Abstract
We evaluated the relationships of red meat, poultry, fish, and shellfish intakes, as well as heme iron intake, with the risk of type 2 diabetes mellitus (T2D).The Singapore Chinese Health Study is a population-based cohort study that recruited 63,257 Chinese adults aged 45–74 years from 1993 to 1998. Usual diet was evaluated using a validated 165-item semiquantitative food frequency questionnaire at recruitment. Physician-diagnosed T2D was self-reported during 2 follow-up interviews in 1999–2004 and 2006–2010. During a mean follow-up of 10.9 years, 5,207 incident cases of T2D were reported. When comparing persons in the highest intake quartiles with those in the lowest, the multivariate-adjusted hazard ratio for T2D was 1.23 (95% confidence interval (CI): 1.14, 1.33) for red meat intake (P for trend < 0.001), 1.15 (95% CI: 1.06, 1.24) for poultry intake (P for trend = 0.004), and 1.07 (95% CI: 0.99, 1.16) for fish/shellfish intake (P for trend = 0.12). After additional adjustment for heme iron, only red meat intake remained significantly associated with T2D risk (multivariate-adjusted hazard ratio = 1.13, 95% CI: 1.01, 1.25; P for trend = 0.02). Heme iron was associated with a higher risk of T2D even after additional adjustment for red meat intake (multivariate-adjusted hazard ratio = 1.14, 95% CI: 1.02, 1.28; P for trend = 0.03). In conclusion, red meat and poultry intakes were associated with a higher risk of T2D. These associations were mediated completely for poultry and partially for red meat by heme iron intake.
Keywords: fish, heme iron, poultry, prospective studies, red meat, type 2 diabetes
Although prospective human studies have consistently shown that a diet rich in processed red meat is associated with an increased risk of type 2 diabetes mellitus (T2D) (1–3), the association has been less consistent for unprocessed red meat (3–7). In a meta-analysis of 9 prospective studies, Pan et al. (3) showed an association with unprocessed red meat that was direct but had a large degree of heterogeneity, and the only Chinese study included in the meta-analysis reported a null association (8). Furthermore, whether higher intakes of poultry or fish/shellfish are associated with T2D risk remains controversial; some meta-analyses indicated null associations (7, 9, 10), whereas studies in Chinese populations reported inverse associations (8, 11).
Heterogeneous findings when comparing Chinese populations with Western populations may be attributed to differences in the absolute intakes and the types of red meat (pork vs. beef) consumed (8, 11, 12). However, few studies have been conducted on the associations of T2D with red meat (8, 13), poultry (8, 13), and fish/shellfish (11) intakes in Asian populations.
Moreover, prospective human studies have consistently shown a positive association between dietary heme iron intake and T2D risk (14), but the question of whether only heme iron intake from red meat increases the risk of diabetes or if heme iron intake from other types of meat could have the same deleterious impact remains unanswered (15), given that poultry and fish/shellfish also contain heme iron (16).
Therefore, in the present large cohort study of middle-aged and elderly Chinese people in Singapore, we examined the relationships of dietary intakes of red meat, poultry, and fish/shellfish with the risk of incident T2D. Moreover, we assessed the association of total heme iron intake with T2D risk and whether heme iron intake mediated the meat consumption–T2D associations.
METHODS
Study population
The Singapore Chinese Health Study is a population-based cohort study established between April 1993 and December 1998 in which investigators recruited 35,303 Chinese women and 27,954 Chinese men aged 45–74 years (17). The participants were descendants from southern China, either from the Fujian province, where the Hokkien dialect is spoken, or from the Guangdong province, where the Cantonese dialect is spoken; both are major dialects common among Chinese in Singapore. Briefly, all participants were interviewed in person using structured questionnaires at recruitment. Surviving participants received a follow-up via telephone call at follow-up I (1999–2004) and follow-up II (2006–2010). The institutional review board at the National University of Singapore approved the study, and informed consent was obtained from all the study subjects.
Assessment of diet and covariates
Information on usual diet for the past year was collected using a semiquantitative food frequency questionnaire that included 165 food items commonly consumed by this population during the baseline interview. The food frequency questionnaire was subsequently validated in a subset of 810 participants by performing a repeat administration, as well as by comparing two 24-hour recalls (17). The validation study using these 2 methods showed similar distributions, with most mean pairs for energy and nutrients within 10% of each other's values. For energy and nutrients, the coefficients for the correlations between these 2 methods ranged between 0.24 and 0.79, values that are comparable to those from previous validation studies in diverse populations (18). Red meat, poultry, and fish/shellfish were among the main sources of protein and fat in this population. Although correlation coefficients were not specifically calculated for poultry, red meat, and fish/shellfish intake, the coefficients for the correlations between these 2 methods ranged from 0.36 to 0.61 for protein intake and from 0.34 to 0.47 for fat intake (17). For dietary intake, the respondents were required to select from 8 categories, ranging from “never or hardly ever” to “2 or more times a day,” and from 3 portion sizes (small, medium, or large) with the actual plate/bowl and photographs of food on the same plate/bowl provided as a guide. We separately assessed intakes (in grams) of red meat (8 items), poultry (7 items), fish/shellfish (6 items), and preserved or processed meat foods (12 items) using 33 items that included these foods (see Web Appendix 1, available at https://academic.oup.com/aje). We further assessed heme iron and nonheme iron intakes (in milligrams) from the Singapore Food Composition Database developed specifically for this cohort (17). We also collected self-reported information about each subject's age, body weight, height, educational level, alcohol consumption, smoking status, physical activity level, and known medical conditions at recruitment. Body mass index was calculated as body weight in kilograms divided by square of height in meters.
Assessment of T2D
We asked participants about any history of physician-diagnosed T2D at recruitment and the 2 follow-up interviews by using the question, “Have you been told by a doctor that you have diabetes (high blood sugar)?” If they answered yes, we followed with, “Please also tell me the age at which you were first diagnosed.” Using standard protocols, we validated the accuracy (at 98.8%) of self-reported diabetes in this cohort in a separate study of 1,651 cohort subjects who self-reported a history of physician-diagnosed T2D at follow-up I (19). Participants with a history of diagnosed T2D at recruitment were excluded from the analysis. Individuals were considered to have incident cases of T2D if they reported being diagnosed anytime between recruitment and the subsequent follow-up interviews.
