Abstract
Previous studies have suggested an association between fetal growth restriction and the risk of spontaneous preterm birth (sPTB). However, addressing this association is methodologically challenging. We conducted a prospective cohort study of nulliparous women with a singleton pregnancy in Cambridge, United Kingdom (2008–2012). Ultrasonic fetal biometry was performed at 20 weeks of gestation as per routine clinical care. Participants also had blinded research ultrasonography performed at approximately 28 weeks. Biometric measurements were expressed as gestational-age-adjusted z scores. Fetal growth velocity was quantified by change in z score between 20 weeks and 28 weeks. Risk of sPTB, defined as delivery at ≥28 weeks and <37 weeks associated with labor in the absence of induction, was analyzed using cause-specific Cox regression. Of 3,892 women, 98 (2.5%) had sPTB. When compared with the other decile groups, the lowest decile of growth velocity of the fetal femur between 20 and 28 weeks was associated with increased risk of sPTB (hazard ratio = 2.37, 95% confidence interval: 1.43, 3.93; P < 0.001). Adjustment for maternal characteristics had no material effect (hazard ratio = 2.50, 95% confidence interval: 1.50, 4.14; P < 0.001). There were no significant associations between other fetal measurements and risk of sPTB. To conclude, slow growth velocity of the fetal femur is associated with an increased risk of sPTB.
Keywords: fetal biometry, fetal growth, growth velocity, preterm birth, spontaneous preterm birth
In the attempt to achieve the United Nations' Millennium Development Goals, a global effort has been made to address the preventable causes of maternal and child mortality and to improve maternal and child health (1–4). As notable gains are being made, poorly understood causes, such as preterm birth (PTB), are contributing to an increasingly large proportion of maternal and child morbidity and mortality (5). The World Health Organization estimates that approximately 15 million babies are born preterm each year, among whom 1 million die, making prematurity the leading cause of neonatal death worldwide and the second-leading cause of under-age-5 mortality (5, 6). Importantly, this figure is on the rise, with increases noted in the numbers of both iatrogenic PTBs (iPTB) and spontaneous PTBs (sPTB) (5–8). The pathophysiology of sPTB is poorly understood (5, 7, 9, 10). Better understanding of the mechanisms involved might allow screening and intervention.
Previous studies have shown associations between placental biomarkers and sPTB, including pregnancy-associated plasma protein A, α-fetoprotein, and corticotropin-releasing hormone (11–17). However, the mechanistic link with the risk of sPTB is unclear. A number of studies have characterized relationships between fetal growth restriction and the risk of sPTB. Because fetal growth restriction is associated with some of the same biomarkers as sPTB (11–18), it could lie within the causal pathway linking biomarker levels to sPTB. Births of growth-restricted fetuses are often cases of iPTB, but less is known about the direct relationship between specific aspects of fetal growth patterns and the timing of sPTB (19–21).
A handful of studies have directly explored the relationship between fetal growth and sPTB. Some have lacked a clear definition of abnormal fetal growth, using birth weight or birth weight for gestational age as a proxy, which does not fully capture the process of growth in utero (22–25). Others have used ultrasonographic measures of fetal biometry taken at 1 time point, which provides a snapshot of fetal size but not the process of growth (26–30). In some cases, gestational age was measured by the date of the last menstrual period, which decreases the reliability of classification of prematurity (26, 28, 31). Importantly, many of the articles describing studies based on ultrasound-measured fetal growth have not explicitly mentioned blinding of fetal growth measurements (13, 22–33), and some have also not specifically distinguished between iPTB and sPTB (34). Some authors have reported not individual biometric measurements but only estimated fetal weight. Varying reference standards have been used, and different cutoffs have been applied to define fetal growth restriction. The studies have varied in their design and analytical methods—for example, logistic regression (13–16, 22–25, 28, 30, 33), linear regression (13, 31), or time-to-event analysis (35).
In the present analysis, we used data from the Pregnancy Outcome Prediction (POP) Study, a prospective cohort study of nulliparous women with a viable singleton pregnancy in Cambridge, United Kingdom, in which women underwent serial blinded ultrasonography throughout pregnancy (36). Previous analyses of the POP Study data have addressed the utility of universal ultrasonography as a screening test for fetal growth restriction (37). In the present analysis, we investigated the association between early fetal growth and the subsequent risk of sPTB.
METHODS
Study population
The POP Study was a prospective cohort study of nulliparous women with a viable singleton pregnancy, based at the Rosie Hospital in Cambridge, United Kingdom. The study was approved by the Cambridge Local Research Ethics Committee. The full study protocol and the study cohort have been described elsewhere (36, 37).
Briefly, between January 14, 2008, and July 31, 2012, women visiting the Rosie Hospital who met the study criteria (nulliparous, viable singleton pregnancy) were invited to enroll in the POP Study. Written informed consent was obtained by research midwives. In addition to the routine ultrasound scans given at approximately 12–14 weeks (for dating) and 20 weeks (for anomaly) of gestation, women underwent ultrasonography for the purposes of research at 28 and 36 weeks of gestation. Participants and their health-care providers were blinded to the results of these scans, unless a major incidental finding was observed (major congenital anomaly, placenta previa, severe oligohydramnios, or noncephalic presentation at 36 weeks).
Gestational age was defined by ultrasound at the time of the dating scan. Maternal age was recorded at recruitment, maternal weight was measured at the dating scan appointment, and maternal height was measured at the 20-week appointment. Body mass index was calculated from weight and height (weight (kg)/height (m)2). Information on maternal characteristics was collected either through a computer-assisted questionnaire at the 20-week scan, from examination of the clinical case record, or through linkage to the hospital's electronic databases (marital status, previous spontaneous and therapeutic abortions, ethnicity, smoking status, age at leaving full-time education, and Index of Multiple Deprivation 2007 (38) score, based on residential area (37)).
Participants who withdrew or delivered their babies elsewhere were excluded from the POP Study. Additional exclusion criteria for the purposes of this analysis were stillbirth, a pregnancy ending before 28 weeks of gestation, a default from any scan, or a history of essential hypertension or preexisting diabetes mellitus.
Statistical analysis
Wilcoxon's rank-sum test was used to compare continuous variables, and Pearson's χ2 test or Fisher's exact test was used to compare variables with binary outcomes. Fetal growth was assessed by 1) z scores of ultrasound measurements of fetal biometry taken at 20 and 28 weeks of gestation and 2) fetal growth velocity, defined as change in z score of fetal biometry between 20 and 28 weeks of gestation. Z scores were adjusted for gestational age, estimated within the POP Study using the method outlined by Altman and Chitty (39). Measures of fetal biometry included head circumference (HC), abdominal circumference (AC), femur length (FL), estimated fetal weight (EFW; calculated using the equation of Hadlock et al. (40)), HC:AC ratio, and AC:FL ratio (37). Additionally, z scores were calculated with respect to the recently published INTERGROWTH-21st references for fetal growth (41) where the given measurement was reported (EFW and HC:AC and AC:FL ratios were not reported). sPTB was defined as birth before 37 weeks' gestation in the absence of induction of labor or elective cesarean section. Preliminary analyses included logistic regression on sPTB, excluding iPTBs. Linearity of associations and the effects of interactions between fetal biometry and maternal characteristics on sPTB were tested using the likelihood ratio test.
