418 donors sampled March 9–23, 2016; 42.2% seropositive among 176 donors sampled June 6–13, 2016) (30).
Figure 5.
Seroprevalence of Zika virus in Martinique, France, 2016. The 2 stars represent independent seroprevalence estimates in blood donors (30). The black line and shaded area represent means and 95% credible intervals obtained from our model.
Sensitivity analyses
Web Figures 6 and 7 show how estimates and predictions were modified if we did not use the outbreak in French Polynesia to inform the priors of model parameters but instead used uniform priors for all parameters. In that situation, the rapid slowdown of epidemic growth around weeks 5–7 in 2016 (Figure 2A) was interpreted as an indication that a large proportion of the population had already been infected, with a low probability of developing GBS following ZIKV infection. This resulted in an overly conservative estimate for the total number of GBS cases (Web Figure 7A). The model started to produce more accurate predictions from week 18 in 2016.
Given the important uncertainty about the probability of seeking care, especially at the beginning of the epidemic, we explored different scenarios where this probability was fixed between 5% and 25% (Web Figures 8 and 9). After week 16 in 2016, all these scenarios provided remarkably consistent estimates.
We estimated that the ZIKV average infectious period was T = 11 days using data from the outbreak in French Polynesia. For T = 11 days, the model was able to correctly reproduce the final attack rate and the epidemic curve. The fit deteriorated substantially when we assumed T = 15 days or T = 20 days (Web Figure 3). Changing this parameter did not affect the forecasts of resources needed to manage GBS cases in Martinique (Web Appendix 5, Web Table 3). Moreover, parameter estimates obtained for Martinique with a model in which the average infectious period was drawn from the posterior distribution obtained for French Polynesia were identical to the estimates obtained when assuming T = 11 days (Web Appendix 5, Web Table 4).
Our results were largely unchanged when we considered a susceptible-exposed-infectious-recovered model with a latency period after infection instead of a susceptible-infectious-recovered model (Web Appendix 6, Web Table 5, Web Figure 4). Assuming a time-varying reproduction number (Web Appendix 7), we obtained a slightly better fit to the epidemic curve (Web Figure 10) without substantially changing or improving the predictions concerning GBS cases (Web Figure 10). Moreover, the predictions of this model were inconsistent with recent seroprevalence estimates (Web Figure 11).
DISCUSSION
We developed a mathematical model to characterize and monitor the ZIKV epidemic in Martinique in real time and assess resources (intensive-care bed capacity and mechanical ventilators) required for the management of GBS patients. These assessments relied on the analysis of epidemiologic data from Martinique and from the 2013–2014 ZIKV outbreak in French Polynesia. We also estimated key parameters (e.g., reproduction numbers, the average infectious period, and probabilities of seeking care) required to characterize ZIKV transmission in Martinique and allow predictions about the overall epidemic dynamics (e.g., proportion of the population infected with ZIKV and timing of resource needs).
To predict the trajectory of an epidemic, it is essential to be able to estimate the number of infections, because this number captures the buildup of immunity in the population. This often proves difficult because surveillance systems track only the number of consultations, and the proportion of infected persons that seek care is often difficult to measure. For French Polynesia, the availability of serological data meant that we were able to reliably estimate that approximately 18% of ZIKV-infected persons sought care. In the absence of serological data, estimation of this parameter remained substantially more uncertain in Martinique and was sensitive to the choice of priors (Figure 3A and Web Figure 6). In our initial assessments, we therefore decided to explore scenarios with the probability of seeking care ranging from 5% to 25% (Web Figures 8 and 9), and we made a separate assessment of the most likely values for this probability based on our data and other information available in the literature. Early in the outbreak, we favored scenarios in which the probability of seeking care was in the range of 5%–10% for 2 reasons. First, it is expected that only a fraction of those with symptoms seek medical care given the mildness of the disease. For example, only approximately half of symptomatic chikungunya cases seek medical care in Martinique (33). So if 20% of ZIKV-infected persons were symptomatic, as was suggested in Yap Island (34), the proportion of ZIKV-infected persons seeking care is unlikely to be much above 10%. Second, the epidemic in Martinique quickly reached a plateau, so early on, the best model fits were obtained for small probabilities of seeking care (Web Figure 6). However, given the prolonged duration of the epidemic (Figure 3A), such a scenario can now be ruled out. The probability of seeking care in Martinique is currently estimated at ρ = 22% (95% CI: 20, 25), in good agreement with what was found for French Polynesia, with ρ = 18% (95% CI: 16, 21). These estimates suggest that the proportion of ZIKV infections that are symptomatic may be higher than initially thought. Given the difficulty of reliably estimating the probability of seeking care early in an epidemic, there is a need to design new ways to monitor the buildup of immunity in the population—for example, with studies that document health care–seeking behaviors (e.g., surveys asking if respondents were sick and if they sought care (33)) or new approaches to measure incidence more directly (e.g., testing for ZIKV in blood donors) (30).
