Abtract
Reports on the associations between multiple clinical and behavioral health indicators and major health outcomes among older adults are scarce. We prospectively examined concordance with guidelines from the American Cancer Society and American Heart Association for disease prevention in relation to cancer, cardiovascular disease (CVD), and mortality among Cardiovascular Health Study enrollees aged 65–98 years who, at baseline assessment in 1989–1996 (n = 3,491), were free of CVD and cancer. Total and cause-specific mortality, as well as incidence of cancer and CVD, were lower with higher guideline concordance. Independent of body mass index, blood pressure, total cholesterol, and fasting plasma glucose, better health behaviors (diet, physical activity, and alcohol consumption) were associated with lower mortality (2-sided P < 0.0001). Among individuals with ideal levels for 3–4 of these 4 cardiometabolic biomarkers, those with poor concordance with health behavior recommendations had higher mortality compared with those who had the highest concordance with these behavioral recommendations (adjusted mortality hazard ratio = 1.82, 95% confidence interval: 1.25, 2.67). Older adults who are concordant with recommendations for cancer and CVD prevention have reduced rates of chronic disease and mortality. Interventions to achieve and maintain healthy lifestyle behaviors may offer benefits both in the presence and absence of adverse traditional clinical risk factors.
Keywords: cancer, cardiovascular disease, epidemiology, health promotion, prevention
Studies from global populations suggest that clinical guidelines put forth to reduce cancer (1–4) and cardiovascular disease (CVD) (5–11) have broad effects on risk of chronic diseases. Both the American Cancer Society (ACS) and the American Heart Association (AHA) have issued guidelines that extend beyond the traditional approach of focusing on adverse levels of risk factors. Instead, these guidelines reflect a recent trend towards promoting and measuring predictors of favorable health status in populations (1–3, 5, 7–10).
As global populations age, clear guidance is needed to quantify for older adults the magnitude of benefit associated with behavior change compared with other clinical goals, such as maintaining favorable levels of cardiometabolic risk factors. Cancer and CVD account for over half of all deaths among individuals aged 65 years or older (12). However, while the potential benefits associated with achieving recommendations put forth in cancer and CVD prevention guidelines have been examined in multiple cohorts with considerable numbers of participants aged ≥65 years (2, 3, 5), only one study had a participant mean age of >65 years (5), and none examined the relationship solely among the elderly subgroup.
The lack of evidence on guideline concordance and major health events is important because age-related physiological changes and an increased burden of comorbid conditions (e.g., hypertension, type 2 diabetes mellitus, and vascular diseases) among older individuals complicate the extrapolation of guidelines to older populations. For example, quitting smoking late in life confers similar benefits among those below or above age 65 years (13), but there is limited evidence on the effects of other modifiable risk factors, such as diet and physical activity, on cancer and CVD outcomes among older adults (14–18). Furthermore, among older adults, associations between traditional cardiovascular disease risk factors and major health outcomes are not consistent with those observed for the population at large (19–22). The present study addressed the hypothesis that behavioral factors might predict health outcomes at least as well as standard clinical measurements such as serum lipids and blood pressure (BP), which have often been found to be relatively weak predictors of major health events in older persons. In addition, we examined the impact of modifiable health behaviors in the context of varying levels of such traditional risk factors in the older adult population.
METHODS
Participants
The Cardiovascular Health Study (CHS) is a prospective study of adults aged 65 years or older living in Sacramento, California; Forsyth County, North Carolina; Washington County, Maryland; and Allegheny County, Pennsylvania (23, 24). Participants (n = 5,201) were enrolled between 1989 and 1990, and an additional predominantly African-American cohort of 687 participants was enrolled in 1992–1993, through random sampling of Medicare eligibility lists. Among eligible adults contacted, 57% were enrolled. We excluded individuals who had a baseline history of CVD (25) or cancer (n = 2,143) and those missing data on diet (n = 196) or other covariates (n = 137). All centers obtained informed consent and approval from institutional review boards.
Data collection
Standardized protocols were used to conduct in-person interviews and collect clinical measurements, including body mass index (BMI, calculated as weight (kg) divided by height (m)2), seated BP, fasting serum total cholesterol (TC), and fasting plasma glucose (FPG), as well as an in-home medication inventory (23, 26, 27). Staff conducted semiannual ascertainment interviews with participants or proxy respondents to identify major health events and deaths. Hospitalizations and deaths were also identified from administrative data (National Death Index, Medicare) and adjudicated by a committee of physicians (28). A picture-sort version of the 99-item food frequency questionnaire from the National Cancer Institute assessed usual intake of relevant nutrients and food groups among the original cohort in 1989–1990 (29, 30), and intakes for the African-American cohort were assessed using the Willett food frequency questionnaire at the third annual visit in 1995–1996 (31). Physical activity, in metabolic equivalent of task (MET)-hours, was estimated using the Minnesota Leisure-Time Physical Activity Questionnaire (32).
Definitions of ACS and AHA guideline concordance
Concordance with each component of the ACS Guidelines for Nutrition and Physical Activity for Cancer Prevention score and the AHA cardiovascular health metrics (Web Table 1, available at https://academic.oup.com/aje) was rated as 0 (poor), 1 (intermediate), or 2 (ideal). Using the AHA (11) and ACS (4) guidelines, the ideal level of ≥150 minutes/week of moderate-intensity physical activity (3.5 METs) translates to 8.75 MET-hours/week. Engaging in some physical activity less than 8.75 MET-hours/week was coded as intermediate. The ACS advises limiting red and processed meats, eating fruits and vegetables each day, and consuming whole rather than refined grains. To operationalize these recommendations, we created a diet subscore ranging from 0–9. Three points were allotted for fruit and vegetable consumption, including 1 point for consuming ≥2.5 cups of fruits and vegetables/day, excluding juice and potatoes. A variety score was created by summing the number of unique fruits and vegetables consumed at least once per month; those in the highest tertile and middle tertile of variety were given 2 points and 1 point, respectively. For red and processed meats, quartiles of servings/day were assigned values from 0 (highest quartile) to 3 (lowest quartile). Quartiles of whole-grain consumption, as a fraction of total grains, were similarly coded as 0–3. Last, individual diet components were summed, and participants who scored 0–2 were considered to have a poor diet; 3–6 was considered intermediate; and 7–9 was considered ideal. AHA dietary guidelines recommend limiting consumption of sodium (“<1,500 mg per day”) and sugar-sweetened beverages (“≤450 kcal (36 oz) per week”), while maintaining a diet rich in fruits and vegetables (“≥4.5 cups per day”), fish (“≥two 3.5-oz servings per week”), and whole grains (“≥three 1-oz-equivalent servings per day”) (11, p. 596). Concordance with ≥4 of 5 criteria was considered ideal cardiovascular health, with 2–3 considered intermediate.