Cause-specific Cox regression was used to estimate the risk of sPTB with respect to each of the measures of fetal biometry or fetal growth velocity. The at-risk period for sPTB was defined as 28 + 0/7 – 36 + 6/7 weeks of gestation (from 28 completed weeks and 0 days to 36 completed weeks and 6 days; “0/7” and “6/7” refer to the additional number of days completed). The number of deliveries at less than 28 weeks was very small, and these births predated the 28-week scan. Clinically indicated preterm deliveries were treated as censored at the time of delivery. The analysis of sPTB was repeated using competing-risks regression (Fine and Gray model) treating indicated preterm deliveries as competing events. Each growth measure was analyzed 1) on its own in relation to sPTB and 2) adjusted for maternal characteristics. Records with missing values were excluded from the regression analysis.
We further examined the relationship between fetal biometry and the risk of sPTB by dichotomizing biometric and growth velocity measures into the most extreme decile of change (that associated with the slowest growth) versus the other 9 decile groups. In most cases, the lowest decile group was clearly the extreme decile associated with poor growth. However, an elevated HC:AC ratio is associated with poor fetal growth, and analysis of this measure compared the highest decile group with the other 9. In addition to regression analyses, cumulative incidence curves were produced using the competing-risks method for each group, and the population attributable fraction related to the extreme decile was calculated using the cause-specific method. All analyses were performed using Stata, version 14.0 (StataCorp LP, College Station, Texas).
RESULTS
Out of 4,512 women enrolled in the study, a total of 620 were excluded for 1 or more of the following reasons: participant formally withdrew (n = 67), child was delivered elsewhere (n = 255), stillbirth (n = 12), pregnancy ended prior to 28 weeks of gestation (n = 42), participant defaulted from any scan (n = 184), or participant reported prior primary hypertension (n = 73) or diabetes mellitus (n = 15). A total of 3,892 women were included in the analyses.
The characteristics of the study population are given by type of birth in Table 1. Ninety-eight (2.5%) births were sPTB, and 59 (1.5%) were iPTB (including 3 women with ruptured membranes whose labor was induced only after 3 days of rupture); data on the nature of 9 (0.2%) births were missing. Women who had term deliveries were taller but were similar regarding age, body mass index, numbers of previous spontaneous and therapeutic abortions, ethnicity, and indicators of socioeconomic status.
Table 1.
Characteristics of Study Participants by Birth Outcome in the POP Study, Cambridge, United Kingdom, 2008–2012
| Type of Birtha |
P Valueb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall |
Term |
Spontaneous Preterm |
||||||||
| No. of Participants | % | Median (IQR) | No. of Participants | % | Median (IQR) | No. of Participants | % | Median (IQR) | ||
| Pregnancy outcome | ||||||||||
| Total births | 3,892 | 100 | 3,726 | 96 | 98 | 2.5 | ||||
| Gestational age at birth, weeks | 40.3 (39.3–41.1) | 40.4 (39.4–41.1) | 35.9 (34.7–36.4) | |||||||
| Maternal characteristics | ||||||||||
| Age, years | 30.3 (26.8–33.3) | 30.3 (26.8–33.3) | 30.8 (27.3–33.7) | 0.23 | ||||||
| Height, cm | 165 (161–169) | 165 (161–170) | 164 (159–167) | 0.005 | ||||||
| Body mass indexc | 24.0 (21.8–27.2) | 24.0 (21.8–27.2) | 24.3 (22.5–26.4) | 0.75 | ||||||
| Previous spontaneous abortion | ||||||||||
| Yes | 396 | 10 | 375 | 10 | 9 | 9.2 | ||||
| No | 3,496 | 90 | 3,351 | 90 | 89 | 91 | 0.78 | |||
| Previous therapeutic abortion | ||||||||||
| Yes | 35 | 0.9 | 35 | 0.9 | 0 | 0 | ||||
| No | 3,857 | 99 | 3,691 | 99 | 98 | 100 | >0.99 | |||
| White ethnicity | ||||||||||
| Yes | 3,620 | 93 | 3,469 | 93 | 89 | 91 | ||||
| No | 210 | 5.4 | 201 | 5.4 | 7 | 7.1 | 0.44 | |||
| Missing data | 62 | 1.6 | 56 | 1.5 | 2 | 2 | ||||
| Smoking during pregnancy | ||||||||||
| Yes | 183 | 4.7 | 174 | 4.7 | 6 | 6.1 | ||||
| No | 3,709 | 95 | 3,552 | 95 | 92 | 94 | 0.5 | |||
| Married | ||||||||||
| Yes | 2,670 | 69 | 2,547 | 68 | 68 | 69 | ||||
| No | 1,220 | 31 | 1,179 | 32 | 30 | 31 | 0.83 | |||
| Age at which full-time education was discontinued, years | 21 (18–23) | 21 (18–23) | 21 (18–23) | 0.81 | ||||||
| IMD scored | 8.9 (5.7–14.2) | 8.9 (5.7–14.2) | 8.9 (5.6–12.2) | 0.71 | ||||||
Abbreviation: IMD, Index of Multiple Deprivation; IQR, interquartile range; POP, Pregnancy Outcome Prediction.
a Overall characteristics include all types of births in the study population: term, spontaneous preterm, iatrogenic preterm, and unknown. Of the total number of births, 59 (1.5%) preterm births were iatrogenic, and data were missing on the nature of 9 (0.2%) births.
b Continuous variables were compared between term births and spontaneous preterm births using the Wilcoxon rank-sum test, and binary variables were compared using Pearson's χ2 test (apart from previous therapeutic abortion, for which Fisher's exact test was used due to small cell counts). With the exception of Pearson's χ2 test, all tests for significance were 2-sided.
c Weight (kg)/height (m)2.
d IMD (38) scores in the POP Study population ranged from 0.67 (least deprived) to 53.5 (most deprived).