Statistical analysis
Of 54,341 participants that were contacted in at least 1 follow-up, a total of 45,411 subjects were eligible for the current analysis (Web Figure 1). We used Cox proportional hazards models to examine associations between quartiles of meat intake (red meat, poultry, and fish/shellfish) and T2D risk using the lowest quartile intake as the reference category. We adjusted food and nutrient intakes for energy intake by using the residual method (20). We calculated person-years for each participant from the date of recruitment until the reported time of T2D diagnosis or the date of last follow-up interview, whichever came first. In the multivariable model, we adjusted for age (continuous), sex, interview year (1993–1995 or 1996–1998), dialect group (Hokkien or Cantonese), level of education (none, primary school, or secondary school or more), physical activity level (<0.5, 0.5–3.9, or ≥4.0 hours/week), body mass index (continuous), cigarette smoking status (never smoker, former smoker, or current smoker), alcohol consumption (never or monthly, weekly, or daily), baseline history of hypertension, total energy intake (continuous), and quartiles of scores indicating adherence to the “vegetable-, fruit-, and soy-rich pattern,” which is a dietary pattern previously identified through principal component analysis and has been shown to be associated with the risk of T2D in this cohort (21). In sensitivity analyses for each meat type, we also adjusted for dietary intakes of eggs, soy, nonsoy legumes, vegetables, fruits and related juice, noodles, rice, nuts and seeds, coffee (all in quartiles), soda (glasses per week, continuous), and the 2 other meat types. We included heme iron intake (quartiles) as a covariate in the final model.
We tested the proportionality assumption using Schoenfeld residuals, and no violation was seen. We tested P values for linear trend by including median intakes of quartiles as continuous variables in models. We also stratified the analyses by sex using sex-specific quartiles. We tested potential interactions using a likelihood ratio test of the cross-product terms for the associations of median intake quartiles and sex, age (categorized based on cohort median of 54 years at recruitment), and body mass index (<23 or ≥23). Moreover, we evaluated the effects of substituting 1 serving of meat with other types of meat (red meat, poultry, and fish/shellfish) using the method described by Kulldorff et al. (22). To facilitate the substitution analysis, we further defined 1 serving as 50 grams red meat, fish/shellfish, or poultry, which is the serving size (“liang”) widely used in Chinese populations (23). Accordingly, we simultaneously included 2 types of meat as continuous variables (serving) in the multivariate Cox model. We calculated the hazard ratio for substitution by using the exponential of the difference between the 2 coefficients, and we used the covariance between them to derive corresponding standard errors and 95% confidence intervals. We also modeled heme iron intake using a restricted cubic spline with 4 knots to test the linearity in multivariate-adjusted Cox regression analyses for men and women separately. Using the second knot as a referent, we calculated corresponding hazard ratios for heme iron values in each model. For mediation analysis, we used the method described by Buis et al. (24) to decompose a total association between quartiles of meat intake and T2D risk into direct and indirect associations (with heme iron intake as a continuous mediator) using a bootstrapping method to calculate the standard errors.
All the statistical analyses were conducted using Stata statistical software, release 11.2 (StataCorp LP, College Station, Texas). A 2-sided P value less than 0.05 was the threshold for statistical significance.
RESULTS
Participants had a mean age of 55.2 (standard deviation, 7.6) years at recruitment, and 57.3% were women. The baseline characteristics of participants in the first and fourth quartiles of meat intakes are shown in Table 1. Participants in the highest quartiles of poultry and fish/shellfish intakes were more likely to be women but less likely to be smokers compared with those with low intakes. Participants in the highest quartile of poultry intake also had a substantially higher education level. The median intake of total meat in this population was 97.4 g/day (interquartile range: 67.9–135 g/day); on average, intake comprised 27.9% red meat, 18.6% poultry, and 53.3% fish/shellfish. The majority of red meat consumed was in the form of fresh meat (87.1%), whereas organ and preserved red meat accounted for 3.6% and 8.5% of total red meat intake, respectively. The energy-adjusted intakes of red meat, poultry, and fish/shellfish were similar in men and women. The pairwise Pearson correlation coefficients for the intakes of different types of meat (grams per day) were 0.28 for red meat and poultry, 0.21 for red meat and fish/shellfish, and 0.10 for poultry and fish/shellfish.
Table 1.
