Abstract
Objective
To report descriptive and normative data for the Montreal Cognitive Assessment (MoCA) in a population-based African American sample.
Method
The MoCA was administered to 1,419 African American participants (mean age 49.89 years, range 18–75, 64% female). After excluding those with subjective cognitive complaints (n = 301), normative data were generated by education and overlapping age ranges (n = 1,118). Pearson correlations and analysis of variance were used to examine the relationship to demographic variables, and frequency of missed items was reviewed.
Results
Total MoCA scores (mean 22.3, SD 3.9) were lower than previously published normative data derived from an elderly Caucasian Canadian population with 80% falling below the suggested cutoff (<26) for impairment. Several MoCA items were missed by a large portion of the sample, including cube drawing (72%), delayed free recall (66% <4/5 words), sentence repetition (63%), and abstraction items (45%).
Conclusion
This is the first study to examine normative performance on the MoCA specific to community-dwelling African Americans. Findings suggest that certain aspects of this measure and previously established cutoff scores may not be well-suited for some populations.
Keywords: Normative studies, Assessment, Aging, Minority, Mild cognitive impairment
Introduction
Cognitive screening measures are effective in discriminating cognitive impairment from normal aging, and the Montreal Cognitive Assessment (MoCA) (Nasreddine, et al., 2005) is a popular, sensitive screening tool. Despite the increasing use of the MoCA in clinical and research settings, normative data for the MoCA specific to African Americans and other ethnic minority groups are lacking, with only one study providing MoCA performance data in a cohort of 414 African Americans with Type 2 diabetes (Sink, et al., 2015). The need for normative information for cognitive assessment measures in minority groups is well established (Welsh, et al., 1995; Casaletto, et al., 2015). For example, the mini-mental state examination (Folstein, Folstein, & McHugh, 1975), perhaps the most widely used cognitive screening tool, has shown diminished diagnostic utility when used to evaluate ethnic minorities (Escobar, et al., 1986; Bohnstedt, Fox, & Kohatsu, 1994). Interpretation of test scores based on data from a sample that is not representative of the individual can result in a disproportionate number of cognitively normal ethnic minority individuals inaccurately classified as impaired and overestimate the level of cognitive impairment in ethnic minorities with dementia (Pedraza, et al., 2012). There may be sociocultural and/or varying educational experiences across groups that are not completely controlled for through statistical covariance or by applying adjustments to scores derived from predominantly white education-based norms (Touradj, Manly, Jacobs, & Stern, 2001). The aim of this study was to extend our prior MoCA normative work (Rossetti, Lacritz, Cullum, & Weiner, 2011) by providing preliminary normative and descriptive data for the MoCA specific to the African American subset of our community-based cohort.
Materials and Methods
Participants
This investigation was conducted as part of a longitudinal, population-based, multi-ethnic study of factors contributing to the development of cardiovascular disease. African Americans were oversampled to ensure approximately 50% of African American representation (Victor, et al., 2004). The MoCA was administered to 2,766 participants. Participants for this study met the following inclusion criteria: self-identified as African American, able to provide informed consent, and completed a valid MoCA test. Thirty-seven individuals were excluded due to stroke history. One participant was excluded because he requested that his data should not be used. Forty-five duplicate entries were removed and another 29 were deleted due to missing data that prevented the calculation of an MoCA total score. Participants who self-identified as White, Hispanic or Other (n = 1235) were excluded. Following these exclusions, there were 1,419 African American subjects available for this study analyses. All participants provided written informed consent to participate, and the study protocol was approved by the Institutional Review Board of UT Southwestern Medical Center.
Measures
The MoCA is a 30-point screening tool that requires approximately 10–15 min to administer and evaluates aspects of attention, orientation, language, verbal memory, visuospatial, and executive function. The individual MoCA items have been described in detail elsewhere (Nasreddine, et al., 2005).
Procedures
The MoCA was administered by trained personnel. The suggested 1-point correction for <12 years of education was not applied, as one aim of the study was to generate normative data across education levels. After scoring, the de-identified MoCA data were entered item by item in the database using only the study identifier. To screen for subjective cognitive impairment, participants were asked 3 Yes/No questions prior to completing the MoCA: whether they believed they had any problems with their memory, if those problems interfered in daily functioning, and if they had any difficulty solving problems; and those who endorsed one or more questions were removed from normative analysis.