We found that the risk of developing GBS following ZIKV infection was 1.63 (95% CI: 1.00, 2.56) times lower in Martinique than in French Polynesia, the difference between the 2 territories being borderline significant. A number of mechanisms could explain this difference. For example, the risk of developing GBS following ZIKV infection could depend on ethnicity (individuals of African descent compose the bulk of the population in Martinique). This hypothesis is supported by a study conducted in the United States during the 1976 National Influenza Immunization Program that showed that vaccinated individuals of African descent had a risk of developing GBS that was 2.4 times lower than individuals of other origins (35). Other population factors could also induce differences in the risk of GBS per infection; GBS is more likely in men and in older individuals (14). However, the population of Martinique is older than that of French Polynesia (Web Figure 12), and the difference in the sex ratio is minimal (49% women in French Polynesia compared with 54% in Martinique). It is essential to better understand why the risk of GBS following ZIKV infection may vary at a population level, because the difference estimated in our analysis may have a substantial impact on health-care planning. To compute the risk of GBS following ZIKV infection in French Polynesia, we assumed in our model that the final attack rate in the general population was similar to the 66% seroprevalence measured in 476 schoolchildren after the epidemic (24). If the attack rate was lower in adults than in children, the estimated risk of GBS following ZIKV infection would increase for French Polynesia, leading to a wider gap between French Polynesia and Martinique. Our estimate of the risk ratio may therefore be seen as conservative. The seroprevalence estimate for French Polynesia is unlikely to be affected by cross-reaction with other flaviviruses, because in a serological survey conducted before the epidemic, less than 1% of the individuals tested positive for ZIKV, despite a high level of dengue seropositivity (28).
Our analysis allowed the estimation of key epidemiologic parameters for ZIKV in both Martinique and French Polynesia. We found that the basic reproduction number of ZIKV was 1.36 in Martinique (compared with 1.61 in French Polynesia), suggesting that the final attack rate in Martinique would be approximately 48%. Interestingly, independent seroprevalence estimates (30) that were consistent with our predictions became available as this article was under review (Figure 5). Our 11-day estimate for the average infectious period was consistent with findings of a previous study (36). For Martinique, at the time when these analyses were performed, it was not possible to estimate the average infectious period due to the absence of serological data, but this parameter did not affect the forecasts of resources needed to manage GBS cases (Web Table 3). Refitting the model for Martinique, accounting for the results from the recent serological studies, gave very similar estimates of the average infectious period (12.7; 95% CI: 9.9, 16.1 days) (Web Appendix 8, Web Table 7). By comparing predictions under different epidemic scenarios with data, our approach made it possible to determine which were most likely. For example, without this framework, we feel it is unlikely that we could have predicted by week 7 in 2016 that the number of GBS patients in Martinique could be expected to be substantially smaller than what was suggested by the experience in French Polynesia.
Our analysis has a number of limitations. First, we hypothesized that ZIKV transmission rates remained constant over time. In practice, they could vary with climatic (Web Appendix 9, Web Figures 13 and 14) or environmental factors or interventions, which might affect the epidemic trajectory. Unfortunately, we could not obtain data documenting the intensity of control measures over time that might correlate with temporal variations in the transmission rate. The epidemic in Martinique was characterized by a quick increase in the number of cases with a subsequent slowdown and plateau. This pattern might potentially be explained by temporal variations in the transmission rate or in the reporting rate. Assuming time-varying transmission rates did not strongly affect predictions about GBS cases but did provide attack-rate estimates inconsistent with recent serological data (Web Figure 11). Part of the minor discrepancies observed between the epidemic curve and model predictions might therefore be due to variations of reporting over time. In practice, it is likely that the truth lies somewhere between the models with time-varying transmission rates and those with constant transmission rates, although the latter received better support from the serological data.
Second, our model assumed homogeneous mixing of individuals, but spatial heterogeneities may introduce more complex dynamics at local scale. Even so, our model provided good fits to both epidemics.
Third, we assumed a simple susceptible-infectious-recovered model structure, with cases being infectious as soon as they were infected, but the results were largely unchanged when we considered a more complex susceptible-exposed-infectious-recovered structure (Web Appendix 6, Web Table 5, Web Figure 4). There is a debate in the modeling community about the level of model complexity that is necessary to correctly capture the epidemic dynamics of vector-born infections such as ZIKV. A previous study found that in a context where no detailed entomological data were available, the susceptible-infectious-recovered model was substantially better at capturing dengue epidemic dynamics in Thailand than was an explicit vector-host model (37). This confirms our natural preference for more parsimonious models in situations such as the present one, where much is unknown about the pathogen vector. For example, while both French Polynesia and Martinique host Aedes aegypti mosquitoes, French Polynesia also hosts Aedes polynesiensis, but the contribution of either species to the spread of ZIKV during the 2013–2014 outbreak is not yet clear (38). Accounting for the short delay between infection and consultation would generate a slight shift in the simulated trajectories that would be unlikely to change our key findings.
