Abstract
Intestinal glucose stimulates secretion of the incretin hormone glucagon-like peptide 1 (GLP-1). The mechanisms underlying this pathway have not been fully investigated in humans. In this study, we showed that a 30-min intraduodenal glucose infusion activated half of all duodenal L cells in humans. This infusion was sufficient to increase plasma GLP-1 levels. With an ex vivo model using human gut tissue specimens, we showed a dose-responsive GLP-1 secretion in the ileum at ≥200 mmol/L glucose. In ex vivo tissue from the duodenum and ileum, but not the colon, 300 mmol/L glucose potently stimulated GLP-1 release. In the ileum, this response was independent of osmotic influences and required delivery of glucose via GLUT2 and mitochondrial metabolism. The requirement of voltage-gated Na+ and Ca2+ channel activation indicates that membrane depolarization occurs. KATP channels do not drive this, as tolbutamide did not trigger release. The sodium–glucose cotransporter 1 (SGLT1) substrate α-MG induced secretion, and the response was blocked by the SGLT1 inhibitor phlorizin or by replacement of extracellular Na+ with N-methyl-d-glucamine. This is the first report of the mechanisms underlying glucose-induced GLP-1 secretion from human small intestine. Our findings demonstrate a dominant role of SGLT1 in controlling glucose-stimulated GLP-1 release in human ileal L cells.
Introduction
The incretin hormone glucagon-like peptide 1 (GLP-1) is secreted postprandially by enteroendocrine L cells to enhance glucose-dependent insulin release from pancreatic β-cells. The two incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide together account for 50–70% of insulin secretion after oral glucose administration (1). Glucose-lowering effects of GLP-1 also occur through increasing insulin-independent glucose disposal (2). The use of GLP-1–based antidiabetic agents, namely dipeptidyl peptidase 4 inhibitors and GLP-1 receptor agonists, highlights the clinical relevance of GLP-1 in maintaining glucose homeostasis. Furthermore, there is increasing acceptance of the notion that the metabolic benefits of gastric bypass surgeries are partly attributable to the elevation of GLP-1 levels (3).
L cells predominantly reside in the epithelia of distal small intestine and colon; however, the early phase of postprandial GLP-1 secretion is likely mediated by smaller populations of L cells dispersed along the duodenum (4). Luminal nutrient exposure potently stimulates GLP-1 release, and the underlying mechanisms have been investigated in various experimental models (5). Seminal studies using primary murine intestinal mixed cell cultures (6–8), in vivo transgenic mouse models (9), and ex vivo rat models (10) have shown that glucose induces GLP-1 release through sodium–glucose cotransporter 1 (SGLT1) and, to a lesser extent, the glucose transporter GLUT2. Intracellular glucose metabolism and subsequent closure of KATP channels are also implicated. In addition, sweet taste receptor (STR) signaling is involved in glucose-induced GLP-1 release in animals (11) and possibly in humans (12).
However, similar mechanistic examinations in human L cells are lacking. In this study, we examine the mechanisms controlling glucose-induced GLP-1 release in humans. We showed marked L-cell activation upon intraduodenal glucose infusion in humans. We then used an ex vivo static secretion model in human gut intestinal mucosa to show that glucose potently triggered GLP-1 release in duodenal, ileal, but not colonic, mucosae at concentrations equivalent to luminal, but not postprandial, blood glucose levels. SGLT1 is predominantly responsible for this glucose-induced GLP-1 secretion from human ileal mucosae.
Research Design and Methods
The detailed protocols of this study are available online in the Supplementary Data. Briefly, healthy subjects (n = 8) were fasted overnight before the endoscopic studies commenced. Mucosal biopsy samples were collected using standard biopsy forceps before and 30 min after an intraduodenal glucose infusion and were immediately placed in 4% paraformaldehyde for 2 h for immunohistochemistry. Immunoreactivity was detected using a polyclonal GLP-1 primary antibody (C-17, 1:400; SC-7782; Santa Cruz Biotechnology) and a polyclonal phospho-Ca2+-calmodulin–dependent protein kinase II primary antibody (pCaMKII) (1:400; AB32678; Abcam) by sequential labeling.