Participant Characteristics According to Extreme Quartiles of Red Meat, Poultry, and Fish/Shellfish Intakes, the Singapore Chinese Health Study, 1993–2010
| Characteristic | Intake Quartile | |||||
|---|---|---|---|---|---|---|
| 1 | 4 | |||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Red Meat | ||||||
| Total | 11,629 | 25.6 | 11,304 | 24.9 | ||
| Age, years | 55.4 (7.5) | 54.6 (7.5) | ||||
| Female sex | 6,070 | 52.2 | 5,912 | 52.3 | ||
| Dialect group | ||||||
| Hokkien | 6,104 | 52.5 | 5,186 | 45.9 | ||
| Cantonese | 5,525 | 47.5 | 6,118 | 54.1 | ||
| Secondary school education or higher | 3,788 | 32.6 | 3,574 | 31.6 | ||
| Ever smoker | 3,313 | 28.5 | 3,677 | 32.5 | ||
| Alcohol intake | ||||||
| None/occasionally | 10,062 | 86.5 | 9,733 | 86.1 | ||
| Weekly | 1,060 | 9.1 | 1,148 | 10.2 | ||
| Daily | 507 | 4.4 | 423 | 3.7 | ||
| Hypertension | 2,262 | 19.5 | 2,034 | 18.0 | ||
| Weekly moderate activity | ||||||
| <0.5 hours/week | 8,668 | 74.5 | 9,196 | 81.4 | ||
| 0.5–3.9 hours/week | 1,803 | 15.5 | 1,369 | 12.1 | ||
| ≥4.0 hours/week | 1,158 | 10.0 | 739 | 6.5 | ||
| Body mass indexa | 22.9 (3.2) | 23.1 (3.3) | ||||
| Total energy intake, kcal/day | 1,779.0 (482.0) | 1,640.0 (570.0) | ||||
| Coffee consumption ≥2 cups/day | 4,245 | 36.5 | 4,241 | 37.5 | ||
| Soda consumption ≥2 glasses/week | 1,176 | 10.1 | 1,534 | 13.6 | ||
| Red meat, g/day | 10.2 (8.2) | 53.6 (15.6) | ||||
| Poultry, g/day | 14.1 (16.6) | 25.6 (17.2) | ||||
| Fish and seafood, g/day | 48.1 (30.5) | 60.9 (25.8) | ||||
| Egg, g/day | 10.4 (13.6) | 14.7 (13.2) | ||||
| Tofu equivalent, g/day | 120.0 (107.0) | 103.0 (72.0) | ||||
| Nonsoy legumes, g/day | 3.3 (5.5) | 3.2 (4.4) | ||||
| Total vegetables, g/day | 116.0 (70.1) | 109.0 (48.3) | ||||
| Total fruit, g/day | 236.0 (197.0) | 171.0 (130.0) | ||||
| Noodles, g/day | 38.4 (36.5) | 66.4 (46.1) | ||||
| Rice, g/day | 471.0 (196.0) | 368.0 (136.0) | ||||
| Nuts and seeds, g/day | 2.6 (4.3) | 3.3 (4.4) | ||||
| Heme iron, mg/day | 0.4 (0.8) | 2.5 (0.7) | ||||
| Nonheme iron, mg/day | 1.4 (1.2) | 1.6 (1.1) | ||||
| Poultry | ||||||
| Total | 11,260 | 24.8 | 11,730 | 25.8 | ||
| Age, years | 55.7 (7.6) | 54.0 (7.2) | ||||
| Female sex | 5,417 | 48.1 | 6,482 | 55.3 | ||
| Dialect group | ||||||
| Hokkien | 5,249 | 46.6 | 6,175 | 52.6 | ||
| Cantonese | 6,011 | 53.4 | 5,555 | 47.4 | ||
| Secondary school education or higher | 3,195 | 28.4 | 4,463 | 38.1 | ||
| Ever smoker | 3,814 | 33.9 | 3,116 | 26.6 | ||
| Alcohol intake | ||||||
| None/occasionally | 9,537 | 84.7 | 10,272 | 87.6 | ||
| Weekly | 1,146 | 10.2 | 1,125 | 9.6 | ||
| Daily | 577 | 5.1 | 333 | 2.8 | ||
| Hypertension | 2,175 | 19.3 | 2,190 | 18.7 | ||
| Weekly moderate activity | ||||||
| <0.5 hours/week | 8,515 | 75.6 | 9,306 | 79.3 | ||
| 0.5–3.9 hours/week | 1,701 | 15.1 | 1,592 | 13.6 | ||
| ≥4.0 hours/week | 1,044 | 9.3 | 832 | 7.1 | ||
| Body mass indexa | 23.0 (3.2) | 23.1 (3.3) | ||||
| Total energy intake, kcal/day | 1,809.0 (487.0) | 1,643.0 (558.0) | ||||
| Coffee consumption ≥2 cups/day | 4,161 | 37.0 | 4,174 | 35.6 | ||
| Soda consumption ≥2 glasses/week | 1,221 | 10.8 | 1,513 | 12.9 | ||
| Red meat, g/day | 21.9 (20.0) | 36.3 (19.2) | ||||
| Poultry, g/day | 4.1 (6.2) | 40.9 (15.5) | ||||
| Fish and seafood, g/day | 50.9 (31.5) | 58.4 (26.1) | ||||
| Egg, g/day | 11.3 ( 14.4) | 13.5 (12.4) | ||||
| Tofu equivalent, g/day | 117.0 (106.0) | 108.0 (75.0) | ||||
| Nonsoy legumes, g/day | 3.4 (5.6) | 3.1 (4.4) | ||||
| Total vegetables, g/day | 113.0 (67.7) | 114 (52.0) | ||||
| Total fruit, g/day | 220.0 (192.0) | 192 (140) | ||||
| Noodles, g/day | 46.1 (45.1) | 60.2 (40.6) | ||||
| Rice, g/day | 462.0 (197.0) | 374.0 (141.0) | ||||
| Nuts and seeds, g/day | 3.1 (4.7) | 2.8 (3.8) | ||||
| Heme iron, mg/day | 0.5 (0.9) | 2.5 (0.7) | ||||
| Nonheme iron, mg/day | 1.4 (1.2) | 1.5 (1.1) | ||||
| Fish/Shellfish | ||||||
| Total | 11,383 | 25.1 | 11,360 | 25.0 | ||
| Age, years | 55.5 (7.8) | 54.5 (7.3) | ||||
| Female sex | 5,527 | 48.6 | 6,632 | 58.4 | ||
| Dialect group | ||||||
| Hokkien | 5,948 | 52.3 | 5,100 | 44.9 | ||
| Cantonese | 5,435 | 47.8 | 6,260 | 55.1 | ||
| Secondary school educatoin or higher | 3,689 | 32.4 | 3,502 | 30.8 | ||
| Ever smoker | 3,661 | 32.2 | 3,143 | 27.7 | ||
| Alcohol intake | ||||||
| None/occasionally | 9,755 | 85.7 | 9,938 | 87.5 | ||
| Weekly | 1,110 | 9.8 | 1,066 | 9.4 | ||
| Daily | 518 | 4.6 | 356 | 3.1 | ||
| Hypertension | 2,101 | 18.5 | 2,202 | 19.4 | ||
| Weekly moderate activity | ||||||
| <0.5 hours/week | 8,853 | 77.8 | 8,824 | 77.7 | ||
| 0.5–3.9 hours/week | 1,683 | 14.8 | 1,568 | 13.8 | ||
| ≥4.0 hours/week | 847 | 7.4 | 968 | 8.5 | ||
| Body mass indexa | 22.8 (3.2) | 23.2 (3.2) | ||||
| Total energy intake, kcal/day | 1,681.0 (543.0) | 1,627.0 (538.0) | ||||
| Coffee consumption ≥2 cups/day | 4,347 | 38.2 | 3,787 | 33.3 | ||
| Soda consumption ≥2 glasses/week | 1,511 | 13.3 | 1,050 | 9.2 | ||
| Red meat, g/day | 24.5 (19.0) | 33.9 (20.0) | ||||
| Poultry, g/day | 17.8 (17.1) | 22.2 (17.2) | ||||
| Fish and seafood, g/day | 25.0 (11.0) | 89.2 (20.9) | ||||
| Egg, g/day | 12.4 (14.7) | 12.6 (11.8) | ||||
| Tofu equivalent, g/day | 101.0 (93.0) | 124.0 (84.0) | ||||
| Nonsoy legumes, g/day | 3.4 (5.3) | 3.2 (4.4) | ||||
| Total vegetables, g/day | 100.0 (59.7) | 127.0 (57.3) | ||||
| Total fruit, g/day | 202.0 (174.0) | 210.0 (152.0) | ||||
| Noodles, g/day | 40.6 (34.4) | 67.1 (47.5) | ||||
| Rice, g/day | 481.0 (182.0) | 357.0 (138.0) | ||||
| Nuts and seeds, g/day | 2.7 (4.1) | 2.9 (4.3) | ||||
| Heme iron, mg/day | 1.0 (1.1) | 1.9 (1.1) | ||||
| Nonheme iron, mg/day | 1.2 (1.1) | 1.8 (1.1) | ||||
Abbreviation: SD, standard deviation
a Weight (kg)/height (m)2.