Fourth, we assumed that GBS patients in Martinique received similar medical care to those in French Polynesia, because the health-care practices and infrastructures in the different French territories are comparable. This hypothesis allowed us to use the detailed data we had from French Polynesia in a setting where, especially at the beginning, a similar level of detail was not available. In principle however, it would also be possible to directly incorporate in our framework the data on ICU and mechanical ventilator needs from Martinique and relax this assumption—for example, to revise the estimates of the probability of requiring intensive care or the probability of requiring mechanical ventilation once in intensive care.
Given the pace at which ZIKV is spreading through the Americas, it is crucial for at-risk countries to be able to assess the adequacy of their available resources to treat patients presenting with ZIKV-related complications. We have provided such an assessment for GBS patients in Martinique, also obtaining important insights about the interpretation of surveillance data, ZIKV epidemic dynamics, and the risk of GBS following ZIKV infection in different populations. Similar analyses should be implemented for other affected areas in order to inform local health policies.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Mathematical Modelling of Infectious Diseases Unit, Institut Pasteur, Paris, France (Alessio Andronico, Henrik Salje, Simon Cauchemez); Centre National de la Recherche Scientifique, Unité de Recherche Associée 3012, Paris, France (Alessio Andronico, Henrik Salje, Simon Cauchemez); Center of Bioinformatics, Biostatistics and Integrative Biology, Institut Pasteur, Paris, France (Alessio Andronico, Henrik Salje, Simon Cauchemez); Santé Publique France, French National Public Health Agency, Cellule d'Intervention en Région (Cire) Antilles, Saint-Maurice, France (Frédérique Dorléans, Elise Daudens-Vaysse, Martine Ledrans); Centre Hospitalier Universitaire de Martinique, Fort-de-France, France (Jean-Louis Fergé, Aissatou Signate, André Cabié); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Henrik Salje); Centre Hospitalier de Polynésie Française, Papeete, Tahiti, French Polynesia (Frédéric Ghawché, Laure Baudouin); Emerging Diseases Epidemiology Unit, Institut Pasteur, Paris, France (Timothée Dub, Arnaud Fontanet); Institut Louis Malardé, Papeete, Tahiti, French Polynesia (Maite Aubry, Van-Mai Cao-Lormeau); Santé Publique France, French National Public Health Agency, Saint-Maurice, France (Harold Noel); Bureau de Veille Sanitaire, Direction de la Santé, Papeete, Tahiti, French Polynesia (Henri-Pierre Mallet); Chimie Vivant Santé (EPN7), Conservatoire National des Arts et Métiers, Paris, France (Arnaud Fontanet); Centre d’Investigation Clinique 1424, Institut National de la Santé et de la Recherche Médicale, Fort-de-France, France (André Cabié); and Equipe d'Accueil 4537, Université des Antilles, Fort-de-France, France (André Cabié).
This work was supported by the European Union's Horizon 2020 Programme through ZIKAlliance (grant 734548), the Investissement d'Avenir program, the Laboratoire d'Excellence Integrative Biology of Emerging Infectious Diseases program (grant ANR-10-LABX-62-IBEID), the Models of Infectious Disease Agent Study of the National Institute of General Medical Sciences, the AXA Research Fund, and the Association Robert Debré.
Conflict of interest: none declared.
REFERENCES
- 1. World Health Organization Zika situation report http://www.who.int/emergencies/zika-virus/situation-report/8-september-2016/en/ Published September8, 2016. Accessed September8, 2016.
- 2. Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21(10):1885–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camacho E, Paternina-Gomez M, Blanco PJ, et al. Detection of autochthonous Zika virus transmission in Sincelejo, Colombia. Emerg Infect Dis. 2016;22(5):927–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention All countries and territories with active Zika virus transmission http://www.cdc.gov/zika/geo/active-countries.html Updated December16, 2016. Accessed December14, 2016.
- 5. Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014;20(10):O595–O596. [DOI] [PubMed] [Google Scholar]
- 6. Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014;19(14):20761. [DOI] [PubMed] [Google Scholar]
- 8. Ioos S, Mallet HP, Leparc Goffart I, et al. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44(7):302–307. [DOI] [PubMed] [Google Scholar]
- 9. Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62. [DOI] [PubMed] [Google Scholar]
- 10. Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brasil P, Pereira JP Jr, Moreira ME, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. França GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388(10047):891–897. [DOI] [PubMed] [Google Scholar]
- 13. Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barré syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19(9):20720. [DOI] [PubMed] [Google Scholar]
- 14. Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arias A, Torres-Tobar L, Hernández G, et al. Guillain-Barré syndrome in patients with a recent history of Zika in Cúcuta, Colombia: a descriptive case series of 19 patients from December 2015 to March 2016. J Crit Care. 2016;37:19–23. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en/ Published February1, 2016. Accessed February22, 2016.