When the results of fetal biometry at 20 weeks were analyzed as continuous variables, the risk of sPTB was directly associated with EFW (Table 2). A 1-standard-deviation (SD) increase in EFW was associated with a 26% increase in the risk of sPTB after adjustment for maternal characteristics. At 28 weeks, the risk of sPTB was inversely associated with FL and directly associated with AC:FL ratio (Table 2). A 1-SD increase in these measures was associated with a 19% decrease and a 23% increase in the risk of sPTB, respectively. However, both associations were attenuated by adjustment for maternal characteristics and were no longer statistically significant. We next assessed the relationship between growth velocity between the 20- and 28-week ultrasound scans and the risk of sPTB (i.e., change in the z score of paired measurements between the 2 scans, with lower values representing smaller relative measurements at the time of the second scan). When they were analyzed as continuous variables, higher growth velocities of FL and EFW were associated with a decreased risk of sPTB (Table 2). A 1-SD increase in these measures was associated with a 27% decrease and a 21% decrease in the risk of sPTB, respectively. Neither association was affected by adjustment for maternal characteristics. All associations were very similar between the cause-specific regression and competing-risks regression and when the INTERGROWTH-21st reference standard was employed (Table 3).
Table 2.
Fetal Growth and the Risk of Spontaneous Preterm Birth in the POP Study, Cambridge, United Kingdom, 2008–2012
| Measurea | No. of Participants | Cause-Specific Regression Analysis |
Competing-Risks Regression Analysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjustedb |
Unadjusted |
Adjustedb |
||||||||||
| HR | 95% CI | P Valuec | HR | 95% CI | P Value | sHR | 95% CI | P Value | sHR | 95% CI | P Value | ||
| Fetal biometry at 20 weeks’ gestation | |||||||||||||
| HC | 3,619 | 1.19 | 0.97, 1.46 | 0.10 | 1.22 | 0.99, 1.49 | 0.06 | 1.19 | 0.98, 1.44 | 0.08 | 1.22 | 1.01, 1.47 | 0.04 |
| AC | 3,660 | 1.18 | 0.96, 1.45 | 0.12 | 1.20 | 0.98, 1.48 | 0.08 | 1.18 | 0.96, 1.44 | 0.12 | 1.21 | 0.98, 1.48 | 0.07 |
| FL | 3,667 | 1.12 | 0.92, 1.36 | 0.28 | 1.18 | 0.96, 1.44 | 0.12 | 1.12 | 0.91, 1.38 | 0.28 | 1.18 | 0.96, 1.46 | 0.13 |
| EFW | 3,658 | 1.20 | 0.99, 1.47 | 0.06 | 1.26 | 1.03, 1.54 | 0.02 | 1.21 | 0.99, 1.47 | 0.06 | 1.27 | 1.04, 1.54 | 0.02 |
| HC:AC ratio | 3,609 | 0.95 | 0.77, 1.18 | 0.66 | 0.95 | 0.77, 1.18 | 0.66 | 0.95 | 0.76, 1.20 | 0.68 | 0.95 | 0.75, 1.20 | 0.68 |
| AC:FL ratio | 3,658 | 1.03 | 0.84, 1.26 | 0.80 | 1.00 | 0.82, 1.23 | 0.98 | 1.03 | 0.84, 1.25 | 0.80 | 1.00 | 0.82, 1.22 | 0.98 |
| Fetal growth velocity from 20 weeks to 28 weeks | |||||||||||||
| ΔHC | 3,445 | 0.85 | 0.67, 1.06 | 0.15 | 0.86 | 0.68, 1.09 | 0.21 | 0.85 | 0.68, 1.08 | 0.18 | 0.87 | 0.68, 1.11 | 0.21 |
| ΔAC | 3,656 | 0.91 | 0.74, 1.10 | 0.33 | 0.90 | 0.74, 1.10 | 0.32 | 0.91 | 0.75, 1.11 | 0.34 | 0.91 | 0.74, 1.11 | 0.34 |
| ΔFL | 3,662 | 0.73 | 0.60, 0.89 | 0.002 | 0.73 | 0.59, 0.89 | 0.002 | 0.74 | 0.60, 0.90 | 0.004 | 0.73 | 0.58, 0.92 | 0.007 |
| ΔEFW | 3,654 | 0.79 | 0.64, 0.99 | 0.04 | 0.79 | 0.63, 0.99 | 0.04 | 0.80 | 0.64, 0.99 | 0.04 | 0.79 | 0.63, 0.99 | 0.05 |
| ΔHC:AC ratio | 3,433 | 0.98 | 0.81, 1.17 | 0.80 | 0.98 | 0.81, 1.18 | 0.83 | 0.98 | 0.80, 1.19 | 0.83 | 0.98 | 0.80, 1.20 | 0.84 |
| ΔAC:FL ratio | 3,651 | 1.16 | 0.97, 1.38 | 0.11 | 1.16 | 0.97, 1.40 | 0.11 | 1.16 | 0.96, 1.40 | 0.13 | 1.16 | 0.96, 1.42 | 0.13 |
| Fetal biometry at 28 weeks | |||||||||||||
| HCd | 3,490 | 1.02 | 0.82, 1.27 | 0.85 | 1.07 | 0.86, 1.33 | 0.57 | 1.03 | 0.81, 1.31 | 0.83 | 1.07 | 0.84, 1.37 | 0.58 |
| AC | 3,670 | 1.06 | 0.86, 1.29 | 0.60 | 1.08 | 0.88, 1.33 | 0.47 | 1.06 | 0.87, 1.30 | 0.58 | 1.09 | 0.88, 1.34 | 0.44 |
| FL | 3,669 | 0.81 | 0.66, 0.99 | 0.04 | 0.85 | 0.68, 1.05 | 0.12 | 0.81 | 0.65, 1.02 | 0.08 | 0.85 | 0.67, 1.09 | 0.20 |
| EFW | 3,670 | 0.99 | 0.81, 1.22 | 0.96 | 1.04 | 0.85, 1.28 | 0.70 | 1.00 | 0.81, 1.23 | 0.96 | 1.05 | 0.84, 1.30 | 0.67 |
| HC:AC ratio | 3,488 | 0.99 | 0.80, 1.23 | 0.96 | 0.99 | 0.80, 1.22 | 0.93 | 1.00 | 0.79, 1.25 | 0.97 | 0.99 | 0.79, 1.24 | 0.93 |
| AC:FL ratio | 3,667 | 1.23 | 1.01, 1.51 | 0.04 | 1.21 | 0.99, 1.49 | 0.06 | 1.23 | 0.99, 1.54 | 0.06 | 1.21 | 0.97, 1.52 | 0.09 |
Abbreviations: AC, abdominal circumference; CI, confidence interval; EFW, estimated fetal weight; FL, femur length; HC, head circumference; HR, hazard ratio; POP, Pregnancy Outcome Prediction; sHR, ratio of the subdistribution hazards.
a Fetal biometry and growth velocity measures are expressed in z scores estimated in the POP Study. Therefore, hazard ratios are given per 1-standard-deviation increase in fetal biometry or growth velocity measure.
b Adjusted for maternal height, age, body mass index, marital status, previous spontaneous abortion, ethnicity, smoking status, age at leaving full-time education, and Index of Multiple Deprivation score.
c All P values in the table were derived from the z test specific to each method (i.e., cause-specific or competing-risks regression). All tests for significance were 2-sided.
d Departure from proportionality for week 28 HC was detected in both the unadjusted cause-specific regression analysis (Schoenfeld test: P = 0.01) and the competing-risks regression analysis (z test for interaction with follow-up time: P = 0.008).