ACS guidelines advise to “maintain a healthy weight throughout life.” (4, p. 32) We used weight at age 50 years (self-reported in the CHS questionnaire at baseline) along with baseline height and weight to calculate BMI at both time points, assuming constant height. The AHA metrics include scores for ideal BMI, BP, TC, and FPG, defined using standard clinical cutpoints. Last, never smoking or quitting >1 year ago is considered ideal by the AHA, and quitting within the past year is considered intermediate (5). Total guideline concordance scores were calculated by summing components (2, 3, 11). For AHA, possible scores ranged from 0 (unfavorable levels of all components) to 14 (ideal levels of all components). With the addition of smoking to the 4 other targets in the ACS guidelines, scores for ACS ranged from 0 to 10.
Outcomes assessment
For potential incident cardiovascular events (28), abstractors from each site reviewed hospital records, and interviews were conducted with participants/proxies to capture symptoms and circumstances preceding incident and fatal CVD events. Events were then adjudicated by the CHS Events Subcommittee. For this analysis, CVD events included incident myocardial infarction, congestive heart failure, and stroke. CHS participants with a primary incident cancer diagnosis were identified through record linkage with 5 population-based cancer registries serving the 4 CHS regions (33). Ascertainment of cause of death was based on the underlying rather than immediate cause and was assigned by a committee of physicians who were without knowledge of prior examination findings (28, 33, 34). Cardiovascular deaths included atherosclerotic coronary disease, cerebrovascular disease (stroke), other atherosclerotic disease (such as aortic aneurysm), and other vascular disease (such as valvular heart disease or pulmonary embolism).
Statistical analysis
Pearson χ2 tests and analysis of variance were used in descriptive analyses to compare levels of covariates across low extreme, moderate (approximately 15th to 85th percentiles), and high extreme levels of guideline concordance. We used Cox proportional hazards models to estimate hazard ratios for incident cancer, incident CVD, and mortality associated with guideline concordance. Scores were collapsed to maintain >5% of the total sample in the extreme high and low categories. The group meeting the fewest guideline recommendations served as the reference. Time at risk was calculated as the time elapsed between the date of baseline diet assessment (1989–1990 for the original cohort and 1995–1996 for the African-American cohort) and the date of the incident event (CVD or cancer), death, loss to follow-up (<13% of participants), or latest event adjudication date (2005 or 2006 for incident cancer and December 2011 for all other outcomes). To assess the linear relationship between guideline concordance and outcomes, concordance scores were entered into Cox models as continuous terms. The proportional hazards assumption was satisfied for each independent variable and outcome variable combination through visual inspection of survival curves and through residual methods (35).
Further analyses examined whether concordance with the health behavior targets established by the ACS guidelines was associated with outcomes independent of the level of concordance with AHA cardiometabolic risk-factor targets, including BMI, BP, TC, and FPG, measurements routinely used in clinical settings. The outcomes of total mortality and incident CVD were judged to have adequate numbers of events to support cross-classification of subjects by ACS and AHA scores simultaneously. First, we created a modified ACS health behavior score having a range between 0 and 6, which reflected level of concordance with physical activity, diet, and alcohol-consumption targets. We then created a modified AHA score giving 1 point for each ideal cardiometabolic risk-factor metric (not on drug treatment and measured levels of FPG <100 mg/dL, BMI <25, BP <120/80, or TC <200 mg/dL). This produced a variable with a range between 0 and 4. Hazard ratios for study outcomes associated with the modified ACS health behavior score were estimated at each level of the modified AHA cardiometabolic risk factor score. Terms for interaction between ACS behavior score and AHA risk-factor score examined whether the association between concordance with ACS health behavior targets and outcomes differed across groups defined by AHA cardiometabolic risk-factor targets.
All models adjusted for variables determined a priori as potential confounders, including age, self-rated health, race/ethnicity, income, education, sex, and marital status. Models of CVD incidence, cardiovascular mortality, and all-cause mortality outcomes additionally adjusted for use of nonsteroidal antiinflammatory drugs and limitations in instrumental activities of daily living. We adjusted for detailed smoking history in analyses of joint associations between health behaviors and traditional risk factors, as smoking was not included as part of the exposure.
Sensitivity analyses examined 2 alternative approaches to defining levels of physical activity, to address the impact of mismeasurement on our conclusions. First, we excluded household chores from the estimation of physical activity, which reduced the overall proportion meeting guideline targets from over two-thirds to 52%. We also redefined adherence to physical activity recommendations according to quantile values; specifically, we defined those in the highest tertile of physical activity to be guideline concordant. These analyses are not shown, because the alternative approaches did not change the overall conclusions of the analyses.
All P values were 2-sided, and a level of significance of 5% was used. Analyses were conducted using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Participant characteristics
A total of 2,145 women and 1,346 men were a mean of 72 years of age at baseline (range, 64–98 years) (5), and most were non-Hispanic white (Table 1). Few participants jointly met ideal levels of all components of either the ACS (n = 81; 2%) or AHA (n = 4; <1%) guidelines. Mean total scores for ACS guidelines were 6.9 (standard deviation, 1.5; range, 0–10) and for AHA guidelines were 8.6 (standard deviation, 2.0; range, 1–14). Individuals with high concordance scores with the ACS and AHA guidelines were more likely to be non-Hispanic white and highly educated (Table 2). They were also more likely to have higher self-rated health, lower BMI, and lower frequency of diabetes, limitations in physical functioning, and use of medications. There were no significant age differences by degree of guideline concordance.
Table 1.