Statistical Analyses
Statistical analyses were conducted using IBM SPSS version 23. The frequency of missed items was reviewed to identify items that might be less discriminating of cognitive impairment. The relationship between MoCA scores and demographic variables was examined using Pearson product–moment correlations (age and education) and independent samples t-test (gender). Additionally, MoCA scores for those who endorsed one or more self-reported cognitive complaints were compared to those with no cognitive complaints using analysis of covariance, covarying for age and education. Assumptions for all tests were reviewed.
After the establishment of demographic-dependent differences, overlapping age ranges were utilized (Pauker, 1988). This method provides larger sample sizes in each group for more reliable data and allows clinicians to choose the age group that corresponds best with the age of a patient (e.g., a 65-year-old individual may be better represented in the midpoint [60–70 years] age range rather than at the extreme [55–65 years]). The age groups were subdivided into 3 groups each, according to educational level: <12 years of education, 12 years of education, and >12 years of education.
Results
Demographic characteristics are presented in Table 1. Of the 1,419 participants, 64% were women. The mean age of the sample was 50 years (SD = 11, range 18–75), and the average education level was 12.92 years (SD = 1.96). MoCA scores were significantly correlated with age (r = −0.31, p ≤ .001) and education (r = 0.37, p ≤ .001) but did not differ by gender (t(1,1417) = −1.83, p = .07).
Table 1.
Sample characteristics (n = 1419)
| % | Minimum | Maximum | M (SD) | |
|---|---|---|---|---|
| Age | 18 | 82 | 49.87 (11.27) | |
| Education | 5 | 20 | 12.92 (1.95) | |
| Female (%) | 64 | |||
| MoCA total score | 7 | 30 | 22.01 (4.00) | |
| Subjective cognitive complaint | 21 | |||
| Diabetes | 8 | |||
| Hypertension | 27 | |||
| Hypercholesterolemia | 7 | |||
| ApoE4 | 38 |
Note: MoCA, Montreal Cognitive Assessment, ApoE4, participants with at least 1 apolipoprotein E4 allele.
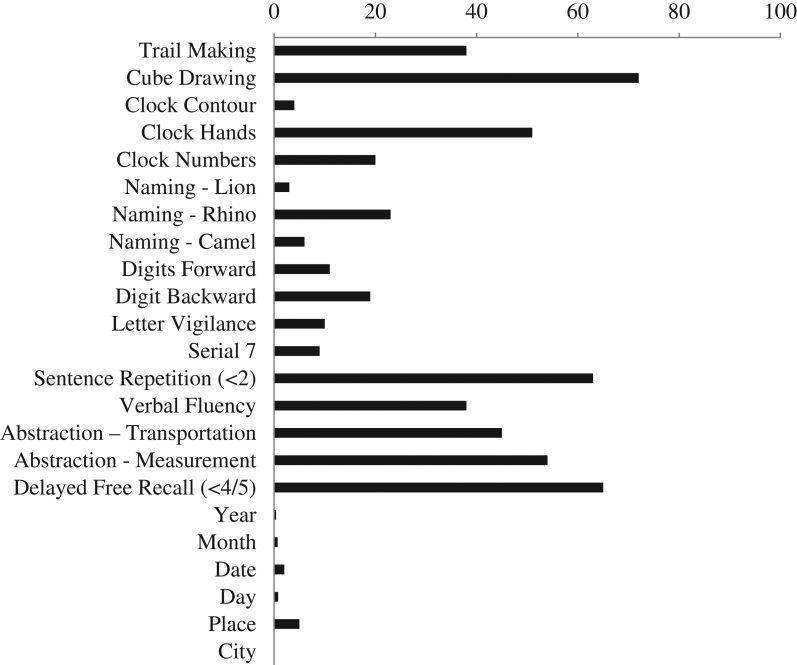
The mean MoCA total score for the sample as a whole (M = 22.01, SD = 4.00) fell below the original suggested cutoff (<26) value for impairment (Nasreddine, et al., 2005), even when the suggested 1-point education correction was applied (M = 22.52, SD = 3.86). The majority of participants, in fact, performed below this published cutoff (80% without education correction; 76% with correction). The most frequently missed items included drawing a cube (72%), delayed free recall (66%; <4/5 words), sentence repetition (63%), and verbal abstraction items (watch/ruler 55%; train/bicycle 45%) (Fig. 1).