- 17. Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388(10045):717–727. [DOI] [PubMed] [Google Scholar]
- 18. Lazarus C, Guichard M, Philippe JM, et al. The French experience of the threat posed by Zika virus. Lancet. 2016;388(10039):9–11. [DOI] [PubMed] [Google Scholar]
- 19. Cauchemez S, Boëlle PY, Thomas G, et al. Estimating in real time the efficacy of measures to control emerging communicable diseases. Am J Epidemiol. 2006;164(6):591–597. [DOI] [PubMed] [Google Scholar]
- 20. Cori A, Ferguson NM, Fraser C, et al. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol. 2013;178(9):1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shaman J, Karspeck A. Forecasting seasonal outbreaks of influenza. Proc Natl Acad Sci USA. 2012;109(50):20425–20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chretien JP, George D, Shaman J, et al. Influenza forecasting in human populations: a scoping review. PLoS One. 2014;9(4):e94130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johansson MA, Reich NG, Hota A, et al. Evaluating the performance of infectious disease forecasts: a comparison of climate-driven and seasonal dengue forecasts for Mexico. Sci Rep. 2016;6:33707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aubry M, Teissier A, Roche C, et al. Serosurvey of dengue, Zika and other mosquito-borne viruses in French Polynesia. 64th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Philadelphia, PA, 2015. [Google Scholar]
- 25. Institut de la Statistique de la Polynésie Française Etat civil: Par âge http://www.ispf.pf/bases/Repertoires/Etatcivil/ParAge.aspx Published December31, 2015. Accessed February22, 2016.
- 26. Santé Publique France Points épidémiologiques: Zika http://www.invs.sante.fr/Actualites/Points-epidemiologiques/%28node_id%29/4639/%28query%29/Zika Published April21, 2011. Accessed August12, 2016.
- 27. Institut National de la Statistique et des Études Économiques Estimation de la population au 1er janvier 2016 http://www.insee.fr/fr/themes/detail.asp?reg_id=99&ref_id=estim-pop Published February10, 2016. Accessed February22, 2016.
- 28. Aubry M, Finke J, Teissier A, et al. Seroprevalence of arboviruses among blood donors in French Polynesia, 2011–2013. Int J Infect Dis. 2015;41:11–12. [DOI] [PubMed] [Google Scholar]
- 29. Gilks WR, Richardson S, Spiegelhalter DJ. Markov Chain Monte Carlo in Practice. London, UK: Chapman and Hall; 1996. [Google Scholar]
- 30. Gallian P, Cabie A, Richard P, et al. Zika virus in asymptomatic blood donors, Martinique. Blood. 2017;129(2):263–266. [DOI] [PubMed] [Google Scholar]
- 31. Mallet H-P, Vial A-L, Musso D. Bilan de l’épidémie à virus ZIKA en Polynésie Française 2013–2014. Bulletin d'Information Sanitaires, Épidémiologiques et Statistiques. 2015;(13):1–4.
- 32. Mallet H-P, Vial A-L, Musso D. Bilan de l’épidémie à virus Zika survenue en Polynésie française entre octobre 2013 et mars 2014. De la description de l’épidémie aux connaissances acquises après l’évènement. Bulletin Épidémiologique Hebdomadaire. 2016;(20-21):367–373. [Google Scholar]
- 33. Blateau A, Cassadou S, Vincent J, et al. Epidémie de chikungunya en Guadeloupe et en Martinique: deux estimations de l'incidence des formes cliniques de la maladie au cours de l’épidémie. Bulletin de veille sanitaire. 2014;3-4-5:29–32. [Google Scholar]
- 34. Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543. [DOI] [PubMed] [Google Scholar]
- 35. Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol. 1979;110(2):105–123. [DOI] [PubMed] [Google Scholar]
- 36. Majumder MS, Cohn E, Fish D, et al. Estimating a feasible serial interval range for Zika fever [published online ahead of print February 9, 2016]. Bull World Health Organ. (doi:10.2471/blt.16.171009). [Google Scholar]
- 37. Pandey A, Mubayi A, Medlock J. Comparing vector-host and SIR models for dengue transmission. Math Biosci. 2013;246(2):252–259. [PubMed] [Google Scholar]
- 38. Richard V, Paoaafaite T, Cao-Lormeau VM. Vector competence of French Polynesian Aedes aegypti and Aedes polynesiensis for Zika virus. PLoS Negl Trop Dis. 2016;10(9):e0005024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.