Characteristics of Participants at Baseline (n = 3,491), Cardiovascular Health Study, 1989–1990 and 1992–1993a
| Characteristic and Prevention Guideline Concordance | Women (n = 2,145) | Men (n = 1,346) | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | No. of Participants | % | Mean (SD) | No. of Participants | % | |
| Age, years | 71.9 (5.2) | 72.8 (5.6) | ||||
| Years of education | 13.8 (4.4) | 14.1 (5.0) | ||||
| Non-Hispanic white | 1,837 | 86 | 1,190 | 88 | ||
| Black | 273 | 13 | 136 | 10 | ||
| Other | 35 | 2 | 20 | 1 | ||
| ACS total score | 7.1 (1.5) | 6.7 (1.5) | ||||
| 0 | 1 | 0 | 0 | 0 | ||
| 1 | 1 | 0 | 1 | 0 | ||
| 2 | 3 | 0 | 2 | 0 | ||
| 3 | 24 | 1 | 24 | 2 | ||
| 4 | 91 | 4 | 75 | 6 | ||
| 5 | 196 | 9 | 184 | 14 | ||
| 6 | 406 | 19 | 285 | 21 | ||
| 7 | 513 | 24 | 346 | 26 | ||
| 8 | 518 | 24 | 302 | 22 | ||
| 9 | 328 | 15 | 110 | 8 | ||
| 10 | 64 | 3 | 17 | 1 | ||
| ACS individual metric, score | ||||||
| Smoking | ||||||
| Poor (0) | 280 | 13 | 160 | 12 | ||
| Intermediate (1) | 30 | 1 | 20 | 1 | ||
| Ideal (2) | 1,835 | 86 | 1,166 | 87 | ||
| Body weight | ||||||
| Poor (0) | 443 | 21 | 240 | 18 | ||
| Intermediate (1) | 919 | 43 | 749 | 56 | ||
| Ideal (2) | 783 | 37 | 357 | 27 | ||
| Drinking | ||||||
| Poor (0) | 214 | 10 | 172 | 13 | ||
| Intermediate (1) | 768 | 36 | 632 | 47 | ||
| Ideal (2) | 1,163 | 54 | 542 | 40 | ||
| Physical activity | ||||||
| Poor (0) | 181 | 8 | 77 | 6 | ||
| Intermediate (1) | 526 | 25 | 319 | 24 | ||
| Ideal (2) | 1,438 | 67 | 950 | 71 | ||
| Diet | ||||||
| Poor (0) | 232 | 11 | 283 | 21 | ||
| Intermediate (1) | 1,321 | 62 | 871 | 65 | ||
| Ideal (2) | 592 | 28 | 192 | 14 | ||
| AHA total score | 8.5 (2.1) | 8.7 (1.9) | ||||
| 0–1 | 1 | 0 | 0 | 0 | ||
| 2 | 3 | 0 | 0 | 0 | ||
| 3 | 11 | 1 | 5 | 0 | ||
| 4 | 45 | 2 | 13 | 1 | ||
| 5 | 92 | 4 | 35 | 3 | ||
| 6 | 188 | 9 | 91 | 7 | ||
| 7 | 310 | 14 | 205 | 15 | ||
| 8 | 389 | 18 | 268 | 20 | ||
| 9 | 395 | 18 | 252 | 19 | ||
| 10 | 333 | 16 | 217 | 16 | ||
| 11 | 224 | 10 | 170 | 13 | ||
| 12 | 111 | 5 | 70 | 5 | ||
| 13 | 39 | 2 | 20 | 1 | ||
| 14 | 4 | 0 | 0 | 0 | ||
| AHA individual metric, score | ||||||
| Smoking | ||||||
| Poor (0) | 280 | 13 | 160 | 12 | ||
| Intermediate (1) | 30 | 1 | 20 | 1 | ||
| Ideal (2) | 1,835 | 86 | 1,166 | 87 | ||
| Physical activity | ||||||
| Poor (0) | 181 | 8 | 77 | 6 | ||
| Intermediate (1) | 526 | 25 | 319 | 24 | ||
| Ideal (2) | 1,438 | 67 | 950 | 71 | ||
| Diet | ||||||
| Poor (0) | 465 | 22 | 441 | 33 | ||
| Intermediate (1) | 1,486 | 69 | 826 | 61 | ||
| Ideal (2) | 194 | 9 | 79 | 6 | ||
| Body weight | ||||||
| Poor (0) | 451 | 21 | 198 | 15 | ||
| Intermediate (1) | 809 | 38 | 671 | 50 | ||
| Ideal (2) | 885 | 41 | 477 | 35 | ||
| Fasting plasma glucose | ||||||
| Poor (0) | 230 | 11 | 203 | 15 | ||
| Intermediate (1) | 812 | 38 | 632 | 47 | ||
| Ideal (2) | 1,103 | 51 | 511 | 38 | ||
| Blood pressure | ||||||
| Poor (0) | 886 | 41 | 535 | 40 | ||
| Intermediate (1) | 889 | 41 | 594 | 44 | ||
| Ideal (2) | 370 | 17 | 217 | 16 | ||
| Total cholesterol | ||||||
| Poor (0) | 627 | 29 | 145 | 11 | ||
| Intermediate (1) | 904 | 42 | 489 | 36 | ||
| Ideal (2) | 614 | 29 | 712 | 53 | ||
Abbreviations: ACS, American Cancer Society; AHA, American Heart Association; SD, standard deviation.
a Dietary data for the second cohort were collected at the 1995–1996 study visit.
Table 2.