Fig. 1.
Frequency of incorrect items in overall sample (in percentage); n = 1419.
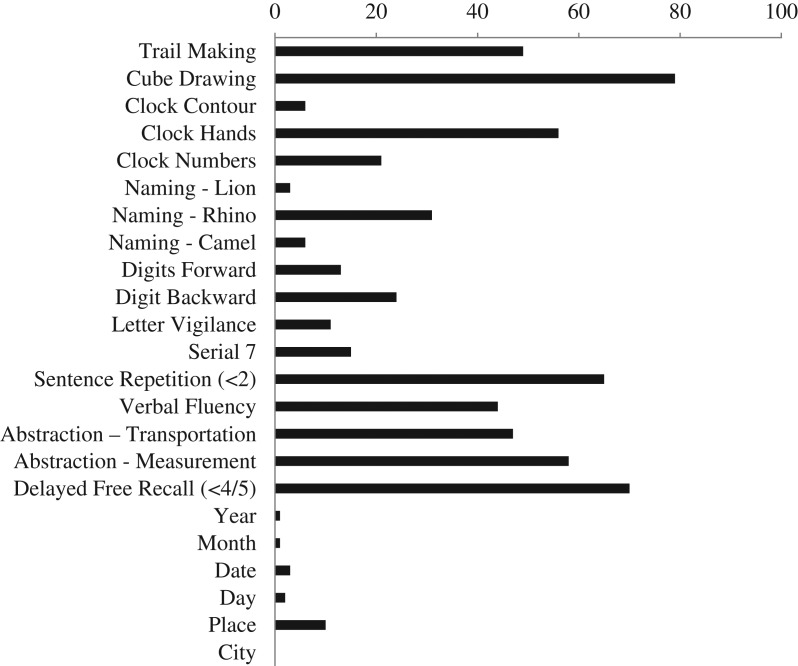
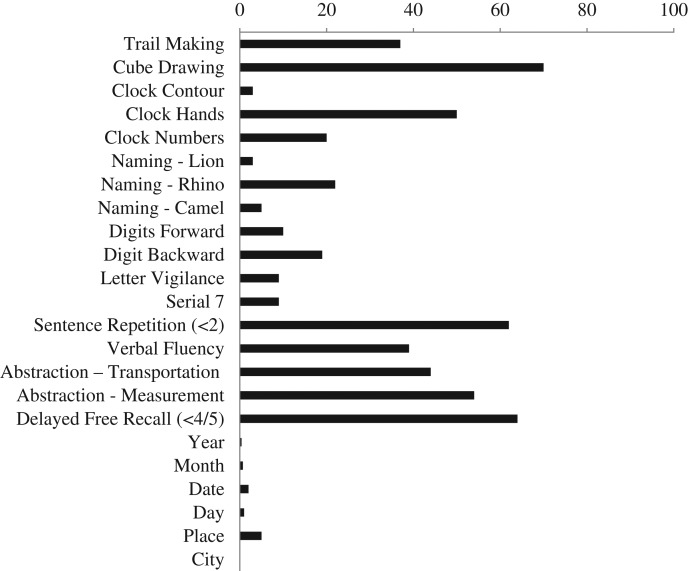
MoCA performance differed significantly based on self-reported memory and executive functioning problems, as those who endorsed one or more of the 3 screening questions scored significantly lower (M = 20.95, SD = 4.25) than those with no cognitive complaints (M = 22.33, SD = 3.89; F(1,1328) = 17.07, p ≤ .0001). Participants with self-identified cognitive complaints were subsequently excluded from the calculation of normative data (n = 301). Normative data stratified by age and education were derived (Table 2). The frequency of missed items in both the cognitive complaint group and the normative sample is presented in Figs. 2 and 3.
Table 2.