During a mean follow-up of 10.9 years (494,741 person-years), we identified 5,207 incident cases of T2D. Table 2 and Web Table 1 present associations between different types of meat intake and T2D risk. After adjustment for potential confounders including dietary pattern, both red meat and poultry intakes showed positive associations with T2D risk. In the sensitivity analyses, we also adjusted these associations for individual food items, including the 2 other meat types, but risk estimates were not materially changed. The hazard ratios for the comparison of extreme quartiles were 1.21 (95% confidence interval (CI): 1.11, 1.32; P for trend < 0.001) for red meat, 1.10 (95% CI: 1.02, 1.20; P for trend = 0.03) for poultry, and 1.02 (95% CI: 0.93, 1.10; P for trend = 0.79) for fish/shellfish. After additional adjustment for heme iron intake, the association between red meat intake and T2D risk was substantially attenuated but remained statistically significant, whereas the association with poultry intake disappeared. In the sensitivity analyses, the association was similar for fresh red meat intake, whereas we found only a weaker, marginally significant association with processed red meat intake, probably because of the very low consumption level. Intake of red organ meat had significant positive association with risk of T2D (hazard ratio = 1.11, 95% CI: 1.02, 1.21; P for trend = 0.02) but not after additional adjustment for heme iron intake (hazard ratio = 1.02, 95% CI: 0.93, 1.12; P for trend = 0.90; Web Table 2).
Table 2.
Hazard Ratios for Incident Type 2 Diabetes According to Intakes of Different Meat Types, the Singapore Chinese Health Study, 1993–2010
| Meat Type and Model | Quartile of Meat Intake | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | ||||||||||
| No. of Cases | No. of Person Years | Median | HR | 95% CI | No. of Cases | No. of Person Years | Median | HR | 95% CI | P for Trenda | |
| Red meat | 1,240 | 127,885 | 12.3 | 1,462 | 121,692 | 48.8 | |||||
| Model 1b | 1.00 | Referent | 1.24 | 1.15, 1.34 | <0.001 | ||||||
| Model 2c | 1.00 | Referent | 1.23 | 1.14, 1.33 | <0.001 | ||||||
| Model 3d | 1.00 | Referent | 1.13 | 1.01, 1.25 | 0.017 | ||||||
| Poultry | 1,224 | 123,045 | 5.8 | 1,408 | 127,377 | 35.9 | |||||
| Model 1b | 1.00 | Referent | 1.14 | 1.06, 1.23 | 0.001 | ||||||
| Model 2c | 1.00 | Referent | 1.15 | 1.06, 1.24 | 0.001 | ||||||
| Model 3d | 1.00 | Referent | 1.01 | 0.91, 1.12 | 0.973 | ||||||
| Fish/shellfish | 1,239 | 122,515 | 27.9 | 1,417 | 124,823 | 82.7 | |||||
| Model 1b | 1.00 | Referent | 1.12 | 1.04, 1.21 | 0.003 | ||||||
| Model 2c | 1.00 | Referent | 1.07 | 0.99, 1.16 | 0.116 | ||||||
| Model 3d | 1.00 | Referent | 1.00 | 0.92, 1.09 | 0.983 | ||||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Linear trend was tested by treating the median intake values of quartiles as continuous variables using Cox proportional hazards models.
b Adjusted for age, sex, dialect, year of interview, and educational level.
c Adjusted for the variables in model 1 and body mass index, physical activity level, smoking status, alcohol use, baseline history of self-reported hypertension, adherence to the vegetable-, fruit-, and soy-rich dietary pattern, and total energy intake.
d Adjusted for the variables in model 2 and heme iron intake.
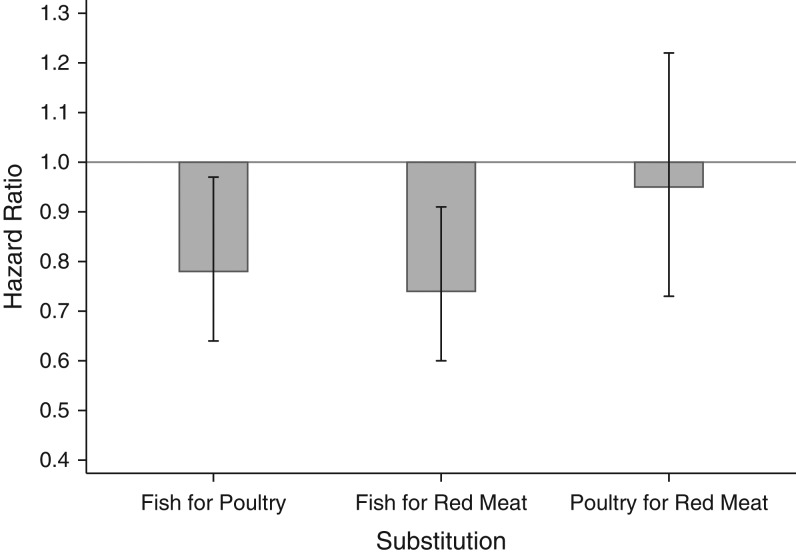
Next, for risk of incident T2D, we evaluated the changes in hazard ratio that resulted from substituting 1 serving of a specific type of meat with the other types (red meat, poultry, and fish/shellfish) (Figure 1). We found that replacing 1 daily serving of red meat with fish/shellfish was significantly associated with a 26% (95% CI: 9, 40) lower risk. Replacement of 1 daily serving of poultry with fish/shellfish was also associated with a 22% (95% CI: 3, 36) lower risk. However, substitution of poultry for red meat was not significantly associated with a change in T2D risk.