For ex vivo secretion experiments, morphologically normal ileal and colonic tissue specimens were collected with consent from patients undergoing bowel resections for cancer or stoma reversal (Supplementary Table 1). The mucosae were isolated and transferred to 96-well plates for static incubations of 15 min in different stimulants (37°C; 95% O2/5% CO2). GLP-1 (active) content in the supernatants was assayed with a commercial ELISA kit according to manufacturer instructions (EGLP-35K; Merck Millipore). GLP-1 secretion was normalized to basal secretion measured in parallel from the same sample. All statistical analysis was conducted as paired analyses, comparing responses in tissues obtained from the same individual to relevant control conditions. A paired ratio Student t test was used for single comparisons and a paired one-way ANOVA with Fisher least significant differences post hoc test used for multiple comparisons. Statistical significance was P < 0.05. All data are shown as the mean ± SEM.
Results
Intraduodenal Glucose Infusion Activated Duodenal L Cells
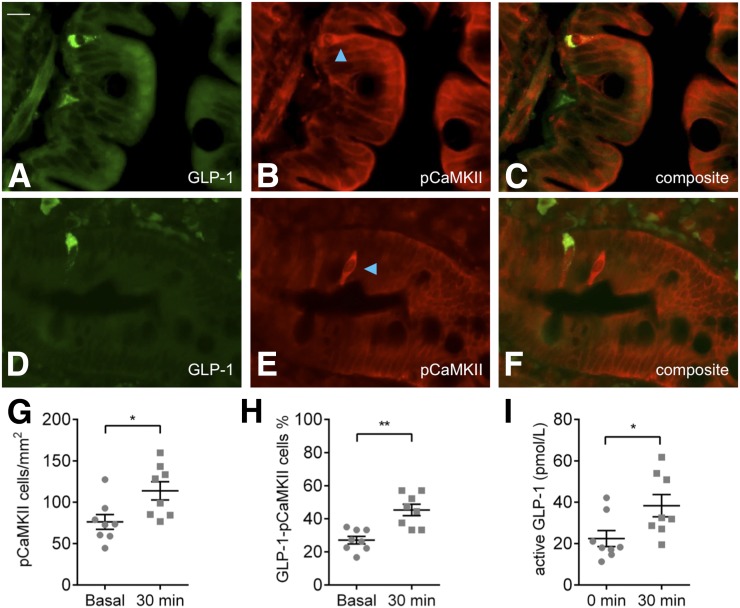
Subjects tolerated the study well, and their characteristics are listed in Table 1. Immunolabeling for GLP-1 was reliably detected in dispersed duodenal epithelial cells (N = 8) (Fig. 1A and D). Immunopositive cells were most abundant in villi and showed cytoplasmic labeling with basal predominance. Immunolabeling for the activation marker pCaMKII was present throughout the cytoplasm of single dispersed cells in the duodenal villi and crypts after intraduodenal glucose infusion (Fig. 1B and E) and under basal conditions (Supplementary Fig. 1). The density of duodenal mucosal cells expressing pCaMKII increased 1.5-fold after 30 min intraduodenal glucose infusion (P < 0.05) (Fig. 1G). The proportion of duodenal L cells coexpressing pCaMKII increased 1.7-fold after intraduodenal glucose (27 ± 2% vs. 45 ± 3%, P < 0.001) (Fig. 1H). Thus, almost half of all L cells are activated by glucose exposure. This glucose infusion in these individuals was sufficient to cause a significant (P < 0.05) increase in plasma active GLP-1 levels (Fig. 1I).
Table 1.