Montreal Cognitive Assessment score by age and education level in an african american sample (N = 1118)
| Years of education | Total by age | |||||||
|---|---|---|---|---|---|---|---|---|
| <12 | 12 | >12 | ||||||
| Age group | N | M (SD) Median | N | M (SD) Median | N | M (SD) Median | N | M (SD) Median |
| <35 | 13 | 22.69 (3.68) | 44 | 23.36 (3.33) | 62 | 25.05 (2.46) | 119 | 24.54 (3.01) |
| 23 | 25 | 26 | 25 | |||||
| 30–40 | 22 | 22.27 (3.40) | 69 | 23.57 (3.03) | 122 | 24.98 (2.85) | 214 | 24.25 (3.09) |
| 22 | 24 | 26 | 25 | |||||
| 35–45 | 27 | 21.33 (3.43) | 121 | 22.45 (3.72) | 161 | 24.34 (3.12) | 310 | 23.35 (3.55) |
| 22 | 23 | 25 | 24 | |||||
| 40–50 | 42 | 20.24 (3.98) | 154 | 21.48 (3.98) | 193 | 23.85 (3.08) | 389 | 22.52 (3.80) |
| 21 | 22 | 24 | 23 | |||||
| 45–55 | 44 | 19.73 (3.83) | 142 | 21.11 (3.95) | 215 | 23.15 (3.24) | 401 | 22.05 (3.78) |
| 20 | 22 | 23 | 23 | |||||
| 50–60 | 37 | 18.62 (4.43) | 105 | 21.47 (3.14) | 194 | 22.72 (3.33) | 336 | 21.88 (3.63) |
| 20 | 22 | 23 | 22 | |||||
| 55–65 | 38 | 18.45 (4.18) | 90 | 20.63 (3.86) | 153 | 22.61 (3.33) | 281 | 21.42 (3.90) |
| 19 | 21 | 23 | 22 | |||||
| 60–70 | 35 | 18.66 (3.58) | 74 | 19.66 (4.34) | 98 | 22.62 (3.23) | 207 | 20.89 (4.07) |
| 19 | 20 | 23 | 21 | |||||
| 65–75 | 23 | 17.39 (3.60) | 42 | 18.86 (4.05) | 42 | 21.76 (3.93) | 107 | 19.68 (4.27) |
| 18 | 20 | 22 | 20 | |||||
| Total by education | 133 | 19.50 (4.16) | 399 | 21.56 (3.97) | 586 | 23.43 (3.29) | 1118 | 22.30 (3.89) |
| 20 | 22 | 24 | 23 | |||||
Note: Use age range in which midpoint best corresponds to age of subject. The minimum age in this sample was 18 years.
Fig. 2.
Frequency of incorrect items in cognitive complaint sample (in percentage); n = 301.
Fig. 3.
Frequency of incorrect items in normative sample (in percentage); n = 1118.
Discussion
The accurate interpretation of cognitive assessment measures depends upon the normative comparison group being utilized, and if the normative sample does not resemble the individual being assessed, there is a risk of misclassification of impairment (Casaletto, et al., 2015). Given that the impact of demographic factors on neurocognitive test performance and the mandate to use appropriate normative standards are well established in the field of neuropsychology (Manly, 2001; Heaton, Lee, & Grant, 2009), there is a need to develop normative data specific to minority groups to establish guidelines for appropriate interpretation of cognitive screening results. This need was highlighted by an analysis of 1,440 articles published in 5 of the most frequently read neuropsychology journals from 1995 to 2000, which showed that demographic variables of age, education, gender, and education were reported 88% of the time, whereas variables such as race or ethnicity were provided only 11% of the time (O'Bryant, O'Jile, & McCaffrey, 2004).
This study is an extension of our prior normative work on the MoCA and provides age and education-stratified MoCA normative data based on the African American sample of the larger, population-based cohort. As anticipated, participants with higher education obtained higher MoCA scores, and scores decreased with age, particularly in the group with less than 12 years of education. In addition to providing interpretive guidelines for healthy aging individuals, these findings also have relevance for the detection of cognitive impairment. For example, prevalence rates for mild cognitive impairment (MCI) vary greatly, with estimates ranging from 3% to 70%, reflecting differences in cohort characteristics and the criteria used to define MCI (Ward, Arrighi, Michels, & Cedarbaum, 2012). A cutoff score of 26 on the MoCA has been proposed to maximize the sensitivity and specificity for identification of MCI based on a sample of 90 healthy Canadian controls (Nasreddine, et al., 2005). Even with the suggested 1-point education correction, the majority (76%) of participants in the present sample scored below the published cutoff for MCI (<26 points), indicating that both this cutoff and the 1-point correction are not appropriate thresholds for defining MCI in this group. This is similar to the pattern reported in the African American–Diabetes Heart MIND study, in which over 90% of participants would be deemed cognitively impaired using the standard 26-point cutoff (Sink, et al., 2015). Such concern was also raised in prior work in a clinical African American sample that found the 26 cutoff score demonstrated unacceptably low specificity (31%; 100% sensitivity) in detecting MCI (Goldstein, et al., 2014). Detection improved to 63% specificity (95% sensitivity) when 24 points was used as the cutoff. Thus, a large portion of African Americans may be inappropriately categorized through the use of “standard” cutoff scores derived from other groups, which has implications for clinical characterization and treatment studies. We suggest utilizing the normative data presented in Table 1 to guide interpretation of individual MoCA performance in similar populations.