Selected Sociodemographic and Health Characteristics According to Concordance With Disease Prevention Guidelines (n = 3,491), Cardiovascular Health Study, 1989–1990 and 1992–1993a
| Characteristic | ACS Guidelines Total Score, %b | AHA Guidelines Total Score, %b | ||||||
|---|---|---|---|---|---|---|---|---|
| Low (0–5) (n = 602) | Moderate (6–8) (n = 2,370) | High (9–10) (n = 519) | P Valuec | Low (0–6) (n = 484) | Moderate (7–10) (n = 2,369) | High (11–14) (n = 638) | P Valuec | |
| ACS guidelines total score | <0.0001 | |||||||
| Low (0–5) | 51 | 15 | 1 | |||||
| Moderate (6–7) | 44 | 50 | 25 | |||||
| High (8–10) | 5 | 35 | 74 | |||||
| AHA guidelines total score | <0.0001 | |||||||
| Low (0–6) | 41 | 10 | 0 | |||||
| Moderate (7–10) | 58 | 73 | 54 | |||||
| High (11–14) | 1 | 17 | 45 | |||||
| Age, yearsd | 72 (5) | 72 (5) | 73 (5) | NS | 72 (5) | 72 (5) | 72 (5) | NS |
| Male sex | 48 | 39 | 24 | <0.0001 | 30 | 40 | 41 | <0.0001 |
| Race | <0.0001 | <0.0001 | ||||||
| Non-Hispanic white | 81 | 87 | 92 | 77 | 87 | 92 | ||
| Black | 16 | 12 | 6 | 21 | 11 | 6 | ||
| Other | 2 | 1 | 2 | 2 | 1 | 2 | ||
| Educational level | 0.0011 | <0.0001 | ||||||
| Less than high-school diploma | 31 | 28 | 23 | 35 | 28 | 18 | ||
| High-school diploma/equivalent | 25 | 28 | 33 | 30 | 29 | 27 | ||
| Some college/vocational | 27 | 23 | 22 | 23 | 23 | 24 | ||
| College graduate | 17 | 22 | 22 | 12 | 20 | 30 | ||
| Annual household income, $ | NS | <0.0001 | ||||||
| <12,000 | 25 | 23 | 17 | 35 | 23 | 14 | ||
| 12,000–24,999 | 33 | 32 | 35 | 35 | 33 | 31 | ||
| 25,000–49,999 | 23 | 25 | 28 | 17 | 26 | 30 | ||
| ≥50,000 | 13 | 13 | 13 | 8 | 12 | 19 | ||
| Missing income | 6 | 6 | 7 | 5 | 6 | 6 | ||
| Marital status | NS | 0.0003 | ||||||
| Married | 66 | 68 | 68 | 59 | 68 | 70 | ||
| Widowed | 23 | 24 | 24 | 29 | 23 | 22 | ||
| Single | 11 | 9 | 8 | 12 | 9 | 7 | ||
| Smoking status | <0.0001 | <0.0001 | ||||||
| Never smoked | 22 | 51 | 66 | 41 | 49 | 54 | ||
| Former smoker | 31 | 42 | 34 | 30 | 39 | 45 | ||
| Current smoker | 47 | 7 | 0 | 30 | 12 | 2 | ||
| BMId,e | 29 (5) | 27 (4) | 23 (3) | <0.0001 | 30 (5) | 27 (4) | 23 (3) | <0.0001 |
| Self-rated health, scored,f | 2.8 (1.1) | 2.6 (1.0) | 2.4 (1.0) | <0.0001 | 3.0 (1.0) | 2.6 (1.0) | 2.3 (1.0) | <0.0001 |
| Alcohol consumption, drinks/weekd | 6.0 (10.1) | 2.2 (5.5) | 0.3 (1.1) | <0.0001 | 2.3 (6.3) | 2.7 (6.9) | 2.2 (4.4) | NS |
| Leisure-time physical activity, MET-hours/weekd | 14 (25) | 27 (31) | 39 (34) | <0.0001 | 11 (20) | 27 (31) | 38 (34) | <0.0001 |
| Functional limitation, IADL ≥1 | 24 | 20 | 15 | 0.0008 | 37 | 29 | 20 | <0.0001 |
| Use of NSAIDS | 12 | 13 | 10 | NS | 16 | 12 | 11 | 0.0156 |
| History of diabetesg | 16 | 14 | 7 | <0.0001 | 36 | 12 | 1 | <0.0001 |
| Medications at baseline | ||||||||
| Lipid-lowering drugs | 3 | 5 | 5 | NS | 7 | 4 | 4 | 0.0078 |
| Antihypertensive | 39 | 40 | 32 | 0.0057 | 53 | 41 | 18 | <0.0001 |
| Oral hypoglycemic agents | 5 | 5 | 3 | NS | 13 | 4 | 1 | <0.0001 |
| Insulin | 3 | 2 | 1 | NS | 4 | 2 | 0 | <0.0001 |
Abbreviations: ACS, American Cancer Society; AHA, American Heart Association; BMI, body mass index; IADL, instrumental activities of daily living; MET, metabolic equivalent of task; NS, not significant; NSAID, nonsteroidal antiinflammatory drug.
a Dietary data for the second cohort were collected at the 1995–1996 study visit.
b Column percentages may not sum to 100 due to rounding.
cP values derived from Pearson χ2 tests or analysis of variance assessing any difference between groups.
d Values are expressed as mean (standard deviation).
e BMI was calculated as weight (kg)/height (m)2.
f Self-rated heath on a scale of 1 to 5, where 1 is “excellent.”
g Prevalent diabetes defined as either pharmacologically treated disease (oral hypoglycemic agents and/or insulin) or fasting plasma glucose >125 mg/dL.
Incident disease, disease specific mortality, and all-cause mortality
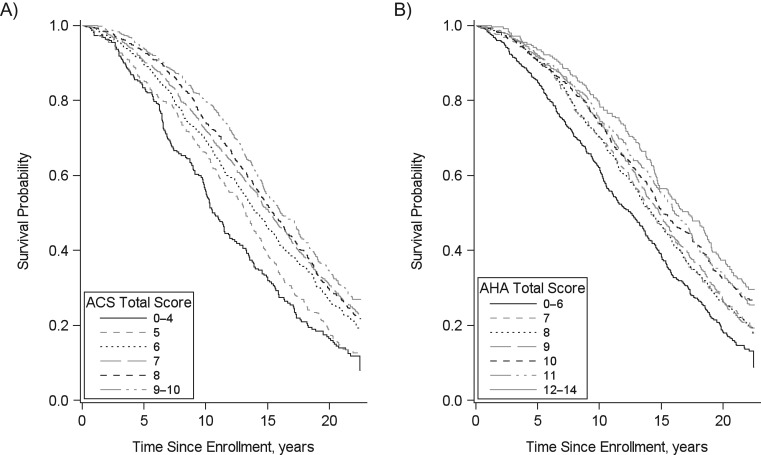
After adjusting for potential confounders, over up to 22 years (median, 15 years) of follow-up, we observed reduced rates of all study outcomes across groups characterized by increased concordance with the ACS cancer prevention guidelines (P < 0.0001 for all outcomes) and AHA ideal health metrics (P < 0.005 for all outcomes; Table 3). Relative to those in the lowest category of ACS guideline concordance (total score 0–4), those with the highest levels of concordance (total score 9–10) had 0.43 times (95% confidence interval (CI): 0.31, 0.60) and 0.30 times (95% CI: 0.21, 0.44) the hazard of incident cancer and cancer mortality, respectively. Comparing those with highest versus lowest levels of AHA guideline concordance, hazard ratios for cancer incidence and mortality were 0.71 (95% CI: 0.50, 1.01) and 0.57 (95% CI: 0.38, 0.84), respectively. For incident CVD and CV-specific mortality, respectively, highest concordance with ACS guidelines was associated with 0.66 (95% CI: 0.53, 0.81) and 0.60 (95% CI: 0.44, 0.82) times the hazard in the lowest levels of concordance. Similar hazard reductions were observed comparing those with the highest versus lowest levels of AHA concordance (for incident CVD, hazard ratio (HR) = 0.50 (95% CI: 0.40, 0.62); for CV-specific mortality, HR = 0.58 (95% CI: 0.42, 0.80)). Increased concordance with both ACS and AHA guidelines was also associated with reduced rates of all-cause mortality (HR = 0.50 (95% CI: 0.42, 0.60) and 0.67 (95% CI: 0.56, 0.80), respectively). Incremental reductions in rates of all-cause mortality across higher concordance with guidelines are illustrated using Kaplan-Meier curves (Figure 1).