Table 3.
Risk of Spontaneous Preterm Birth According to Measures of Fetal Growth, Estimated Using an International Reference Standard, POP Study, Cambridge, United Kingdom, 2008–2012
| Measurea | No. of Participants | Cause-Specific Regression Analysis |
Competing-Risks Regression Analysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjustedb |
Unadjusted |
Adjustedb |
||||||||||
| HR | 95% CI | P Valuec | HR | 95% CI | P Value | sHR | 95% CI | P Value | sHR | 95% CI | P Value | ||
| Fetal biometry at 20 weeks’ gestation | |||||||||||||
| HC | 3,619 | 1.24 | 0.95, 1.62 | 0.12 | 1.28 | 0.98, 1.67 | 0.07 | 1.24 | 0.97, 1.59 | 0.09 | 1.28 | 1.00, 1.64 | 0.05 |
| AC | 3,660 | 1.22 | 0.94, 1.57 | 0.13 | 1.25 | 0.97, 1.62 | 0.09 | 1.22 | 0.95, 1.57 | 0.12 | 1.26 | 0.98, 1.61 | 0.08 |
| FL | 3,667 | 1.14 | 0.89, 1.45 | 0.29 | 1.21 | 0.95, 1.55 | 0.12 | 1.14 | 0.89, 1.46 | 0.30 | 1.22 | 0.94, 1.57 | 0.13 |
| Fetal growth velocity from 20 weeks to 28 weeks | |||||||||||||
| ΔHC | 3,445 | 0.87 | 0.67, 1.11 | 0.26 | 0.89 | 0.69, 1.15 | 0.38 | 0.87 | 0.67, 1.15 | 0.33 | 0.90 | 0.68, 1.19 | 0.46 |
| ΔAC | 3,656 | 0.92 | 0.75, 1.14 | 0.47 | 0.93 | 0.75, 1.15 | 0.49 | 0.93 | 0.75, 1.15 | 0.49 | 0.93 | 0.74, 1.16 | 0.52 |
| ΔFL | 3,662 | 0.72 | 0.58, 0.89 | 0.002 | 0.72 | 0.58, 0.90 | 0.003 | 0.72 | 0.58, 0.91 | 0.006 | 0.73 | 0.57, 0.93 | 0.01 |
| Fetal biometry at 28 weeks’ gestation | |||||||||||||
| HCd | 3,490 | 1.02 | 0.82, 1.26 | 0.86 | 1.06 | 0.86, 1.32 | 0.57 | 1.03 | 0.81, 1.30 | 0.83 | 1.07 | 0.84, 1.36 | 0.59 |
| AC | 3,670 | 1.05 | 0.86, 1.28 | 0.62 | 1.08 | 0.88, 1.32 | 0.48 | 1.06 | 0.87, 1.29 | 0.59 | 1.08 | 0.88, 1.33 | 0.46 |
| FL | 3,669 | 0.82 | 0.67, 1.00 | 0.05 | 0.86 | 0.70, 1.05 | 0.13 | 0.83 | 0.67, 1.02 | 0.08 | 0.86 | 0.69, 1.08 | 0.20 |
Abbreviations: AC, abdominal circumference; CI, confidence interval; FL, femur length; HC, head circumference; HR, hazard ratio; IQR, interquartile range; POP, Pregnancy Outcome Prediction; sHR, ratio of the subdistribution hazards.
a Fetal biometry and growth velocity measures are expressed in z scores with respect to the INTERGROWTH-21st Project reference (41). HRs are given per 1-standard-deviation increase in fetal biometry or growth velocity measure according to the international reference. Median percentiles in the POP Study were 52.8 (IQR, 32.8–71.7) for HC, 66.6 (IQR, 44.4–83.0) for AC, and 48.0 (IQR, 30.7–73.3) for FL at 20 weeks’ gestation and 73.4 (IQR, 46.9–90.2) for HC, 66.1 (IQR, 40.3–86.7) for AC, and 53.2 (IQR, 27.4–79.1) for FL at 28 weeks’ gestation. Median percentiles of change from 20 weeks to 28 weeks were 70.8 (IQR, 50.0–86.1) for ΔHC, 51.9 (IQR, 27.1–74.3) for ΔAC, and 53.8 (IQR, 27.6–76.4) for ΔFL.
b Adjusted for maternal height, age, body mass index, marital status, previous spontaneous abortion, ethnicity, smoking status, age at leaving full-time education, and Index of Multiple Deprivation score.
c All P values in the table were derived from the z test specific to each method (i.e., cause-specific or competing-risks regression). All tests for significance were 2-sided.
d Departure from proportionality for week 28 HC was detected in both the unadjusted cause-specific regression analysis (Schoenfeld test: P = 0.01) and the competing-risks regression analysis (z test for interaction with follow-up time: P = 0.008).
When biometric measurements were analyzed comparing the extreme decile associated with poor growth with the other decile groups, there was no association between any measurement taken at 28 weeks and the risk of sPTB. When growth velocity was assessed, babies in the lowest decile of FL growth velocity between 20 and 28 weeks of gestation had a 2- to 3-fold risk of sPTB (Table 4, Figure 1). Adjustment for maternal characteristics had no material effect, and 12% (95% confidence interval: 3, 21) of the sPTBs were estimated to be attributable to fetuses' being in the lowest decile of FL growth velocity. None of the other extreme deciles of fetal growth velocity were associated with the risk of sPTB (Table 4). There were no effects of interactions between the lowest decile of FL growth velocity and any of the maternal characteristics on sPTB (likelihood ratio test: P > 0.05 in all tests). None of the infants in the lowest decile of FL growth velocity who delivered preterm had skeletal dysplasia. Eight babies with sPTB were delivered by cesarean section. Our main findings persisted when they were excluded: Babies in the lowest decile of FL growth velocity had a 2.88-fold (95% confidence interval: 1.73, 4.81) risk of sPTB in the cause-specific regression analysis after adjustment for maternal characteristics.
Table 4.