Figure 1.
Estimated change in hazard ratio for the associations of the substitution of 1 serving of red meat, poultry, or fish with risk of developing type 2 diabetes, the Singapore Chinese Health Study, 1993–2010. The model was adjusted for age, sex, dialect, year of interview, educational level, body mass index, physical activity level, smoking status, alcohol use, baseline history of self-reported hypertension, adherence to the vegetable-, fruit-, and soy-rich dietary pattern, and total energy intake.
Compared with a heme iron intake in the lowest quartile, intakes in higher quartiles were associated with higher T2D risk in a dose-response manner (Table 3 and Web Table 3). This association remained statistically significant after further adjustment for other lifestyle and dietary factors and remained unchanged with additional adjustment for poultry and fish/shellfish intakes. However, it was attenuated with further adjustment for red meat. No significant association was observed between intake of nonheme iron and T2D risk.
Table 3.
Hazard Ratios for of Incident Type 2 Diabetes According to Intake of Heme Iron, the Singapore Chinese Health Study, 1993–2010
| Iron Type and Model | Quartile of Iron Intake | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | |||||||||||
| No. of Cases | No. of Person Years | Median | HR | 95% CI | No. of Cases | No. of Person Years | Median | HR | 95% CI | P for Trenda | ||
| Heme iron | 1,181 | 124,549 | 0.21 | 1,446 | 124,505 | 0.64 | ||||||
| Model 1b | 1.00 | Referent | 1.24 | 1.15, 1.34 | <0.001 | |||||||
| Model 2c | 1.00 | Referent | 1.24 | 1.14, 1.34 | <0.001 | |||||||
| Model 3d | 1.00 | Referent | 1.23 | 1.13, 1.34 | <0.001 | |||||||
| Model 3d with poultry and fish | 1.00 | Referent | 1.23 | 1.09, 1.38 | 0.001 | |||||||
| Model 3d with red meat | 1.00 | Referent | 1.14 | 1.02, 1.28 | 0.034 | |||||||
| Nonheme iron | 1,347 | 127,128 | 6.07 | 1,295 | 120,180 | 9.38 | ||||||
| Model 1b | 1.00 | Referent | 1.04 | 0.96, 1.12 | 0.454 | |||||||
| Model 2c | 1.00 | Referent | 1.02 | 0.94, 1.11 | 0.770 | |||||||
| Model 3d | 1.00 | Referent | 0.94 | 0.83, 1.06 | 0.256 | |||||||
| Model 3d with poultry and fish | 1.00 | Referent | 0.96 | 0.85, 1.08 | 0.436 | |||||||
| Model 3d with red meat | 1.00 | Referent | 0.97 | 0.86, 1.09 | 0.556 | |||||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Linear trend was tested by treating the median intake values of quartiles as continuous variables using Cox proportional hazards models.
b Adjusted for age, sex, dialect, year of interview, and educational level.
c Adjusted for the variables in model 1 and body mass index, physical activity level, smoking status, alcohol use, baseline history of self-reported hypertension, adherence to the vegetable-, fruit-, and soy-rich dietary pattern, and total energy intake.
d Adjusted for the variables in model 2 (except for dietary patterns) and dietary intakes of egg, soy, nonsoy legumes, vegetables, fruit, noodles, rice, nuts and seeds, coffee, and soda.
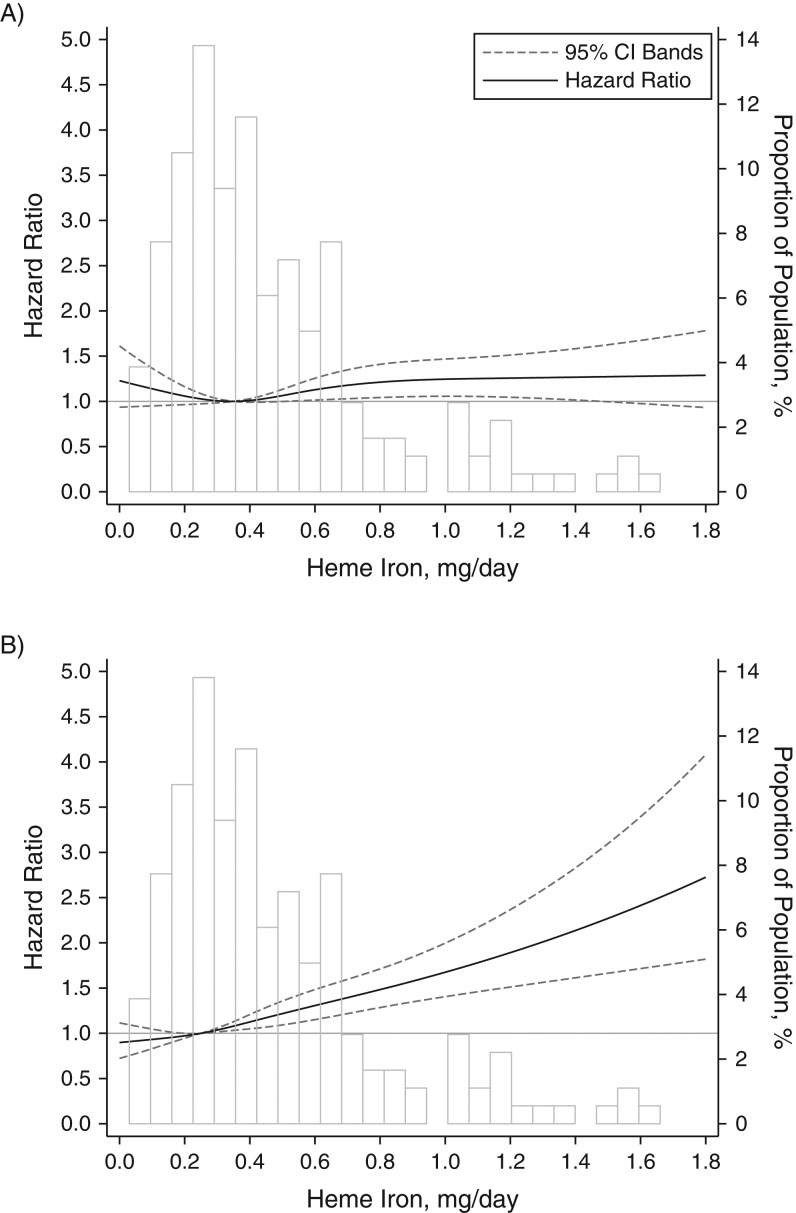
We further noticed that the association of T2D risk with red meat intake was attenuated after adjustment for heme iron intake in women but not in men, although the interaction between sex and red meat intake did not reach statistical significance (P for interaction = 0.13). As for mediation analysis, when comparing extreme quartiles, we found that red meat intake was associated with T2D risk either directly (P = 0.035) or indirectly through heme iron intake, with a 43.3% of total effect mediated (P = 0.026). The association with poultry intake was substantially mediated by heme iron intake (mediation proportion = 89.4%). There was no significant difference in the associations with poultry and fish/shellfish between men and women; the P values for interaction were 0.17 and 0.70, respectively (Web Table 4). We did not find any significant interaction between meat types and age or body mass index either. However, we found that the association between heme iron intake and T2D risk was stronger in women (for extreme quartiles, hazard ratio = 1.33, 95% CI: 1.18, 1.49; P for trend < 0.001) than men (for extreme quartiles, hazard ratio = 1.14, 95% CI: 1.00, 1.31; P for trend = 0.09) (P for interaction = 0.008; Web Table 5). Restricted cubic analysis showed a linear association between heme iron intake and T2D risk in both men (P for nonlinearity = 0.09) and women (P for nonlinearity = 0.78; Figure 2).
Figure 2.
Dose-response relationship between heme iron intake and the risk of type 2 diabetes in men (A) and women (B) using restricted cubic analysis, the Singapore Chinese Health Study, 1993–2010 (P for interaction = 0.001). The model was adjusted for age, sex, dialect, year of interview, educational level, body mass index, physical activity level, smoking status, alcohol use, baseline history of self-reported hypertension, a diet with a high consumption of meat and dim sum dishes, vegetable-, fruit-, and soy-rich dietary pattern, and total energy intake.