Table 3.
Concordance With American Heart Association or American Cancer Society Guidelines and Subsequent Incidence of Cancer, Cardiovascular Disease, and Disease-Specific and All-Cause Mortality Over Long-Terma Follow-up (n = 3,491), Cardiovascular Health Study
| Disease Prevention Guideline and Concordance | Cancerb | CVD | All-Cause Mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Person-Years | No. of Cases | HRc | 95% CI | Person-Years | No. of Cases | HRc | 95% CI | Person-Years | No. of Cases | HRc | 95% CI | |
| Incidence | ||||||||||||
| ACS total score | ||||||||||||
| 0–4 | 1,921 | 70 | 1.00 | Referent | 1,964 | 131 | 1.00 | Referent | ||||
| 5 | 3,572 | 97 | 0.72 | 0.53, 0.98 | 3,764 | 230 | 0.89 | 0.71, 1.10 | ||||
| 6 | 6,803 | 149 | 0.61 | 0.46, 0.81 | 7,483 | 407 | 0.75 | 0.62, 0.92 | ||||
| 7 | 8,908 | 192 | 0.61 | 0.46, 0.80 | 9,927 | 474 | 0.68 | 0.56, 0.83 | ||||
| 8 | 8,574 | 171 | 0.58 | 0.44, 0.77 | 9,759 | 448 | 0.68 | 0.56, 0.83 | ||||
| 9–10 | 5,744 | 80 | 0.43 | 0.31, 0.60 | 6,444 | 288 | 0.66 | 0.53, 0.81 | ||||
| P-continuousd | <0.0001 | <0.0001 | ||||||||||
| AHA total score | ||||||||||||
| 0–6 | 4,528 | 108 | 1.00 | Referent | 4,378 | 306 | 1.00 | Referent | ||||
| 7 | 5,128 | 122 | 0.93 | 0.72, 1.21 | 5,405 | 335 | 0.87 | 0.74, 1.01 | ||||
| 8 | 6,565 | 154 | 0.92 | 0.72, 1.18 | 7,214 | 383 | 0.73 | 0.63, 0.85 | ||||
| 9 | 6,678 | 143 | 0.85 | 0.66, 1.10 | 7,413 | 367 | 0.69 | 0.59, 0.81 | ||||
| 10 | 5,793 | 106 | 0.72 | 0.55, 0.95 | 6,637 | 281 | 0.60 | 0.51, 0.71 | ||||
| 11 | 4,124 | 79 | 0.76 | 0.56, 1.02 | 4,962 | 197 | 0.54 | 0.45, 0.65 | ||||
| 12–14 | 2,705 | 47 | 0.71 | 0.50, 1.01 | 3,333 | 109 | 0.50 | 0.40, 0.62 | ||||
| P-continuousd | 0.0044 | <0.0001 | ||||||||||
| Mortalitye | ||||||||||||
| ACS total score | ||||||||||||
| 0–4 | 2,574 | 60 | 1.00 | Referent | 2,574 | 60 | 1.00 | Referent | 2,574 | 193 | 1.00 | Referent |
| 5 | 4,854 | 81 | 0.66 | 0.47, 0.92 | 4,854 | 120 | 0.94 | 0.69, 1.29 | 4,854 | 327 | 0.81 | 0.68, 0.97 |
| 6 | 9,553 | 121 | 0.49 | 0.36, 0.67 | 9,553 | 197 | 0.71 | 0.53, 0.95 | 9,553 | 537 | 0.60 | 0.51, 0.71 |
| 7 | 12,439 | 135 | 0.44 | 0.32, 0.59 | 12,439 | 211 | 0.61 | 0.46, 0.82 | 12,439 | 642 | 0.57 | 0.49, 0.67 |
| 8 | 12,128 | 125 | 0.42 | 0.31, 0.58 | 12,128 | 211 | 0.65 | 0.48, 0.87 | 12,128 | 620 | 0.57 | 0.49, 0.68 |
| 9–10 | 8,025 | 58 | 0.30 | 0.21, 0.44 | 8,025 | 135 | 0.60 | 0.44, 0.82 | 8,025 | 371 | 0.50 | 0.42, 0.60 |
| P-continuousd | <0.0001 | <0.0001 | <0.0001 | |||||||||
| AHA total score | ||||||||||||
| 0–6 | 5,975 | 99 | 1.00 | Referent | 5,975 | 155 | 1.00 | Referent | 5,975 | 406 | 1.00 | Referent |
| 7 | 7,180 | 82 | 0.64 | 0.48, 0.86 | 7,180 | 163 | 0.86 | 0.69, 1.07 | 7,180 | 404 | 0.79 | 0.69, 0.91 |
| 8 | 9,172 | 116 | 0.70 | 0.53, 0.92 | 9,172 | 179 | 0.68 | 0.54, 0.84 | 9,172 | 519 | 0.74 | 0.65, 0.85 |
| 9 | 9,332 | 108 | 0.66 | 0.50, 0.87 | 9,332 | 170 | 0.66 | 0.53, 0.83 | 9,332 | 510 | 0.75 | 0.65, 0.85 |
| 10 | 8,092 | 89 | 0.64 | 0.48, 0.86 | 8,092 | 116 | 0.54 | 0.42, 0.69 | 8,092 | 394 | 0.68 | 0.59, 0.79 |
| 11 | 5,954 | 50 | 0.47 | 0.33, 0.66 | 5,954 | 98 | 0.59 | 0.46, 0.77 | 5,954 | 287 | 0.63 | 0.54, 0.74 |
| 12–14 | 3,867 | 36 | 0.57 | 0.38, 0.84 | 3,867 | 53 | 0.58 | 0.42, 0.80 | 3,867 | 170 | 0.67 | 0.56, 0.80 |
| P-continuousd | <0.0001 | <0.0001 | <0.0001 | |||||||||
Abbreviations: ACS, American Cancer Society; AHA, American Heart Association; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
a Long-term follow-up was up to 22 years (median, 15 years). Dietary data were collected in 1989–1990 and 1995–1996.
b Incident cancers included: 148 prostate, 119 lung/respiratory, 107 breast, 97 colorectal, 70 hematologic, 68 noncolorectal gastrointestinal, 43 female genital tract, 42 urinary, and 65 other, unknown, or ill-defined.
c Adjusted for age (continuous), self-rated health (continuous), race/ethnicity, income, education, sex, and marital status. Models of CVD incidence, cardiovascular mortality, and all-cause mortality outcomes additionally adjusted for nonsteroidal antiinflammatory drug use and limitations in instrumental activities of daily living.
dP values derived from Wald tests in which scores for concordance with ACS and AHA guidelines were entered into models as continuous variables.
e Cardiovascular disease deaths included those from atherosclerotic coronary disease, cerebrovascular disease (stroke), other atherosclerotic disease (such as aortic aneurysm), and other vascular disease (such as valvular heart disease or pulmonary embolism). Noncardiovascular deaths were first classified into 19 disease and organ-system categories and then collapsed into 5 categories: dementia, cancer, pulmonary disease, infection, and other causes.