There are study limitations, with the first being that participants in this population-based cohort were not formally screened for conditions (other than stroke) that could have an effect on cognition. As such, the sample may have included individuals with neurologic comorbidities or incipient MCI or dementia, although we attempted to address this issue by excluding individuals with subjective cognitive complaints. We have also examined other potential confounds in this cohort, including cerebrovascular risk factors and the presence of the apoE4 allele (Rossetti, et al., 2015; Srinivasa, et al., 2015) and found little to no impact on MoCA scores. Second, the MoCA is subject to the limitations of any brief cognitive screening tool; it is designed to assess global cognition and may fail to detect cognitive impairment. As with other brief cognitive screening tools, it is not a substitute for a thorough neuropsychological evaluation (Coen, Robertson, Kenny, & King-Kallimanis, 2015). Certain items are scored using a Yes/No (i.e., credit or no credit) format (e.g., verbal fluency), which limits the measure's ability to gauge levels of impairment, and some items may suffer from greater socioeducational and/or cultural bias than others. Third, we acknowledge that years of education may be an inadequate measure of educational experience, particularly among minorities, and adjusting for quality of education by using an estimate of reading level can improve the specificity of cognitive measures (Manly, Byrd, Touradji, & Stern, 2004). Unfortunately, a measure of reading ability was not available and as such these norms are subject to the limitations of all education-based normative data sets. Last, without the benefit of clinical evaluations in this study, we were unable to relate MoCA scores to clinical diagnosis.
This is the first study to report normative data for the MoCA specific to a population-based African American sample. Our results suggest the need for caution when applying previously published cut-scores on the MoCA derived from smaller and non-diverse populations. These findings also suggest that the MOCA may not be optimally designed for cognitive assessment among African Americans. The high failure rate on certain test items in this study, evidenced by failure rates >30% for 7 of the test items, strongly suggests that certain items within this instrument lack psychometric utility.
Although the MoCA is an increasingly popular screening tool in both clinical and research settings, our findings suggest caution when using this test to screen for MCI in African American individuals. Future research should include development of a clinically useful MoCA cutoff for MCI and dementia for specific sociodemographic groups, and continued efforts to develop culture-fair cognitive assessment tools are needed. Until such measures are available, we recommend selecting normative reference data for the existing MOCA that best reflects the characteristics of the individual being evaluated to minimize risk of misclassification or misdiagnosis.
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding
This work was supported in part by the Alzheimer's Association (NIRG-14-322666) by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number UL1TR001105, and UT Southwestern Alzheimer's Disease Center (NIH/NIA P30 AG12300-21).
Conflict of Interest
None declared.