Characteristics and blood glucose responses of subjects for intraduodenal glucose infusion study
| N | 8 |
| Age (years) | 41 ± 6 |
| Sex (n) | 7 M/1 F |
| BMI (kg/m2) | 28 ± 2 |
| HbA1c (%) | 5.7 ± 0.1 |
| Fasting BGL (mmol/L) | 5.9 ± 0.2 |
| T = 30 BGL (mmol/L) | 9.0 ± 0.7 |
| BGL AUC30 (mmol/L/min) | 85 ± 6 |
Data are shown as the mean ± SEM unless otherwise stated. AUC30, area under the curve for blood glucose 30 min post-infusion; BGL, blood glucose level; F, female; M, male; T, time.
Figure 1.
Functional activation of duodenal cells after intraduodenal glucose infusion in healthy subjects. A and D: GLP-1–immunopositive cells in the duodenal mucosa. B and E: pCaMKII-immunopositive cells in the duodenum after 30 min of intraduodenal glucose infusion, highlighted by blue arrows. Composite images of a pCaMKII-immunopositive L cell (C) and separate pCaMKII and L cells (F). G: An increase in density of duodenal pCaMKII-immunopositive cells after glucose infusion in healthy subjects; *P < 0.05. H: Increased proportion of duodenal L cells coexpressing pCaMKII in healthy subjects after glucose infusion; **P < 0.01. I: Increased plasma GLP-1 after a duodenal glucose infusion of 30 min in these individuals; *P < 0.05. Scale bar (in A for all images) = 20 µm. Data are the mean ± SEM.
Glucose Triggers GLP-1 Release in the Duodenum and Ileum
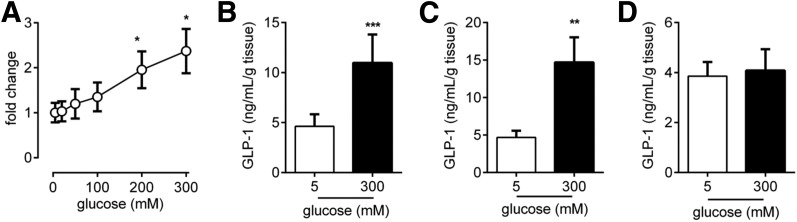
To test the glucose range that L cells respond to, we tested a range of glucose concentrations in ileal epithelial tissue (n = 5) (Fig. 2A). Release was triggered only once as concentrations reached 200 mmol/L, and a concentration of 300 mmol/L gave the highest GLP-1 secretion of the glucose concentrations tested. From all the samples in which we tested 300 mmol/L glucose, we observed a significant increase in GLP-1 secretion in the ileum (Fig. 2B) (n = 19) and duodenum (Fig. 2C) (n = 6). In contrast, a glucose concentration of 300 mmol/L did not trigger GLP-1 release from colonic epithelial tissue (n = 24) (Fig. 2D). These data from the ileum and colon were pooled both from individuals who do not have diabetes and from individuals who have type 2 diabetes because we observe no difference in basal, stimulated release or the fold change in release between the groups (Supplementary Fig. 2). A 300 mmol/L concentration of d-mannitol did not increase GLP-1 release (n = 8) (Supplementary Fig. 3), indicating that osmotic stress did not drive the observed glucose response. All ex vivo mechanistic analysis was conducted in the ileum as this was the glucose-responsive section of the gastrointestinal tract most available to us in this study, and the size of biopsy samples, such as those from the duodenum, severely limits the number of ex vivo secretion experiments possible in a single-patient sample.
Figure 2.
GLP-1 secretion upon glucose stimulation in human gut mucosae. A: Concentration–response curve for GLP-1 secretion in response to increasing glucose levels in human ileum tissue (n = 5). A glucose concentration of 300 mmol/L potently triggered GLP-1 secretion from L cells in human ileal (n = 19) (B), duodenal (n = 6) (C), and colonic (D) mucosae (n = 24). Bar graph data are the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared with respective control groups.