Figure 1.
Adherence to American Cancer Society (ACS) and American Heart Association (AHA) prevention guidelines and overall survival (n = 3,491), Cardiovascular Health Study, 1989–2011. Kaplan-Meier curves for overall survival by adherence to American Cancer Society Guidelines for Nutrition and Physical Activity for Cancer Prevention (A) and American Heart Association cardiovascular health metrics (B). Participants were free of cancer or cardiovascular disease at baseline.
We also examined the multivariable-adjusted association of concordance with individual disease-prevention targets and rates of incident cancer and CVD (Web Table 2), as well as cause-specific and all-cause mortality (Web Table 3). Although not all targets were significantly associated with each outcome, most associations were in the expected direction and showed the expected dose-response relationship with outcomes.
Subsequent analyses examined the joint associations of concordance with the health behavior targets established by the ACS guidelines for physical activity, diet, and alcohol consumption, and concordance with AHA recommended levels of cardiometabolic risk factors including BMI, BP, TC, and FPG (Table 4). Numbers of individuals achieving the targets of ACS and AHA appear in Web Table 4. Irrespective of the number of achieved AHA cardiometabolic risk-factor targets, individuals with the least favorable score for ACS health behavior concordance tended to have the highest rates of all-cause mortality and incident CVD. Even among those with ideal levels of 3 or 4 AHA cardiometabolic risk markers, better concordance with ACS health behavior targets was associated with reduced mortality. Within this stratum we observed an 82% higher hazard of mortality (95% CI: 25, 167) when comparing subjects with the lowest versus highest concordance with health behavior targets. For the outcome of incident CVD, in the group with ideal levels of 3 or 4 AHA cardiometabolic risk makers, the hazard ratio was 1.36 (95% CI: 0.84, 2.22) when comparing individuals with the lowest versus highest concordance with health behavior targets. We did not observe significant interaction between ACS health behavior targets and AHA clinical cardiometabolic risk-factor targets in their ability to predict all-cause mortality (P-interaction = 0.25) and incident CVD (P-interaction = 0.65). In contrast to the health behaviors, the cardiometabolic variables did not display a consistent gradient of rates with increasing number of targets met. When smoking was incorporated into the modified ACS health behavior concordance score (Web Table 5), the gradient of all-cause mortality and incident CVD rates was even greater across the range of concordance with behavioral recommendations.
Table 4.
Modified American Cancer Society Health Behaviors Score as a Predictor of All-Cause Mortality and Incident Cardiovascular Disease, According to Number of Ideal Cardiometabolic Risk-Factor Metrics Over Long-Term Follow-upa (n = 3,491), Cardiovascular Health Study
| Concordance With ACS Health Behavior Guidelines | No. of AHA Cardiometabolic Risk-Factor Targets Met (FPG, BMI, BP, TC) | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3–4 | P Valuec | |||||||
| HRb | 95% CI | HRb | 95% CI | HRb | 95% CI | HRb | 95% CI | HRb | 95% CI | ||
| All-cause mortality | 0.25 | ||||||||||
| ACS behaviors sumd | |||||||||||
| 0–2 | 1.19 | 0.88, 1.62 | 1.46 | 1.12, 1.90 | 1.23 | 0.90, 1.68 | 1.82 | 1.25, 2.67 | 1.39 | 1.20, 1.61 | |
| 3–4 | 1.08 | 0.89, 1.31 | 1.04 | 0.87, 1.24 | 1.21 | 1.01, 1.46 | 1.18 | 0.97, 1.45 | 1.13 | 1.04, 1.23 | |
| 5–6 | 1.10 | 0.89, 1.36 | 0.90 | 0.75, 1.10 | 1.00 | 0.82, 1.22 | 1 | Referent | 1 | Referent | |
| P continuouse | 0.642 | <0.001 | 0.022 | 0.033 | <0.001 | ||||||
| Incident CVD | 0.65 | ||||||||||
| ACS behaviors sumd | |||||||||||
| 0–2 | 1.45 | 1.02, 2.05 | 1.41 | 1.03, 1.92 | 1.14 | 0.78, 1.68 | 1.36 | 0.84, 2.22 | 1.23 | 1.03, 1.47 | |
| 3–4 | 1.33 | 1.06, 1.66 | 1.15 | 0.93, 1.42 | 1.06 | 0.85, 1.32 | 0.91 | 0.71, 1.18 | 1.04 | 0.95, 1.15 | |
| 5–6 | 1.14 | 0.89, 1.47 | 1.16 | 0.93, 1.45 | 0.98 | 0.77, 1.24 | 1 | Referent | 1 | Referent | |
| P continuouse | 0.048 | 0.496 | 0.279 | 0.954 | 0.054 | ||||||
Abbreviations: ACS, American Cancer Society; AHA, American Heart Association; BMI, body mass index; BP, blood pressure; CI, confidence interval; CVD, cardiovascular disease; FPG, fasting plasma glucose; HR, hazard ratio; TC, total cholesterol.
a Long-term follow-up was up to 22 years (median, 15 years). Dietary data were collected in 1989–1990 and 1995–1996.
b Hazard ratios were derived from Cox proportional hazards models with predictors for the sum of ACS behaviors, the number of AHA cardiometabolic risk-factor targets met, and the interaction between them, adjusted for age, detailed smoking history (never smoked, quit >20 years ago, quit 6–20 years ago, quit 0–5 years ago, and currently smoked), self-rated health, race/ethnicity, income, education, sex, marital status, limitations in instrumental activities of daily living, and use of nonsteroidal antiinflammatory drugs, antihypertensive agents, oral hypoglycemic agents, insulin, and lipid-lowering drugs.
c Interaction P values test for heterogeneity in the adjusted hazard ratio for the sum of ACS behaviors across subgroups defined by the number of AHA cardiometabolic risk-factor targets met.
d ACS behaviors sum is the sum of ACS subscores for alcohol consumption, physical activity, and diet.
e Derived from Wald tests for ACS behaviors sum treated as a continuous variable within subgroups defined by the number of AHA cardiometabolic risk-factor targets met, while adjusting for all variables in the model.