Risk of Spontaneous Preterm Birth According to Measures of Fetal Growth, With Growth Expressed as the Most Extreme Decilea Indicative of Impaired Growth, POP Study, Cambridge, United Kingdom, 2008–2012
| Measureb | No. of Participants | Cause-Specific Regression Analysis |
Competing-Risks Regression Analysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjustedc |
Unadjusted |
Adjustedc |
||||||||||
| HR | 95% CI | P Valued | HR | 95% CI | P Value | sHR | 95% CI | P Value | sHR | 95% CI | P Value | ||
| Fetal biometry at 20 weeks’ gestation | |||||||||||||
| HC | 3,619 | 0.45 | 0.17, 1.23 | 0.12 | 0.41 | 0.15, 1.13 | 0.09 | 0.45 | 0.17, 1.23 | 0.12 | 0.41 | 0.15, 1.15 | 0.09 |
| AC | 3,660 | 0.79 | 0.36, 1.70 | 0.54 | 0.75 | 0.34, 1.62 | 0.46 | 0.77 | 0.36, 1.67 | 0.52 | 0.74 | 0.34, 1.58 | 0.43 |
| FL | 3,667 | 0.79 | 0.37, 1.72 | 0.56 | 0.72 | 0.33, 1.55 | 0.40 | 0.79 | 0.37, 1.71 | 0.55 | 0.71 | 0.33, 1.54 | 0.39 |
| EFW | 3,658 | 0.89 | 0.43, 1.83 | 0.74 | 0.84 | 0.41, 1.74 | 0.64 | 0.88 | 0.42, 1.81 | 0.72 | 0.83 | 0.40, 1.71 | 0.62 |
| HC:AC ratioe | 3,609 | 1.19 | 0.62, 2.30 | 0.60 | 1.22 | 0.63, 2.35 | 0.56 | 1.19 | 0.62, 2.30 | 0.60 | 1.22 | 0.63, 2.36 | 0.56 |
| AC:FL ratio | 3,658 | 1.01 | 0.51, 2.00 | 0.98 | 1.05 | 0.53, 2.10 | 0.89 | 1.01 | 0.51, 2.00 | 0.99 | 1.05 | 0.53, 2.10 | 0.89 |
| Fetal growth velocity from 20 weeks to 28 weeks | |||||||||||||
| ΔHC | 3,445 | 1.30 | 0.67, 2.53 | 0.43 | 1.26 | 0.65, 2.45 | 0.49 | 1.27 | 0.66, 2.47 | 0.48 | 1.24 | 0.64, 2.42 | 0.53 |
| ΔAC | 3,656 | 1.12 | 0.58, 2.16 | 0.74 | 1.12 | 0.58, 2.17 | 0.73 | 1.12 | 0.58, 2.16 | 0.74 | 1.13 | 0.58, 2.18 | 0.73 |
| ΔFL | 3,662 | 2.37 | 1.43, 3.93 | <0.001 | 2.50 | 1.50, 4.14 | <0.001 | 2.36 | 1.42, 3.91 | 0.001 | 2.48 | 1.48, 4.17 | 0.001 |
| ΔEFW | 3,654 | 1.24 | 0.66, 2.33 | 0.50 | 1.26 | 0.67, 2.37 | 0.47 | 1.23 | 0.65, 2.31 | 0.53 | 1.25 | 0.66, 2.35 | 0.49 |
| ΔHC:AC ratioe | 3,433 | 0.85 | 0.39, 1.85 | 0.69 | 0.87 | 0.40, 1.89 | 0.73 | 0.86 | 0.39, 1.86 | 0.69 | 0.87 | 0.40, 1.91 | 0.73 |
| ΔAC:FL ratio | 3,651 | 0.85 | 0.41, 1.75 | 0.65 | 0.86 | 0.42, 1.78 | 0.69 | 0.85 | 0.41, 1.75 | 0.66 | 0.87 | 0.42, 1.81 | 0.70 |
| Fetal biometry at 28 weeks’ gestation | |||||||||||||
| HC | 3,490 | 1.13 | 0.56, 2.25 | 0.73 | 1.04 | 0.52, 2.08 | 0.92 | 1.11 | 0.55, 2.21 | 0.77 | 1.02 | 0.51, 2.06 | 0.95 |
| AC | 3,670 | 0.66 | 0.29, 1.51 | 0.32 | 0.62 | 0.27, 1.43 | 0.27 | 0.64 | 0.28, 1.48 | 0.30 | 0.61 | 0.27, 1.40 | 0.24 |
| FL | 3,669 | 1.55 | 0.88, 2.73 | 0.13 | 1.41 | 0.79, 2.51 | 0.24 | 1.53 | 0.87, 2.70 | 0.14 | 1.39 | 0.78, 2.49 | 0.26 |
| EFW | 3,670 | 1.02 | 0.51, 2.02 | 0.96 | 0.93 | 0.46, 1.86 | 0.83 | 0.99 | 0.50, 1.98 | 0.99 | 0.91 | 0.44, 1.85 | 0.79 |
| HC:AC ratioe | 3,488 | 0.98 | 0.48, 2.04 | 0.97 | 0.97 | 0.47, 2.02 | 0.95 | 0.98 | 0.47, 2.04 | 0.96 | 0.97 | 0.47, 2.02 | 0.93 |
| AC:FL ratio | 3,667 | 0.89 | 0.43, 1.83 | 0.75 | 0.93 | 0.45, 1.93 | 0.84 | 0.89 | 0.43, 1.83 | 0.75 | 0.93 | 0.45, 1.92 | 0.85 |
Abbreviations: AC, abdominal circumference; CI, confidence interval; EFW, estimated fetal weight; FL, femur length; HC, head circumference; HR, hazard ratio; POP, Pregnancy Outcome Prediction; sHR, ratio of the subdistribution hazards.
a Extreme decile cutoff points of z scores were −1.2835 for HC, −1.2807 for AC, −1.2136 for FL, −1.2640 for EFW, 1.3296 for HC:AC ratio, and −1.3030 for AC:FL ratio at 20 weeks and −1.2588 for HC, −1.2780 for AC, −1.2517 for FL, −1.2378 for EFW, 1.2717 for HC:AC ratio, and −1.2638 for AC:FL ratio at 28 weeks. Extreme decile cutoff points of z score changes from 20 weeks to 28 weeks were −1.1949 for ΔHC, −1.3289 for ΔAC, −1.3020 for ΔFL, −1.1640 for ΔEFW, 1.4960 for ΔHC:AC ratio, and −1.4823 for ΔAC:FL ratio.
b Fetal biometry and growth velocity measures were expressed in z scores estimated in the POP Study and dichotomized to the clinically relevant extreme decile versus the other decile groups.
c Adjusted for maternal height, age, body mass index, marital status, previous spontaneous abortion, ethnicity, smoking status, age at leaving full-time education, and Index of Multiple Deprivation score.
d All P values in the table were derived from the z test specific to each method (i.e., cause-specific or competing-risks regression). All tests for significance were 2-sided.
e The HR associated with being in the lowest decile of each measurement versus the other decile groups was calculated for all measures except HC:AC ratio and ΔHC:AC ratio, where the highest decile group was compared with the other 9.
Figure 1.