DISCUSSION
In the present large-scale cohort of middle-aged and elderly Chinese persons, we found positive associations between intakes of red meat and poultry and risk of T2D. Furthermore, we also found that substitution with fish/shellfish could reduce the risk of T2D associated with red meat and poultry intakes. The association with poultry seemed to be mediated via its heme iron content, whereas the association with red meat was only partially explained by its heme iron content. Heme iron intake was positively associated with T2D risk, with a stronger association in women than in men.
Various studies had consistently shown a positive association between processed red meat intake and risk of developing T2D (1–3), whereas the relationship of unprocessed red meat intake with T2D risk has been less consistent (3–7). In a meta-analysis that included 9 prospective studies (8 conducted in Western populations and 1 in a Chinese population), investigators reported a pooled fully adjusted relative risk of 1.19 (95% CI: 1.04, 1.37) per 100 g/day of unprocessed red meat intake, although there was great heterogeneity (I2 = 93.3%) (3). In the only study in Chinese populations, researchers compared median intakes of 67.6 g/day versus 24.5 g/day and reported a null association (relative risk = 0.94, 95% CI: 0.79, 1.12) (8). The authors hypothesized that this might have been due to the low amount and different type of red meat (90% pork) consumed among the participants of the study based in China (8). However, we found a positive association with red meat intake in our study population, who seemed to consume quantities and type of red meat similar to what was seen in the study from China. We found similar strengths of association between red meat intake and T2D risk in both men and women; however, the association in women but not in men was attenuated after adjustment for heme iron. In another large cohort study in Japanese adults, investigators found that red meat intake was associated with a higher risk of T2D in men but not in women (13).
As for poultry intake, results in the previous literature show a mix of inverse (8), null (7, 13), and positive (7) associations with T2D risk. In contrast to our findings, those from a recent meta-analysis that included 10 prospective studies from Europe showed a pooled relative risk of 1.04 (95% CI: 0.82, 1.32) (25), and 2 additional studies from China and Japan found inverse (8) and null associations (13), respectively. To the best of our knowledge, a harmful association has only been reported in 2 studies in European populations, but neither of them has evaluated the association with heme iron (7). In the present study, the positive association with poultry was attenuated and not statistically significant after adjustment for heme iron intake, indicating the possible role of heme iron as a mediator of the association between poultry and T2D development. Interestingly, heme iron contents differ in different parts of poultry; for example, chicken thighs contain 5 μg/g of heme iron, whereas chicken breasts contain approximately 2 μg/g of heme iron (16). Hence, 1 potential explanation for the inconsistency in the evidence of an association between poultry and T2D risk in different populations might be differences in preferences for different parts of poultry.
Investigators in a previous meta-analyses showed that the effect of fish/shellfish intake may be modified by study location (9, 10). In 1 meta-analysis, researchers showed a higher risk among participants in studies conducted in the United States, null association among European populations, and an inverse association among Asian and Australian populations (10). In a recent analysis in 8 European countries undertaken by the InterAct Consortium of the European Prospective Investigation Into Cancer and Nutrition, no significant association was found between total fish/shellfish consumption and T2D risk (26). Another meta-analysis similarly showed a null association in Western population but a protective association in populations in Asian studies (9). However, the 2 Asian studies in the meta-analyses have reported inconsistent results themselves; Villegas et al. (11) reported a significant inverse association in Chinese women but not in men, whereas Nanri et al. (27) reported a significant inverse association in Japanese men but not in women. We did not find any significant association between fish/shellfish consumption and T2D risk in this Chinese population. However, the substitution analysis showed that fish could be a beneficial alternative to red meat and poultry.