DISCUSSION
Among adults aged 65 years or older, we examined the degree of concordance with recommended targets put forth by the AHA ideal metrics for cardiovascular health and the ACS Guidelines on Nutrition and Physical Activity for Cancer Prevention statements, as well as the association of guideline concordance with rates of major health outcomes. Over long-term follow-up, a higher level of concordance with the AHA and ACS prevention guidelines was associated with lower rates of cancer and CVD, as well as lower cause-specific and all-cause mortality. Comparing those in the highest category versus the lowest category of concordance defined an approximately 2-fold gradient in incidence, after adjustment for potential confounding factors. Favorable rates of outcomes were amplified by concordance with multiple recommendations. To our knowledge, this is among the largest long-term follow-up studies of older adults to confirm that achieving recommendations of widely disseminated cardiovascular and cancer prevention guidelines is associated with reductions in multiple major health outcomes and mortality. To our knowledge, this analysis is unique in that we jointly examine the AHA and ACS scores and examine the associations of behavioral metrics beyond the standard physiologic and cardiometabolic metrics. We performed cross-classification on multiple sets of guideline targets. These analyses suggested that behavioral recommendations set forth by the ACS, including diet and physical activity targets, in aggregate, might add to standard cardiometabolic risk factors in the prediction of all-cause mortality and incident CVD.
Several recent studies have shown improvements in chronic disease outcomes and survival among individuals meeting health and lifestyle goals set by health organizations (1–3, 5, 6, 8, 36). We confirmed here, among older adults, that the ACS guidelines predict CVD-specific mortality (2) and the AHA’s ideal cardiovascular health metrics were associated with cancer incidence (6). Elements of AHA and ACS risk prevention guidelines are partially overlapping, although each guideline was most strongly associated with its intended target outcome.
One of our most important findings is the observation that favorable health behaviors were associated with reduced mortality regardless of the level of cardiometabolic risk factors. This was seen even among those with “ideal” levels of BMI, BP, TC, and/or FPG, although in the elderly, low levels of these factors may, paradoxically, be a marker of ill health. Thus, despite being free of elevated cardiometabolic risk factors late in life, it may still be important for older adults to follow diet and physical activity recommendations.
Other notable findings concerned the disease associations for individual risk factors in older adults. While FPG was a robust predictor of CVD and all-cause mortality, we did not find a significant association of FPG with cancer incidence or mortality. Although prior studies had suggested increased cancer risk in people with obesity and diabetes (37), our data suggest that this may not hold in older adulthood. As expected, we found that elevated TC was not an adverse CVD risk factor in older adults (38, 39). Smoking was the most prominent single risk factor for CVD, cancer, and mortality, which confirms the long-term adverse health effects of smoking even over 20-plus years’ follow-up in a cohort aged >65 years.
Relatively few prior studies have examined guideline-established targets and major health events in adults older than 65 years of age, although some available data sets include large numbers of elderly and provide concordant results about the association of health behaviors and clinical risk factors with incidence of CVD (5). Strengths of our study include a well-characterized population, allowing adjustment for multiple confounders, and rigorous assessment of incident and fatal events. Extended prospective follow-up for more than 20 years and a large number of events allowed us to evaluate associations of health behaviors and outcomes within levels of traditional CVD risk-factor targets. However, a relatively small sample size for cancer outcomes limited the precision of associations in this study. Other limitations include the use of information on lifestyle that was self-reported by participants. For instance, self-reported physical activity levels were higher than might be expected (7), which may represent mismeasurement due to desirability biases. Such community-based studies composed of volunteer participants may also preferentially select for healthier individuals, thereby limiting the generalizability of our findings. Because behaviors were measured before the occurrence of study events, selective recall bias was unlikely to have induced false associations with study outcomes. In addition, our study was conducted before widespread uptake of recommendations for physical activity, which may have increased internal validity compared with contemporary cohorts, by 1) reducing social desirability bias in reporting of health behaviors, and 2) reducing biases whereby behavior modification might have been widely prescribed to patients at high disease risk, therefore introducing reverse causation between event rates and health behavior patterns. Nevertheless, participants who reported having healthier levels of the specific behaviors included in the guidelines may have had a propensity toward a healthful lifestyle in general, so that other unmeasured factors may have also contributed to their favorable outcomes. The tendency to engage in healthy behaviors may either persist or vary over time, and we were not able to adjust for the effects of health behaviors at younger ages when examining the benefits of healthy lifestyle practices in this older population. The study was not designed to quantify the effects of drug treatments, which may be most beneficial when used in the context of a healthy lifestyle. In addition, secular increases in medication use in the primary prevention setting since these data were collected may affect applicability of findings to present-day populations (40, 41). We excluded participants with prior CVD or cancer to reduce confounding and to remove individuals who may have had the most pronounced changes in risk factors over time. This approach, as well as the fact that only US adults were studied, may limit generalizability. These observational data do not substitute for intervention study designs that establish the risks and benefits of guideline-driven strategies for risk reduction.
In conclusion, adults who were 65 years of age or older and who were concordant with ACS and AHA professional guidelines had reduced rates of chronic disease and mortality. Our findings suggest that interventions to improve diet and increase physical activity have a place in the recommendations to older adults for prevention of CVD and cancer. We followed a large population-based study cohort for more than 20 years, showing that both incident disease and mortality may be preventable into the most advanced stage of life. Even among those who had ideal levels of cardiometabolic biomarkers, prolonged survival was associated with meeting recommended targets for moderate-to-vigorous physical activity, avoidance of excessive alcohol consumption, and following a healthy dietary pattern. These findings support increasing efforts to develop efficient clinical approaches to assist older adults in achieving and maintaining healthy lifestyle behaviors.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (Heather Greenlee, Gina S. Lovasi, John Richardson, Linda P. Fried); Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, New York (Heather Greenlee); Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York (Garrett Strizich, Robert C. Kaplan); Department of Biostatistics, School of Public Health, University of Washington, Seattle, Washington (Mary L. Biggs); Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington (Christopher I. Li); Division of Public Health Sciences, Wake Forest School of Medicine, Winston-Salem, North Carolina (Gregory L. Burke); Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Annette L. Fitzpatrick, Amanda M. Fretts, Bruce M. Psaty); Division of General Internal Medicine, Department of Medicine, University of Washington, Seattle, Washington (Bruce M. Psaty); and Group Health Research Institute, Group Health Cooperative, Seattle, Washington (Bruce M. Psaty). H.G. is currently at the Cancer Prevention Program, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington.