Mechanism Regulating Glucose-Induced GLP-1 Secretion
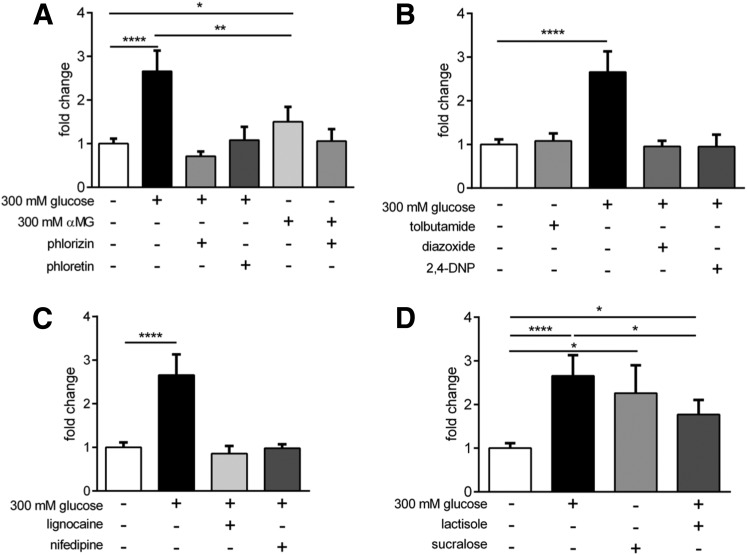
The SGLT1 inhibitor phlorizin (1 mmol/L, n = 9), and the GLUT2 inhibitor phloretin (1 mmol/L, n = 9) both blocked glucose-induced GLP-1 secretion (Fig. 3A). The nonmetabolizable SGLT1 substrate α-MG (300 mmol/L) induced GLP-1 secretion (n = 8, P < 0.05) but was less potent than glucose (P < 0.01). The blockade of SGLT1 with phlorizin (1 mmol/L) attenuated α-MG–induced GLP-1 release (n = 8) (Fig. 3A). Replacement of external Na+ with N-methyl-d-glucamine blocked the response to 300 mmol/L glucose in ileal tissue (n = 5) (Supplementary Fig. 4), further demonstrating the need for Na+ transport via SGLT1 for glucose-induced GLP-1 release. Glucose failed to induce GLP-1 secretion in the presence of the KATP channel opener diazoxide (500 μmol/L, n = 9) or the ATP synthesis inhibitor 2,4-DNP (100 μmol/L, n = 8). The KATP channel antagonist tolbutamide (500 μmol/L, n = 9) did not stimulate GLP-1 release (Fig. 3B). The voltage-gated Na+ channel blocker lignocaine (100 μmol/L, n = 9) and the L-type Ca2+ channel blockade by nifedipine (10 μmol/L) both returned GLP-1 secretion to basal level in the presence of 300 mmol/L glucose (n = 9) (Fig. 3C). The noncaloric sweetener sucralose stimulated GLP-1 release in the absence of high glucose levels (n = 9, P < 0.05), indicating a role for STRs in GLP-1 secretion. Glucose-stimulated GLP-1 release remained in the presence of the STR antagonist lactisole (n = 9, P < 0.05) (Fig. 3D), indicating that glucose-induced GLP-1 secretion is independent of this pathway.
Figure 3.
Mechanisms controlling glucose-induced GLP secretion in human ileal L cells. A: SGLT1 and GLUT2 blockade by phlorizin and phloretin, respectively, abolished the stimulatory effect of high glucose levels on GLP-1 secretion. The nonmetabolizable SGLT1 substrate α-MG caused significant GLP-1 secretion but was less potent than equimolar glucose, and its stimulatory effect was reversed by phlorizin. B: The KATP channel opener and ATP synthesis inhibitor diazoxide and 2,4-DNP, respectively, completely abolished the effect of high glucose levels on GLP-1 secretion, but the KATP channel closer tolbutamide did not cause significant GLP-1 secretion from basal levels. C: Blockade of voltage-gated Na+ and Ca2+ channels by lignocaine and nifedipine, respectively, significantly inhibited the stimulatory effect of high glucose. D: STR activation by the noncaloric artificial sweetener sucralose caused significant GLP-1 secretion from basal levels, but the STR blocker lactisole did not attenuate the stimulatory effect of high glucose levels. Bar graph data are the mean ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001 compared with respective control groups (n = 7–9).