We found that intake of heme iron but not nonheme iron was associated with T2D risk, and this was consistent with findings from previous studies (14, 28). In a meta-analysis of 5 prospective studies in which median intakes of 2.39 mg/day and 0.56 mg/day were compared, the pooled relative risk was 1.33 (95% CI: 1.19, 1.48), with a low degree of heterogeneity (I2 = 27.4) (14). Iron is a strong pro-oxidant that catalyzes the production of reactive oxygen species, which may damage body tissues, particularly insulin-producing pancreatic cells (29). Several prospective human studies have shown a positive association between body iron store and higher T2D risk (30). The distinct effects of heme iron and nonheme iron intake on T2D risk may be explained by the differences in bioavailability and their effects on body iron stores, because heme iron is more readily absorbed than nonheme iron (14).
In line with our findings, researchers conducing a pooled analysis of data from the Health Professionals Follow-Up Study and Nurses’ Health Studies I and II found that the association between red meat consumption and T2D was attenuated largely by further adjustment for heme iron, although it was still positive (3). These findings indicate that the association of T2D with red meat is only partly mediated through the heme iron content of the latter and that other components in red meat, such as advanced glycation end products and trimethylamine N-oxide, also have strong mechanistic links to insulin resistance (31). Red meat intake may also be linked to T2D risk via the promotion of inflammation (4, 32). Altogether, these pathways explain the residual association of red meat that is independent of heme iron.
We reported a stronger association of dietary heme iron with T2D risk in women. Most previous studies on the heme iron–T2D risk association included single sex (14) or did not provide stratified analysis (28). There has been only 1 study in a Chinese population in which researchers evaluated sex-specific associations, but contrary to our findings, they reported a stronger association in men based on a limited number of events (n = 131) (33). The observed sex difference in our study may be due to heterogeneity in iron absorption regulated by intestinal mucosa. Evidence has shown that women, who have generally greater requirements for iron, absorb approximately twice as much iron as men from the same dietary intake (34). Nevertheless, more studies are still needed to confirm sex difference in the association between heme iron and T2D risk and to explore the potential mechanisms.
The strengths of our study include the large sample size, long follow-up period, high response and follow-up rates, and detailed collection of data through face-to-face interviews using a food frequency questionnaire that was specifically developed and validated in this population (17). Furthermore, we controlled for a number of dietary and nondietary covariates to reduce the confounding effects, although unmeasured and residual confounding are still possible in epidemiologic studies. However, our findings have some limitations. First, self-report of meat intake could result in nondifferential misclassification because of measurement errors. Because such nondifferential misclassification for a dichotomous exposure could lead to bias toward the null value (35), we tested binary meat intake variables (based on median) and consistently found significantly higher risks of T2D associated with higher intakes of red meat, poultry, and heme iron (data not shown), which suggests that any nondifferential misclassification would have likely led to an underestimation of the actual risk estimate. Second, diet was only assessed at baseline, and we lack information about potential changes in exposure that occurred later. Third, we only inquired about physician-diagnosed T2D in our study, and it is expected that asymptomatic diabetes existed in the study population. We do not perceive any reason for meat intake to be related to the likelihood of disease diagnosis in our study population. Hence, we believe that such misclassification of diagnosis for asymptomatic diabetes cases at baseline or during follow-up was likely to be nondifferential in either situation and would have resulted in underestimation of the association. Finally, this population had high fish/shellfish consumption; thus, our results may not be generalizable to the populations with lower intakes of fish/shellfish.
In this Chinese population, we found that high intakes of red meat and poultry were associated with a higher risk of T2D, and the association with poultry may be mediated by heme iron. In addition, heme iron only partially explained the detrimental effect of red meat consumption, and other chemicals in red meat may also account for the higher risk. Replacement of red meat and poultry with fish/shellfish may reduce T2D risk, and it is worth testing this theory in experimental studies.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Saw Swee Hock School of Public Health, National University of Singapore, Singapore (Mohammad Talaei, Ye-Li Wang, Woon-Puay Koh); Division of Cancer Control and Population Sciences, University of Pittsburgh Cancer Institute, Pittsburgh, Pennsylvania (Jian-Min Yuan); Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania (Jian-Min Yuan); Department of Epidemiology and Statistics, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (An Pan); and Duke-NUS Graduate Medical School Singapore, Singapore (Woon-Puay Koh).
The first 2 authors contributed equally to this work.
This study was supported by the National Medical Research Council, Singapore (grant NMRC/CIRG/1354/2013) and National Institutes of Health (grants RO1 CA144034 and UM1 CA182876). W.-P.K. is supported by the National Medical Research Council, Singapore (grant NMRC/CSA/0055/2013), M.T. is supported by the SINGA Scholarship, and Y.-L.W. is supported by the Lee Kong Chian Scholarship.
We thank Siew-Hong Low of the National University of Singapore for supervising the fieldwork in the Singapore Chinese Health Study and Renwei Wang for the maintenance of the cohort study database. We also thank the founding principal investigator of the Singapore Chinese Health Study, Mimi C. Yu.