This work was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086, and U01HL130014 and grant HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by grants R01AG023629 and R01AG031890 from the National Institute on Aging and grants K23CA141052 and R01CA116393 from the National Cancer Institute.
A full list of principal Cardiovascular Health Study investigators and institutions can be found at CHS-NHLBI.org.
The sponsors/funders had no role in the research described here.
Conflict of interest: none declared.
REFERENCES
- 1. Kabat GC, Matthews CE, Kamensky V, et al. Adherence to cancer prevention guidelines and cancer incidence, cancer mortality, and total mortality: a prospective cohort study. Am J Clin Nutr. 2015;101(3):558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCullough ML, Patel AV, Kushi LH, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1089–1097. [DOI] [PubMed] [Google Scholar]
- 3. Thomson CA, McCullough ML, Wertheim BC, et al. Nutrition and physical activity cancer prevention guidelines, cancer risk, and mortality in the Women’s Health Initiative. Cancer Prev Res (Phila). 2014;7(71):42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30–67. [DOI] [PubMed] [Google Scholar]
- 5. Dong C, Rundek T, Wright CB, et al. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the Northern Manhattan study. Circulation. 2012;125(24):2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rasmussen-Torvik LJ, Shay CM, Abramson JG, et al. Ideal cardiovascular health is inversely associated with incident cancer the atherosclerosis risk in communities study. Circulation. 2013;127(12):1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307(12):1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Chi HJ, Cui LF, et al. The ideal cardiovascular health metrics associated inversely with mortality from all causes and from cardiovascular diseases among adults in a Northern Chinese industrial city. PLoS One. 2014;9(2):e89161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Artero EG, España-Romero V, Lee DC, et al. Ideal cardiovascular health and mortality : Aerobics Center Longitudinal Study. Mayo Clin Proc. 2012;87(10):944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 12. Kochanek KD, Xu J, Murphy SL, et al. Deaths: Final Data for 2014 Hyattsville, MD: National Center for Health Statistics; 2016. (National vital statistics reports; vol. 65 no. 4) (DHHS Publication no. 2016–1120). https://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_04.pdf. Accessed October 22, 2016. [PubMed]
- 13. Hermanson B, Omenn GS, Kronmal RA, et al. Beneficial six-year outcome of smoking cessation in older men and women with coronary artery disease. Results from the CASS registry. N Engl J Med. 1988;319(21):1365–1369. [DOI] [PubMed] [Google Scholar]
- 14. Physical Activity Guidelines Advisory Committee Physical Activity Guidelines Advisory Committee Report, 2008 Washington, DC: US Department of Health and Human Services; 2008. https://health.gov/paguidelines/report/pdf/committeereport.pdf. Accessed October 22, 2016.
- 15. Soares-Miranda L, Siscovick DS, Psaty BM, et al. Physical activity and risk of coronary heart disease and stroke in older adults. Circulation. 2016;133(2):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ford DW, Jensen GL, Hartman TJ, et al. Association between dietary quality and mortality in older adults: a review of the epidemiological evidence. J Nutr Gerontol Geriatr. 2013;32(2):85–105. [DOI] [PubMed] [Google Scholar]
- 18. Heuberger RA. Alcohol and the older adult: a comprehensive review. J Nutr Elder. 2009;28(3):203–235. [DOI] [PubMed] [Google Scholar]
- 19. Winter JE, MacInnis RJ, Wattanapenpaiboon N, et al. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99(4):875–890. [DOI] [PubMed] [Google Scholar]
- 20. Schatz IJ, Masaki K, Yano K, et al. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: a cohort study. Lancet. 2001;358(9279):351–355. [DOI] [PubMed] [Google Scholar]
- 21. Odden MC, Peralta CA, Haan MN, et al. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med. 2012;172(15):1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. [DOI] [PubMed] [Google Scholar]
- 24. Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(4):358–366. [DOI] [PubMed] [Google Scholar]
- 25. Psaty M, Kuller H, Burke L, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):270–277. [DOI] [PubMed] [Google Scholar]
- 26. Psaty BM, Lee M, Savage PJ, et al. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45(6):683–692. [DOI] [PubMed] [Google Scholar]
- 27. Cushman M, Cornell ES, Howard PR, et al. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41(2):264–270. [PubMed] [Google Scholar]
- 28. Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–285. [DOI] [PubMed] [Google Scholar]
- 29. Kumanyika S, Tell GS, Shemanski L, et al. Eating patterns of community-dwelling older adults: the Cardiovascular Health Study. Ann Epidemiol. 1994;4(5):404–415. [DOI] [PubMed] [Google Scholar]
- 30. Kumanyika S, Tell GS, Fried L, et al. Picture-sort method for administering a food frequency questionnaire to older adults. J Am Diet Assoc. 1996;96(2):137–144. [DOI] [PubMed] [Google Scholar]
- 31. Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1136. [DOI] [PubMed] [Google Scholar]
- 32. Taylor HL, Jacobs DR, Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–755. [DOI] [PubMed] [Google Scholar]
- 33. Chen C, Lewis SK, Voigt L, et al. Prostate carcinoma incidence in relation to prediagnostic circulating levels of insulin-like growth factor I, insulin-like growth factor binding protein 3, and insulin. Cancer. 2005;103(1):76–84. [DOI] [PubMed] [Google Scholar]
- 34. Newman AB, Sachs MC, Arnold AM, et al. Total and cause-specific mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2009;64(12):1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. [Google Scholar]
- 36. Vergnaud AC, Romaguera D, Peeters PH, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: results from the European Prospective Investigation into Nutrition and Cancer cohort study. Am J Clin Nutr. 2013;97(5):1107–1120. [DOI] [PubMed] [Google Scholar]
- 37. Tsilidis KK, Kasimis JC, Lopez DS, et al. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. [DOI] [PubMed] [Google Scholar]
- 38. Krumholz HM, Seeman TE, Merrill SS, et al. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all-cause mortality in persons older than 70 years. JAMA. 1994;272(17):1335–1340. [PubMed] [Google Scholar]
- 39. Psaty BM, Anderson M, Kronmal RA, et al. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(10):1639–1647. [DOI] [PubMed] [Google Scholar]
- 40. Carroll MD, Kit BK, Lacher DA, et al. Trends in lipids and lipoproteins in US adults, 1988–2010. JAMA. 2012;308(15):1545–1554. [DOI] [PubMed] [Google Scholar]
- 41. Gu Q, Burt VL, Dillon CF, et al. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health and Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126(17):2105–2114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.