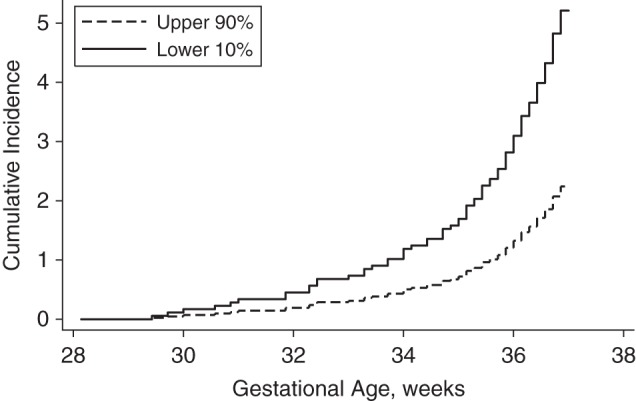
Cumulative incidence (number of births/100 women) of spontaneous preterm birth between 28 + 0/7 and 36 + 6/7 weeks of gestation (see “Statistical analysis” section in text) in the Pregnancy Outcome Prediction (POP) Study, Cambridge, United Kingdom, 2008–2012. Fetuses in the lowest decile of femur length growth velocity between 20 and 28 weeks of gestation (solid line) were compared with all other fetuses (dashed line) using the competing-risks method (P = 0.001).
DISCUSSION
The key finding of the present study was that reduced growth velocity of the fetal femur between 20 and 28 weeks of gestation was associated with an increased risk of sPTB. The association was evident both when FL growth velocity was treated as a continuous variable and when it was dichotomized as the lowest decile of growth velocity versus the other 9. In the latter case, the risk of sPTB was increased 2- to 3-fold. There was also a weak inverse association between an increase in EFW between 20 and 28 weeks and sPTB. However, because EFW incorporates FL, this may simply be due to the same association, as there were no independent relationships between the other biometric measures used to calculate EFW and the risk of PTB. These data imply that the factors which lead to reduced growth of the fetal femur between 20 and 28 weeks of gestation are also associated with the risk of sPTB.
A number of previous studies have addressed the relationship between first- and second-trimester fetal growth and the risk of sPTB. The study that is most directly comparable with the present analysis is that of the Generation R cohort, in which Gaillard et al. (33) also performed serial ultrasonic fetal biometry. The investigators reported a number of associations, some of which were also observed in the present study and some of which were not. Consistent with our study, they found that a decrease in the relative size of the fetal femur between 20 and 30 weeks of gestation was associated with an increased risk of sPTB (33). The observation and magnitude of the association were very similar to our findings. They also observed that higher fetal biometry values at 20 weeks' gestation were associated with an increased risk of sPTB. In our study, associations of a similar magnitude were observed, but they were statistically significant only in multivariate analysis. Moreover, the researchers observed that reduced growth velocities (between approximately 20 and approximately 30 weeks) for both HC and AC were also associated with increased risks of sPTB, whereas we did not.
There are multiple potential explanations for the discrepant results of our study and that of Gaillard et al. (33). The estimates for the association between AC growth velocity and sPTB were similar, but since the Generation R cohort was larger than ours, the statistical power to identify associations of this magnitude was higher in that study. However, the estimated association between HC growth velocity and sPTB was clearly different. Some of the key differences in the Generation R cohort were that it included women of mixed parity, that 43% of the cohort was of non-European ethnic origin, and that gestational age in that study was based on either menstrual history or early ultrasound, depending on the availability of a reliable menstrual record. Further studies, or further analysis of the Generation R cohort, may help to explain the differences. However, the consistency of the findings for FL in both studies indicates that this association is very likely to be real and to be generalizable.
When we analyzed the association between biometry and the risk of sPTB, we studied the measurements both as continuous variables and by dichotomizing them into the extreme decile associated with fetal growth restriction versus the other decile groups. We used this approach because there may be different etiological associations between factors which cause variation across the whole range of the population and factors which lead to a few pregnancies’ having very low values. For example, if we assume that there is some underlying pathological process that impairs growth of the fetal femur, it is most likely that only a small proportion of pregnancies would be so affected. It would also be expected that the 10% of pregnancies with the lowest value of FL growth velocity would include a much higher proportion of these pathological cases than the other 9 decile groups. Hence, it might be expected that analysis of measures as continuous variables addresses primarily the effect of physiological variation in the parameter, whereas analysis of measures by the most extreme decile group addresses primarily pathological variation in the parameter. Interestingly, when we analyzed fetal biometry using the most extreme decile, the only association we identified was between reduced FL growth velocity and sPTB.
The above observations suggest that there is a strong association between pathological determinants of an isolated short femur in the second trimester of pregnancy and the risk of sPTB. Interestingly, a series of papers have highlighted the association between isolated short femur and the risk of severe early-onset fetal growth restriction (34, 42–44). Collectively, these observations suggest that poor growth of the femur during the second trimester may be a marker of an important underlying determinant of adverse pregnancy outcome. Previous studies have found an association between levels of blood biomarkers during pregnancy, such as pregnancy-associated plasma protein A and α-fetoprotein, and both fetal growth restriction and sPTB (11–18). Both the biochemical and ultrasonic data indicate that the factors which lead to fetal growth restriction may also lead to sPTB. There is a potentially plausible biological link. Growth-restricted fetuses have activation of the stress pathway of the hypothalamic-pituitary-adrenal axis (45), and there is extensive evidence linking the hypothalamic-pituitary-adrenal axis to physiological control of the timing of parturition (46).
Studies of growth restriction distinguish between asymmetrical growth restriction, where there is an increase in the size of the head relative to the size of the body, and symmetrical growth restriction, where the baby is small but HC and AC are in proportion. Bocca-Tjeertes et al. (47) recently reported that symmetrically growth-restricted babies had the greatest deviation from normal height and weight at age 4 years, and they speculated that symmetrical growth restriction leading to PTB is due to early-onset placental dysfunction. We previously reported an association between low first-trimester levels of pregnancy-associated plasma protein A and PTB (12). Early-onset placental dysfunction might explain the observed association between reduced FL growth velocity and sPTB, and we are planning to test this hypothesis in the future.
A key strength of the POP Study design is its prospective investigation of serial fetal biometric measures at consistent time points. Moreover, gestational age was measured using early-pregnancy ultrasonography. Both sPTB and iPTB were clearly defined and were ascertained by trained midwives. To better capture the growth process, we assessed fetal growth velocity, defined as the change in fetal biometric measure z score, between 20 and 28 weeks of gestation. Most importantly, patients and health-care providers were blinded to the results of the 28-week scan. Hence, the associations between growth velocity and sPTB cannot be explained by biases related to knowledge of the scan result.
We analyzed the data using both cause-specific and competing-risks approaches. The former is preferred for answering etiological questions (estimation of hazard ratios), whereas the latter is preferred for prognostic questions (calculation of cumulative incidence). The focus of the present paper is on etiology, but we present results derived from both approaches, as recommended (48, 49). The number of competing events was small (n = 59), and the 2 approaches gave very similar results. The proportion of missing values in the regression analyses varied between 5.7% and 11.7%, and we considered that imputation was not necessary since the bias resulting from missing values was probably small (50–52). Furthermore, we did not make adjustments to statistical significance levels, although we tested multiple hypotheses. We adopted this approach because our exposure measures of fetal biometry were correlated and our approach was hypothesis-driven. Moreover, the P value for the association between the lowest decile of FL growth and sPTB was less than 0.001, and it is very unlikely that this could be a chance finding due to multiple comparisons.