The funders had no role in the design and conduct of the study; collection; management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1. Aune D, Ursin G, Veierød MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia. 2009;52(11):2277–2287. [DOI] [PubMed] [Google Scholar]
- 2. Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121(21):2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pan A, Sun Q, Bernstein AM, et al. . Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94(4):1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Woudenbergh GJ, Kuijsten A, Tigcheler B, et al. . Meat consumption and its association with C-reactive protein and incident type 2 diabetes: the Rotterdam Study. Diabetes Care. 2012;35(7):1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fretts AM, Howard BV, McKnight B, et al. . Associations of processed meat and unprocessed red meat intake with incident diabetes: the Strong Heart Family Study. Am J Clin Nutr. 2012;95(3):752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lajous M, Tondeur L, Fagherazzi G, et al. . Processed and unprocessed red meat consumption and incident type 2 diabetes among French women. Diabetes Care. 2012;35(1):128–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bendinelli B, Palli D, Masala G, et al. . Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct Study. Diabetologia. 2013;56(1):47–59. [DOI] [PubMed] [Google Scholar]
- 8. Villegas R, Shu XO, Gao YT, et al. . The association of meat intake and the risk of type 2 diabetes may be modified by body weight. Int J Med Sci. 2006;3(4):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wallin A, Di Giuseppe D, Orsini N, et al. . Fish consumption, dietary long-chain n-3 fatty acids, and risk of type 2 diabetes: systematic review and meta-analysis of prospective studies. Diabetes Care. 2012;35(4):918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xun P, He K. Fish consumption and incidence of diabetes: meta-analysis of data from 438,000 individuals in 12 independent prospective cohorts with an average 11-year follow-up. Diabetes Care. 2012;35(4):930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villegas R, Xiang YB, Elasy T, et al. . Fish, shellfish, and long-chain n-3 fatty acid consumption and risk of incident type 2 diabetes in middle-aged Chinese men and women. Am J Clin Nutr. 2011;94(2):543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koh WP, Yang HN, Yang HQ, et al. . Potential sources of carcinogenic heterocyclic amines in the Chinese diet: results from a 24-h dietary recall study in Singapore. Eur J Clin Nutr. 2005;59(1):16–23. [DOI] [PubMed] [Google Scholar]
- 13. Kurotani K, Nanri A, Goto A, et al. . Red meat consumption is associated with the risk of type 2 diabetes in men but not in women: a Japan Public Health Center-based prospective study. Br J Nutr. 2013;110(10):1910–1918. [DOI] [PubMed] [Google Scholar]
- 14. Bao W, Rong Y, Rong S, et al. . Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and meta-analysis. BMC Med. 2012;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White DL, Collinson A. Red meat, dietary heme iron, and risk of type 2 diabetes: the involvement of advanced lipoxidation endproducts. Adv Nutr. 2013;4(4):403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cross AJ, Harnly JM, Ferrucci LM, et al. . Developing a heme iron database for meats according to meat type, cooking method and doneness level. Food Nutr Sci. 2012;3(7):905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hankin JH, Stram DO, Arakawa K, et al. . Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39(2):187–195. [DOI] [PubMed] [Google Scholar]
- 18. Stram DO, Hankin JH, Wilkens LR, et al. . Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol. 2000;151(4):358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Odegaard AO, Pereira MA, Koh WP, et al. . Coffee, tea, and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr. 2008;88(4):979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Willett W, Stampfer MJ. Implications of total energy intake for epidemiologic analysis In: Willett W, ed. Nutritional Epidemiology. New York, NY: Oxford University Press; 2013:260–286. [Google Scholar]
- 21. Odegaard AO, Koh WP, Butler LM, et al. . Dietary patterns and incident type 2 diabetes in chinese men and women: the singapore chinese health study. Diabetes Care. 2011;34(4):880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kulldorff M, Sinha R, Chow WH, et al. . Comparing odds ratios for nested subsets of dietary components. Int J Epidemiol. 2000;29(6):1060–1064. [DOI] [PubMed] [Google Scholar]
- 23. Pan A, Franco OH, Ye J, et al. . Soy protein intake has sex-specific effects on the risk of metabolic syndrome in middle-aged and elderly Chinese. J Nutr. 2008;138(12):2413–2421. [DOI] [PubMed] [Google Scholar]
- 24. Buis ML. Direct and indirect effects in a logit model. Stata J. 2010;10(1):11–29. [PMC free article] [PubMed] [Google Scholar]
- 25. Feskens EJ, Sluik D, van Woudenbergh GJ. Meat consumption, diabetes, and its complications. Curr Diab Rep. 2013;13(2):298–306. [DOI] [PubMed] [Google Scholar]
- 26. Patel PS, Forouhi NG, Kuijsten A, et al. . The prospective association between total and type of fish intake and type 2 diabetes in 8 European countries: EPIC-InterAct Study. Am J Clin Nutr. 2012;95(6):1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nanri A, Mizoue T, Noda M, et al. . Fish intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr. 2011;94(3):884–891. [DOI] [PubMed] [Google Scholar]
- 28. Fernandez-Cao JC, Arija V, Aranda N, et al. . Heme iron intake and risk of new-onset diabetes in a Mediterranean population at high risk of cardiovascular disease: an observational cohort analysis. BMC Public Health. 2013;13:1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rajpathak SN, Crandall JP, Wylie-Rosett J, et al. . The role of iron in type 2 diabetes in humans. Biochim Biophys Acta. 2009;1790(7):671–681. [DOI] [PubMed] [Google Scholar]
- 30. Kunutsor SK, Apekey TA, Walley J, et al. . Ferritin levels and risk of type 2 diabetes mellitus: an updated systematic review and meta-analysis of prospective evidence. Diabetes Metab Res Rev. 2013;29(4):308–318. [DOI] [PubMed] [Google Scholar]
- 31. Kim Y, Keogh J, Clifton P. A review of potential metabolic etiologies of the observed association between red meat consumption and development of type 2 diabetes mellitus. Metabolism. 2015;64(7):768–779. [DOI] [PubMed] [Google Scholar]
- 32. Lee CC, Adler AI, Sandhu MS, et al. . Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia. 2009;52(6):1040–1047. [DOI] [PubMed] [Google Scholar]
- 33. Shi Z, Zhou M, Yuan B, et al. . Iron intake and body iron stores, anaemia and risk of hyperglycaemia among Chinese adults: the prospective Jiangsu Nutrition Study (JIN). Public Health Nutr. 2010;13(9):1319–1327. [DOI] [PubMed] [Google Scholar]
- 34. Grauer AL, Stuart-Macadam P. Sex and gender in paleopathological perspective In: Grauer AL, Stuart-Macadam P, eds. Iron Deficiency Anemia: Exploring the Difference. Cambridge, UK: Cambridge University Press; 2005:128–147. [Google Scholar]
- 35. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.