Discussion
This study is the first to demonstrate that approximately half of all duodenal L cells in humans is activated acutely by intraduodenal glucose infusion. We further established that levels of glucose equivalent to those seen postprandially in plasma did not stimulate small intestinal GLP-1 release in our human ex vivo model. Rather, glucose concentrations equivalent to dietary intraluminal glucose were capable of triggering ex vivo GLP-1 secretion from the human ileum but not the colon. Finally, we identified the mechanisms by which this glucose response occurs in human ileal L cells and that SGLT1 is central to this pathway.
Although it has previously been reported that GLP-1 is secreted in response to duodenal glucose infusions at even lower doses than tested in this study (13), our in vivo investigation provides the first quantification of the proportion of L cells that are activated by glucose infusion. Our ex vivo preparations also demonstrate duodenal responsiveness to high glucose levels that is similar to that observed in ileal tissue. This finding is consistent with a role of the duodenal L cell in the increased plasma GLP-1 levels we observed upon duodenal glucose infusion in our study. It needs to be noted that this assay using biopsy tissue from subjects administered glucose is not necessarily a means by which to detect the direct effects of glucose at L cells, as it could be that pCaMKII is regulated by factors delivered from adjacent non–L cells or by extraintestinal factors.
Our ex vivo model demonstrates that the exposure of human ileal mucosa to glucose triggers GLP-1 secretion, independent of secondary influences such as neural inputs or gut contraction. We were able to pool our data in the ileum and colon both from individuals without diabetes and with type 2 diabetes as secretion was similar in both groups. Furthermore, glucose-stimulated GLP-1 release was not secondary to osmotic stress as equimolar amounts of mannitol failed to induce secretion. Our data are similar to responses in perfused rat small intestine, where luminal, but not vascular, infusions of high glucose triggered substantial GLP-1 secretion (10). We also showed that L cells in the human duodenum, but not the colon, are glucose sensitive in our assay. The result in colon contrasts the in vitro glucose-induced GLP-1 release observed at very low glucose ranges in fluorescently tagged L cells from the mouse colon (6,8). Whether these differences are species, experiment, or preparation dependent remains unknown. These data support clinical findings that resection of the L cell–rich distal colon did not affect glucose-induced GLP-1 release and that rectally administered glucose did not trigger GLP-1 release (14). One limitation of our study was that we were unable to acquire total GLP-1 content from our samples to observe whether this was altered in these two groups.
We defined pivotal roles of electrogenic and facilitative glucose transport via SGLT1 and GLUT2, respectively, in GLP-1 release from the human ileum. The significant GLP-1 release triggered by equimolar amounts of the nonmetabolizable SGLT1 substrate α-MG reversal of α-MG induced GLP-1 release by phlorizin and blockade of the glucose response by substituting external Na+ with N-methyl-d-glucamine all support a central role of SGLT1 in driving this glucose response. Thus, our results confirm the critical role of electrogenic sodium-dependent glucose uptake by SGLT1 in causing membrane depolarization and the subsequent GLP-1 release in human L cells, which is similar to that shown in various rodent models (7–10,15). Blockade of GLUT2 by phloretin was also sufficient to block glucose-stimulated GLP-1 secretion. This may be a species-specific pathway as it is also observed in ex vivo rat small intestine perfusion models (10,16) but not in mouse in vivo and in vitro studies using Glut2 knockout mice (9) and primary murine small intestine cell cultures (8). Such differences further highlight the need for caution when translating findings in animal models to humans. GLUT2 may be important in human L cells by facilitating glycolytic and/or mitochondrial metabolism so that metabolism-dependent but KATP channel-independent glucose-induced GLP-1 release can occur. In addition to this, GLUT2 function is also implicated in mediating KATP channel–independent GLP-1 secretion by other secretagogues such as lipids and bile acids (16).