The cohort included the first pregnancies of predominantly healthy women from a relatively affluent area in the United Kingdom, which may partly explain the relatively low risk of sPTB. It is well recognized that rates of sPTB are much higher in the United States than in the majority of high-income countries (5, 6), and the reasons for this are incompletely understood. However, the association between fetal growth and sPTB will not necessarily be influenced by the overall prevalence of PTB. Slow growth in FL is unlikely to be simply a marker of maternal characteristics, such as maternal height or ethnicity. Although maternal height was associated with both FL growth velocity and sPTB, adjustment for it had very little impact. We did not observe ethnic differences between the sPTB group and babies born at term. The proportion of nonwhite women in the population was low, and we had inadequate statistical power to detect ethnic differences. Recent data from the INTERGROWTH-21st Project have indicated that ethnicity per se has a relatively modest effect on variation in fetal growth (41). Therefore, it is unlikely to be a strong confounder, even in ethnically heterogeneous populations. Maternal body mass index and socioeconomic status were not associated with sPTB in our study, and this may have been due to insufficient statistical power.
Two main recognized subsets of sPTB include sPTB with intact membranes and preterm premature rupture of membranes. It has been recognized that the pathophysiologies of these conditions are distinct (53, 54). While it might further be hypothesized that fetal growth may play a different role in each, we did not make a distinction between the two, nor have most other studies in this field. Given the low number of PTBs observed in the present study, additional splitting of birth outcomes in the study population would have compromised power. This may be a useful avenue for future studies.
In conclusion, we have shown that reduced growth velocity of the fetal femur between 20 and 28 weeks of gestation is associated with an increased risk of sPTB. These data add to a body of evidence indicating that an isolated short femur could be a marker of early-onset fetal growth restriction and that fetal growth restriction may be an important determinant of apparently unexplained sPTB.
ACKNOWLEDGMENTS
Author affiliations: Department of Public Health and Primary Care, School of Clinical Medicine, University of Cambridge, Cambridge, United Kingdom (Uttara Partap); Department of Obstetrics and Gynaecology, School of Clinical Medicine, University of Cambridge, Cambridge, United Kingdom (Ulla Sovio, Gordon C. S. Smith); and NIHR Cambridge Biomedical Research Centre, Cambridge, United Kingdom (Ulla Sovio, Gordon C. S. Smith).
U.P. and U.S. contributed equally to this work.
This study was funded by the NIHR Cambridge Biomedical Research Centre and the Stillbirth and Neonatal Death Society. U.P. was supported by a Dr. Herchel Smith Fellowship.
We are extremely grateful to all staff at the Rosie Hospital and the NIHR Cambridge Clinical Research Facility, who provided assistance for the study by hosting research visits for the cohort members.
GE Healthcare (Fairfield, Connecticut) donated 2 ultrasound machines for use in the POP Study.
The funders played no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1. Requejo J, Bryce J, Victora C. Countdown to 2015: Maternal, Newborn & Child Survival. Building a Future for Women and Children: The 2012 Report. Geneva, Switzerland: World Health Organization and United Nations Children's Fund; 2012. [Google Scholar]
- 2. Lawn JE, Blencowe H, Oza S et al. Every Newborn: progress, priorities, and potential beyond survival. Lancet. 2014;3849938:189–205. [DOI] [PubMed] [Google Scholar]
- 3. Kenny LC, Black MA, Poston L et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: the Screening for Pregnancy Endpoints (SCOPE) international cohort study. Hypertension. 2014;643:644–652. [DOI] [PubMed] [Google Scholar]
- 4. Kenny LC, Broadhurst DI, Dunn W et al. Robust early pregnancy prediction of later preeclampsia using metabolomic biomarkers. Hypertension. 2010;564:741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. March of Dimes; The Partnership for Maternal, Newborn & Child Health; Save the Children et al. Born Too Soon: The Global Action Report on Preterm Birth. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 6. Goldenberg RL, Culhane JF, Iams JD et al. Epidemiology and causes of preterm birth. Lancet. 2008;3719606:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bloomfield FH. How is maternal nutrition related to preterm birth? Annu Rev Nutr. 2011;31:235–261. [DOI] [PubMed] [Google Scholar]
- 8. Steer P. The epidemiology of preterm labour. BJOG. 2005;112(suppl 1):1–3. [DOI] [PubMed] [Google Scholar]
- 9. Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;876:590–600. [DOI] [PubMed] [Google Scholar]
- 10. Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology. 2013;982:106–115. [DOI] [PubMed] [Google Scholar]
- 11. Dugoff L, Hobbins JC, Malone FD et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol. 2004;1914:1446–1451. [DOI] [PubMed] [Google Scholar]
- 12. Smith GC, Stenhouse EJ, Crossley JA et al. Early pregnancy levels of pregnancy-associated plasma protein A and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab. 2002;874:1762–1767. [DOI] [PubMed] [Google Scholar]
- 13. Kirkegaard I, Uldbjerg N, Petersen OB et al. PAPP-A, free β-hCG, and early fetal growth identify two pathways leading to preterm delivery. Prenat Diagn. 2010;3010:956–963. [DOI] [PubMed] [Google Scholar]
- 14. Smith GC, Shah I, Crossley JA et al. Pregnancy-associated plasma protein A and alpha-fetoprotein and prediction of adverse perinatal outcome. Obstet Gynecol. 2006;1071:161–166. [DOI] [PubMed] [Google Scholar]
- 15. Wadhwa PD, Garite TJ, Porto M et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;1914:1063–1069. [DOI] [PubMed] [Google Scholar]
- 16. Smith GC, Crossley JA, Aitken DA et al. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol. 2007;1096:1316–1324. [DOI] [PubMed] [Google Scholar]
- 17. Chellakooty M, Vangsgaard K, Larsen T et al. A longitudinal study of intrauterine growth and the placental growth hormone (GH)-insulin-like growth factor I axis in maternal circulation: association between placental GH and fetal growth. J Clin Endocrinol Metab. 2004;891:384–391. [DOI] [PubMed] [Google Scholar]
- 18. Proctor LK, Toal M, Keating S et al. Placental size and the prediction of severe early onset intrauterine growth restriction in women with low pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 2009;343:274–282. [DOI] [PubMed] [Google Scholar]