References
- Bohnstedt M., Fox P. J., & Kohatsu N. D. (1994). Correlates of mini-mental status examination scores among elderly demented patients: The influence of race-ethnicity. Journal of Clinical Epidemiology, 47, 1381–1387. [DOI] [PubMed] [Google Scholar]
- Casaletto K. B., Umlauf A., Beaumont J., Gershon R., Slotkin J., Akshoomoff N., et al. (2015). Demographically corrected normative standards for the English version of the NIH toolbox cognition battery. Journal of the International Neuropsychological Society, 21, 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen R. F., Robertson D. A., Kenny R. A., & King-Kallimanis B. L. (2015). Strengths and limitations of the MoCA for assessing cognitive functioning: Findings from a large representative sample of Irish older adults. Journal of geriatric psychiatry and neurology, 29, 18–24. [DOI] [PubMed] [Google Scholar]
- Escobar J. I., Burnam A., Karno M., Forsythe A., Landsverk J., & Golding J. M. (1986). Use of the mini-mental state examination (MMSE) in a community population of mixed ethnicity. Cultural and linguistic artifacts. Journal of Nervous and Mental Disease, 174, 607–614. [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., & McHugh P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Goldstein F. C., Ashley A. V., Miller E., Alexeeva O., Zanders L., & King V. (2014). Validity of the montreal cognitive assessment as a screen for mild cognitive impairment and dementia in African Americans. Journal of Geriatric Psychiatry and Neurology, 27, 199–203. [DOI] [PubMed] [Google Scholar]
- Heaton R., Lee R., & Grant I. (2009). Demographic influences and use of demographically corrected norms in neuropsychologial assessment In A. K. Grant I. (Ed.) Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders (pp. 127–155). New York: Oxford University Press. [Google Scholar]
- Manly J. J. (2001). Future directions in neuropsychological assessment with African Americans In Ferraro F. (Ed.) Minority and cross-cultural aspects of neuropsychological assessment (pp. 79–96). Lisse, Netherlands: Swets and Zeitlinger. [Google Scholar]
- Manly J. J., Byrd D. A., Touradji P., & Stern Y. (2004). Acculturation, reading level, and neuropsychological test performance among African American elders. Applied neuropsychology, 11, 37–46. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bedirian V., Charbonneau S., Whitehead V., Collin I., et al. (2005). The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment In J Am Geriatr Soc, 53 (pp. 695–9). [DOI] [PubMed] [Google Scholar]
- O'Bryant S. E., O'Jile J. R., & McCaffrey R. J. (2004). Reporting of demographic variables in neuropsychological research: Trends in the current literature. Clinical Neuropsychologist, 18, 229–233. [DOI] [PubMed] [Google Scholar]
- Pauker J. D. (1988). Constructing overlapping cell tables to maximize the clinical usefulness of normative test data: Rationale and an example from neuropsychology. Journal of clinical psychology, 44, 930–933. [DOI] [PubMed] [Google Scholar]
- Pedraza O., Clark J. H., O'Bryant S. E., Smith G. E., Ivnik R. J., Graff-Radford N. R., et al. (2012). Diagnostic validity of age and education corrections for the mini-mental state examination in older African Americans. Journal of the American Geriatrics Society, 60, 328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti H. C., Lacritz L. H., Cullum C. M., & Weiner M. F. (2011). Normative data for the montreal cognitive assessment (MoCA) in a population-based sample. Neurology, 77, 1272–5. [DOI] [PubMed] [Google Scholar]
- Rossetti H. C., Weiner M., Hynan L. S., Cullum C. M., Khera A., & Lacritz L. H. (2015). Subclinical atherosclerosis and subsequent cognitive function. Atherosclerosis, 241, 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink K. M., Craft S., Smith S. C., Maldjian J. A., Bowden D. W., Xu J., et al. (2015). Montreal cognitive assessment and modified mini mental state examination in African Americans. Journal of Aging Research, 2015, 872018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasa R. N., Rossetti H. C., Gupta M. K., Rosenberg R. N., Weiner M. F., Peshock R. M., et al. (2015). Cardiovascular risk factors associated with smaller brain volumes in regions identified as early predictors of cognitive decline. Radiology. doi: 10.1148/radiol.2015142488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touradj P., Manly J. J., Jacobs D. M., & Stern Y. (2001). Neuropsychological test performance: A study of non-Hispanic White elderly. Journal of Clinical and Experimental Neuropsychology, 23, 643–649. [DOI] [PubMed] [Google Scholar]
- Victor R. G., Haley R. W., Willett D. L., Peshock R. M., Vaeth P. C., Leonard D., et al. (2004). The Dallas Heart Study: A population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. The American journal of cardiology, 93, 1473–1480. [DOI] [PubMed] [Google Scholar]
- Ward A., Arrighi H. M., Michels S., & Cedarbaum J. M. (2012). Mild cognitive impairment: Disparity of incidence and prevalence estimates. Alzheimer's & dementia: the journal of the Alzheimer's Association, 8, 14–21. [DOI] [PubMed] [Google Scholar]
- Welsh K. A., Fillenbaum G., Wilkinson W., Heyman A., Mohs R. C., Stern Y., et al. (1995). Neuropsychological test performance in African-American and white patients with Alzheimer's disease. Neurology, 45, 2207–2211. [DOI] [PubMed] [Google Scholar]