It has been proposed that glucose induces GLP-1 release through glucose internalization, the production of ATP via oxidative phosphorylation to close KATP channels, and subsequent membrane depolarization (5). We demonstrate that the KATP channel opener diazoxide potently reduced glucose-induced GLP-1 secretion in humans, which is consistent with in vitro (6,17) and ex vivo (10) rodent data. Inhibiting intracellular ATP synthesis with the proton ionophore 2,4-DNP abolished the stimulatory effect of glucose on GLP-1 secretion in the human small intestine, which is consistent with results in rat small intestine (10). In contrast to that same study and other in vitro experiments (6), we did not observe increased GLP-1 secretion with tolbutamide, which is consistent with the finding that sulfonylureas are ineffective in triggering GLP-1 secretion in human in vivo (18,19). Diazoxide increases K+ permeability and subsequently clamps membrane potential below the K+ equilibrium potential. This hyperpolarization must override any membrane depolarization induced by the inward Na+ current associated with SGLT1 activity and block what is normally a KATP channel–independent, SGLT1-dependent, glucose-induced GLP-1 secretion.
A role for Na+-dependent action potentials and voltage-gated L-type Ca2+ currents in mediating basal and stimulated GLP-1 release has been reported in an in vitro study of mouse L cells (17). Our study supports the role of both of these channels in glucose-induced GLP-1 release from the human ileum. Blockade of voltage-gated Na+ channels after intravenous lignocaine administration failed to attenuate glucose-induced GLP-1 secretion in the perfused rat small intestine model (10). Such differing results may highlight a potential shortcoming of the approach used in our study, as cell polarization is lost upon dissection of the epithelial tissue. A major limitation of our study was the inability to differentiate between pathways derived from apical and basolateral membranes. This could potentially also explain the lack of glucose response in human colonic tissue previously observed in mice (6). Experiments using human vascularly perfused tissue or using Ussing chambers could mitigate these shortcomings but were not possible in our present study.
Intestinal STRs are potential regulators of gut hormone secretion. GLP-1 colocalizes with the STRs T1R2 and T1R3, and its signal transduction protein α-gustducin in human small intestine and the STR antagonist lactisole dose-dependently inhibit glucose-stimulated GLP-1 release in vivo in humans (12,20). GLP-1 secretion from primary murine small intestine mixed-cell cultures increased upon stimulation by the artificial sweetener sucralose (6). In contrast to results in a clinical study (21) and in a rat ex vivo perfusion model (10), we observed significant sucralose-induced GLP-1 secretion from human ileal mucosae. However, blockade of the STR by lactisole did not significantly reduce glucose-stimulated GLP-1 secretion. This could be due to the fact that sucralose is a more potent activator of the STRs at this dose or that a small portion, if any, of the glucose-stimulated GLP-1 secretion is mediated by this pathway.
It should be noted that L cells within the ileum are not likely to be exposed to significant, if any, levels of ingested glucose under normal physiological conditions. Likely it would be the duodenal and the more prevalent jejunal L cells involved in this nutrient response. Because of the tissue from those sites not being readily available to us in large quantities for this study, we can only assume at this stage that the mechanisms underlying glucose-induced GLP-1 secretion are the same across these different areas of the gastrointestinal tract. The ability to respond to ingested glucose by ileal L cells may therefore represent a backup system used under certain pathophysiological conditions, rather than being a primary physiological response system.
Our study highlights the importance of species differences in studying L cell physiology. Although several pathways that have been shown to govern GLP-1 secretion in rodents were implicated in our study, some were not. Our data demonstrate that glucose-induced GLP-1 secretion in human small intestine is mediated by the electrogenic activity of SGLT1. It additionally involves a component reliant on intracellular glucose metabolism and is dependent on voltage-gated Na+ and Ca2+ channels. Although STRs can also regulate GLP-1 release in the human ileum, these receptors do not appear to be involved in glucose-induced GLP-1 release.