- 19. Westgren M, Beall M, Divon M et al. Fetal femur length/abdominal circumference ratio in preterm labor patients with and without successful tocolytic therapy. J Ultrasound Med. 1986;55:243–245. [DOI] [PubMed] [Google Scholar]
- 20. Secher NJ, Kern Hansen P, Thomsen BL et al. Growth retardation in preterm infants. Br J Obstet Gynaecol. 1987;942:115–120. [DOI] [PubMed] [Google Scholar]
- 21. Carreno CA, Costantine MM, Holland MG et al. Approximately one-third of medically indicated late preterm births are complicated by fetal growth restriction. Am J Obstet Gynecol. 2011;2043:263.e261–263.e264. [DOI] [PubMed] [Google Scholar]
- 22. Zeitlin J, Ancel PY, Saurel-Cubizolles MJ et al. The relationship between intrauterine growth restriction and preterm delivery: an empirical approach using data from a European case-control study. BJOG. 2000;1076:750–758. [DOI] [PubMed] [Google Scholar]
- 23. Lackman F, Capewell V, Richardson B et al. The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am J Obstet Gynecol. 2001;1845:946–953. [DOI] [PubMed] [Google Scholar]
- 24. Morken NH, Källen K, Jacobsson B. Fetal growth and onset of delivery: a nationwide population-based study of preterm infants. Am J Obstet Gynecol. 2006;1951:154–161. [DOI] [PubMed] [Google Scholar]
- 25. Bukowski R, Gahn D, Denning J et al. Impairment of growth in fetuses destined to deliver preterm. Am J Obstet Gynecol. 2001;1852:463–467. [DOI] [PubMed] [Google Scholar]
- 26. Smith GC, Smith MF, McNay MB et al. First-trimester growth and the risk of low birth weight. N Engl J Med. 1998;33925:1817–1822. [DOI] [PubMed] [Google Scholar]
- 27. Bukowski R, Smith GC, Malone FD et al. Fetal growth in early pregnancy and risk of delivering low birth weight infant: prospective cohort study. BMJ. 2007;3347598:836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kazemier BM, Kleinrouweler CE, Oudijk MA et al. Is short first-trimester crown-rump length associated with spontaneous preterm birth? Ultrasound Obstet Gynecol. 2012;406:636–641. [DOI] [PubMed] [Google Scholar]
- 29. Hediger ML, Scholl TO, Schall JI et al. Fetal growth and the etiology of preterm delivery. Obstet Gynecol. 1995;852:175–182. [DOI] [PubMed] [Google Scholar]
- 30. Severi FM, Bocchi C, Imperatore A et al. Ultrasound estimated fetal weight slightly below the median is associated with increased risk of spontaneous preterm birth. Prenat Diagn. 2012;326:588–591. [DOI] [PubMed] [Google Scholar]
- 31. Johnsen SL, Wilsgaard T, Rasmussen S et al. Fetal size in the second trimester is associated with the duration of pregnancy, small fetuses having longer pregnancies. BMC Pregnancy Childbirth. 2008;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lampl M, Kusanovic JP, Erez O et al. Early rapid growth, early birth: accelerated fetal growth and spontaneous late preterm birth. Am J Hum Biol. 2009;212:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaillard R, Steegers EA, de Jongste JC et al. Tracking of fetal growth characteristics during different trimesters and the risks of adverse birth outcomes. Int J Epidemiol. 2014;434:1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goetzinger KR, Cahill AG, Macones GA et al. Isolated short femur length on second-trimester sonography: a marker for fetal growth restriction and other adverse perinatal outcomes. J Ultrasound Med. 2012;3112:1935–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang J, Sundaram R, Sun W et al. Fetal growth and timing of parturition in humans. Am J Epidemiol. 2008;1688:946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pasupathy D, Dacey A, Cook E et al. Study protocol. A prospective cohort study of unselected primiparous women: the Pregnancy Outcome Prediction Study. BMC Pregnancy Childbirth. 2008;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sovio U, White IR, Dacey A et al. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet. 2015;38610008:2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noble M, McLennan D, Wilkinson K et al. The English Indices of Deprivation 2007. London, United Kingdom: Department for Communities and Local Government; 2008. [Google Scholar]
- 39. Altman DG, Chitty LS. Charts of fetal size: 1. Methodology. Br J Obstet Gynaecol. 1994;1011:29–34. [DOI] [PubMed] [Google Scholar]
- 40. Hadlock FP, Harrist RB, Sharman RS et al. Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. Am J Obstet Gynecol. 1985;1513:333–337. [DOI] [PubMed] [Google Scholar]
- 41. Papageorghiou AT, Ohuma EO, Altman DG et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet. 2014;3849946:869–879. [DOI] [PubMed] [Google Scholar]
- 42. Vermeer N, Bekker MN. Association of isolated short fetal femur with intrauterine growth restriction. Prenat Diagn. 2013;334:365–370. [DOI] [PubMed] [Google Scholar]
- 43. de Carvalho AA, Carvalho JA, Figueiredo I Jr et al. Association of midtrimester short femur and short humerus with fetal growth restriction. Prenat Diagn. 2013;332:130–133. [DOI] [PubMed] [Google Scholar]
- 44. Papageorghiou AT, Fratelli N, Leslie K et al. Outcome of fetuses with antenatally diagnosed short femur. Ultrasound Obstet Gynecol. 2008;315:507–511. [DOI] [PubMed] [Google Scholar]
- 45. Marciniak B, Patro-Małysza J, Poniedziałek-Czajkowska E et al. Glucocorticoids in pregnancy. Curr Pharm Biotechnol. 2011;125:750–757. [DOI] [PubMed] [Google Scholar]
- 46. Nathanielsz PW. Comparative studies on the initiation of labor. Eur J Obstet Gynecol Reprod Biol. 1998;782:127–132. [DOI] [PubMed] [Google Scholar]
- 47. Bocca-Tjeertes I, Bos A, Kerstjens J et al. Symmetrical and asymmetrical growth restriction in preterm-born children. Pediatrics. 2014;1333:e650–e656. [DOI] [PubMed] [Google Scholar]
- 48. Andersen PK, Geskus RB, de Witte T et al. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;413:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Latouche A, Allignol A, Beyersmann J et al. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. 2013;666:648–653. [DOI] [PubMed] [Google Scholar]
- 50. Peng C-YJ, Harwell M, Liou S-M et al. Advances in missing data methods and implications for educational research. In: Sawilowsky SS, ed. Real Data Analysis. Charlotte, NC: Information Age Publishing; 2006:31–78. [Google Scholar]
- 51. Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health. 2001;255:464–469. [PubMed] [Google Scholar]
- 52. Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;81:3–15. [DOI] [PubMed] [Google Scholar]
- 53. Dekker GA, Lee SY, North RA et al. Risk factors for preterm birth in an international prospective cohort of nulliparous women. PLoS One. 2012;77:e39154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gotsch F, Gotsch F, Romero R et al. The preterm parturition syndrome and its implications for understanding the biology, risk assessment, diagnosis, treatment and prevention of preterm birth. J Matern Fetal Neonatal Med. 2009;22(suppl 2):5–23. [DOI] [PubMed] [Google Scholar]