Supplementary Material
Article Information
Acknowledgments. The authors thank the voluntary participants who made this work possible. The authors also thank the staff of the Gastrointestinal Investigation Unit, Royal Adelaide Hospital and Discipline of Surgery, Flinders Medical Centre, for their assistance with the study.
Funding. This work was supported by the Australian Research Council (LP150100419) and the National Health and Medical Research Council (APP1088737).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.W.S. designed and performed the ex vivo experiments, undertook the statistical analyses, and wrote the manuscript. D.d.F., P.R., P.H., L.S., S.L.D., and D.A.W. performed surgeries and provided tissue for the ex vivo experiments. C.K.R. and A.M.D. recruited patients for the clinical study and performed clinical experiments at the Royal Adelaide Hospital. R.L.Y. designed the clinical study, recruited patients for the clinical study, and performed immunohistochemistry analysis. D.J.K. designed the ex vivo experiments, undertook the statistical analyses, and wrote the manuscript. All authors critically reviewed the manuscript and have approved the final version. D.J.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0058/-/DC1.
See accompanying article, p. 2063.
References
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 2.D’Alessio DA, Kahn SE, Leusner CR, Ensinck JW. Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest 1994;93:2263–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holst JJ, Madsbad S. Mechanisms of surgical control of type 2 diabetes: GLP-1 is key factor. Surg Obes Relat Dis 2016;12:1236–1242 [DOI] [PubMed] [Google Scholar]
- 4.Theodorakis MJ, Carlson O, Michopoulos S, et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 2006;290:E550–E559 [DOI] [PubMed] [Google Scholar]
- 5.Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol 2009;587:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab 2008;8:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorboulev V, Schürmann A, Vallon V, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012;61:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker HE, Adriaenssens A, Rogers G, et al. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 2012;55:2445–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Röder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One 2014;9:e89977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhre RE, Frost CR, Svendsen B, Holst JJ. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes 2015;64:370–382 [DOI] [PubMed] [Google Scholar]
- 11.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A 2007;104:15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerspach AC, Steinert RE, Schönenberger L, Graber-Maier A, Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab 2011;301:E317–E325 [DOI] [PubMed] [Google Scholar]
- 13.Schirra J, Katschinski M, Weidmann C, et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 1996;97:92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Printz H, Reiter S, Samadi N, et al. GLP-1 release in man after lower large bowel resection or intrarectal glucose administration. Digestion 1998;59:689–695 [DOI] [PubMed] [Google Scholar]
- 15.Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab 2009;297:E1358–E1365 [DOI] [PubMed] [Google Scholar]
- 16.Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol 2012;590:2917–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers GJ, Tolhurst G, Ramzan A, et al. Electrical activity-triggered glucagon-like peptide-1 secretion from primary murine L-cells. J Physiol 2011;589:1081–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson ER, Flechtner I, Njølstad PR, et al.; Neonatal Diabetes International Collaborative Group . Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 2006;355:467–477 [DOI] [PubMed] [Google Scholar]
- 19.El-Ouaghlidi A, Rehring E, Holst JJ, et al. The dipeptidyl peptidase 4 inhibitor vildagliptin does not accentuate glibenclamide-induced hypoglycemia but reduces glucose-induced glucagon-like peptide 1 and gastric inhibitory polypeptide secretion. J Clin Endocrinol Metab 2007;92:4165–4171 [DOI] [PubMed] [Google Scholar]
- 20.Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Clin Nutr 2011;30:524–532 [DOI] [PubMed] [Google Scholar]
- 21.Steinert RE, Frey F, Töpfer A, Drewe J, Beglinger C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br J Nutr 2011;105:1320–1328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.